Continuous Ultrasound-Assisted Esterification and Transesterification of Palm Fatty Acid Distillate for Ethyl Ester Production
Abstract
1. Introduction
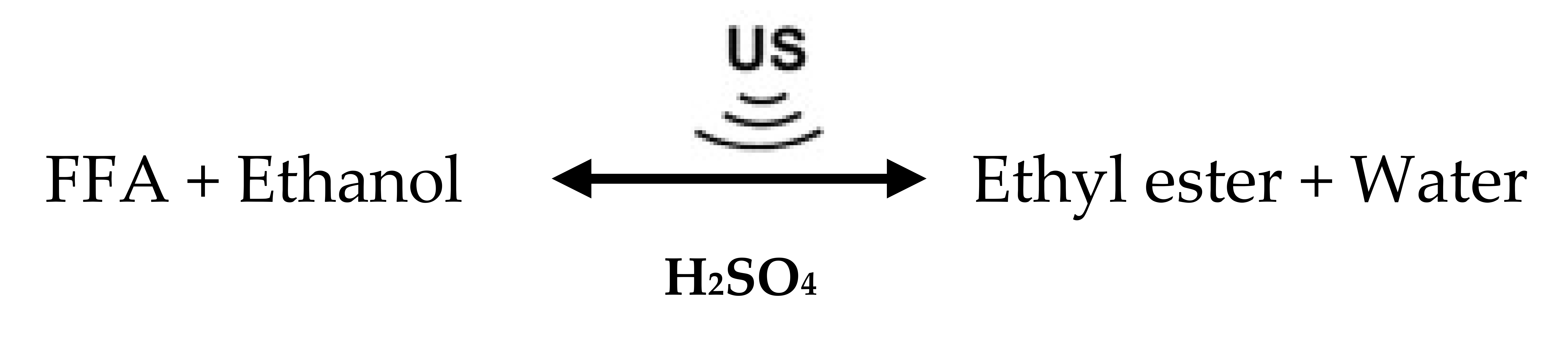

2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Procedure
2.4. Experimental Design
3. Results
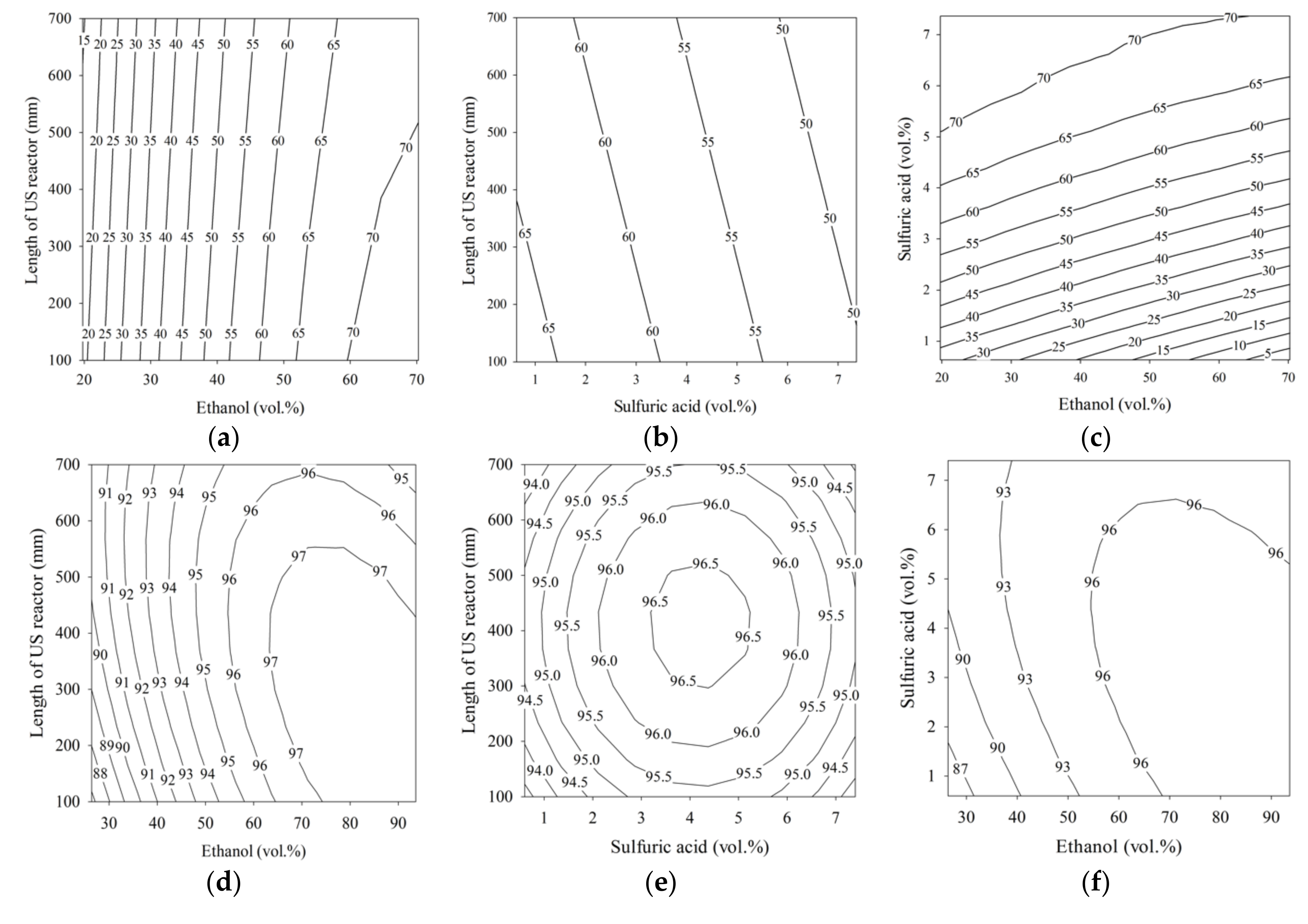
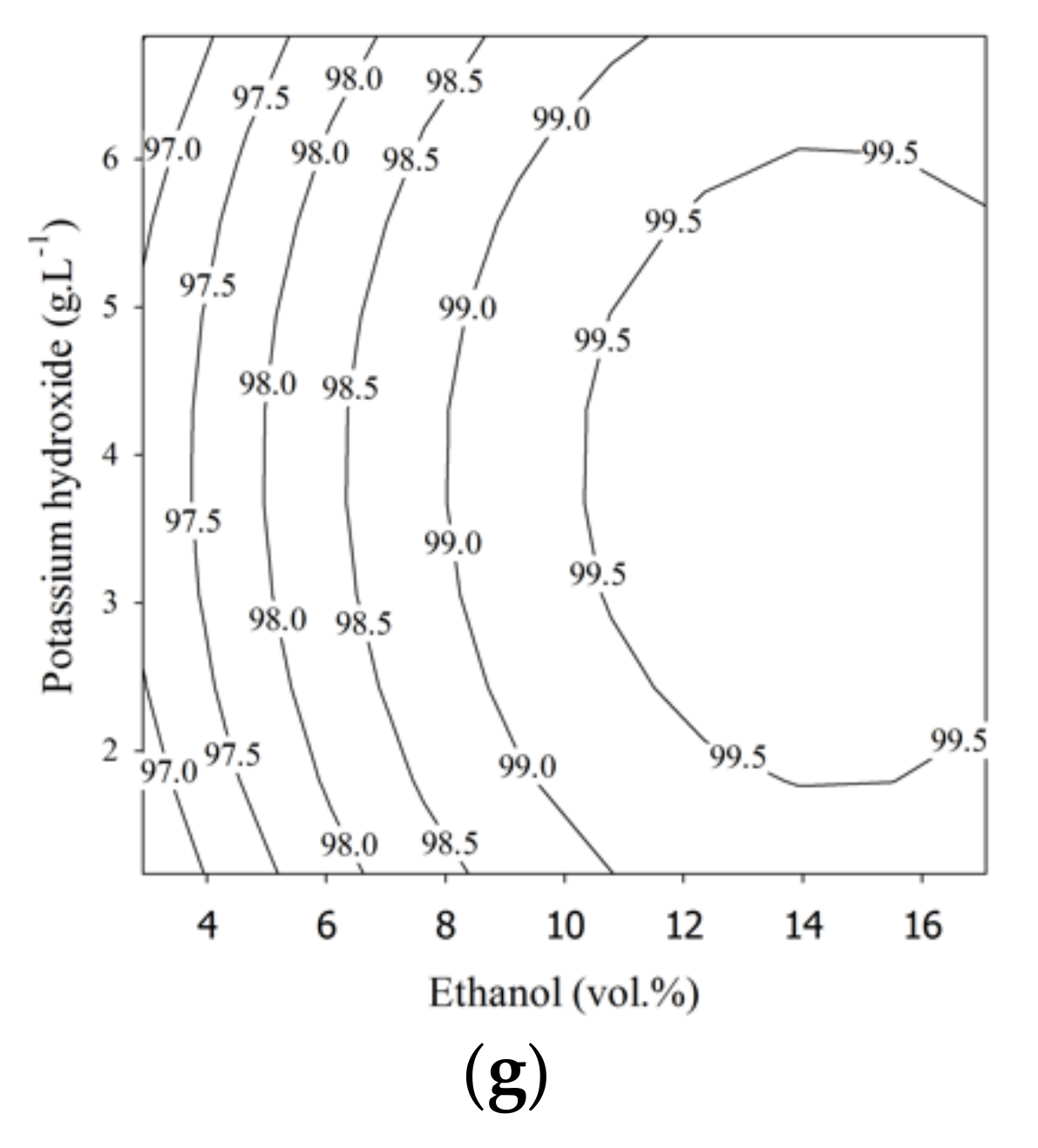
3.1. Response Surface Methodology (RSM) Models and Optimal Operating Points
3.2. Statistical Data Analysis of Predictive Models
3.3. Response Surface Plots
3.4. Compositions and Yields of Oils in Each Continuous Processing Step
3.5. Electricity Consumption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shuit, S.H.; Tan, S.H. Feasibility study of various sulphonation methods for transforming carbon nanotubes into catalysts for the esterification of palm fatty acid distillate. Energy Convers. Manag. 2014, 88, 1283–1289. [Google Scholar] [CrossRef]
- Idris, N.A.; Lau, H.L.N.; Wafti, N.S.A.; Mustaffa, N.K.; Loh, S.K. Glycerolysis of palm fatty acid distillate (PFAD) as Biodiesel feedstock using heterogeneous catalyst. Waste Biomass Valorization 2020, 12, 735–744. [Google Scholar] [CrossRef]
- Ping, B.T.Y.; Yosof, M. Characteristics and properties of fatty acid distillates from palm oil. Oil Palm Bull. 2009, 59, 5–11. [Google Scholar]
- Thawornprasert, J.; Somnuk, K.; Oo, Y.M.; Prateepchaikul, G. Feasibility of using diesel–palm fatty acid distillate ethyl ester–hydrous ethanol blend in an unmodified DI diesel engine: An assessment of stability, fuel properties, and emissions. ACS Omega 2021, 5, 20021–20033, in press. [Google Scholar]
- United State Department of Agriculture, Foreign Agricultural Service. Oil, Palm Explorer. 2020. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=4243000&sel_year=2020&rankby=Production (accessed on 8 February 2021).
- Top, A.G.M. Production and utilization of palm fatty acid distillate (PFAD). Lipid Technol. 2010, 22, 11–13. [Google Scholar]
- Tan, S.X.; Lim, S.; Ong, H.C.; Pang, Y.L. State of the art review on the development of ultrasound-assisted catalytic transesterification process for biodiesel production. Fuel 2019, 235, 886–907. [Google Scholar] [CrossRef]
- Stavarache, C.; Vinatoru, M.; Nishimura, R.; Maeda, Y. Fatty acids methyl esters from vegetable oil by means of ultrasonic energy. Ultrason. Sonochem. 2005, 12, 367–372. [Google Scholar] [CrossRef]
- Sáez-Bastante, J.; Pinzi, S.; Arzamendi, G.; de Castro, M.D.L.; Priego-Capote, F.; Dorado, M.P. Influence of vegetable oil fatty acid composition on ultrasound-assisted synthesis of biodiesel. Fuel 2014, 125, 183–191. [Google Scholar] [CrossRef]
- Majid, I.; Nayik, G.A.; Nanda, V. Ultrasonicatian and food technology: A review. Cogent Food Agric. 2015, 1, 1071022. [Google Scholar] [CrossRef]
- Hayyan, A.; Hashim, M.A.; Mirghani, M.E.S.; Hayyan, M.; AlNashef, I.M. Treatment of industrial low grade palm oil via esterification reaction using sonoreactor. J. Ind. Eng. Chem. 2014, 20, 2066–2070. [Google Scholar] [CrossRef]
- Mohod, A.V.; Subudhi, A.S.; Gogate, P.R. Intensification of esterification of non edible oil as sustainable feedstock using cavitational reactors. Ultrason. Sonochem. 2017, 36, 309–318. [Google Scholar] [CrossRef]
- Andrade-Tacca, C.A.; Chang, C.-C.; Chen, Y.-H.; Ji, D.-R.; Wang, Y.-Y.; Yen, Y.-Q.; Chang, C.Y. Reduction of FFA in Jatropha Curcas oil via sequential direct-ultrasonic irradiation and dosage of methanol/sulfuric acid catalyst mixture on esterification process. Energy Convers. Manag. 2014, 88, 1078–1085. [Google Scholar] [CrossRef]
- Trinh, H.; Yusup, S.; Uemura, Y. Optimization and kinetic study of ultrasonic assisted esterification process from rubber seed oil. Bioresour. Technol. 2018, 247, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.M.; Gogate, P.R.; Kumar, S.S. Intensification of esterification of karanja oil for production of biodiesel using ultrasound assisted approach with optimization using response surface methodology. Chem. Eng. Process. 2018, 124, 186–198. [Google Scholar] [CrossRef]
- Pruszko, R. Biodiesel Production. In Bioenergy; Dahiya, A., Ed.; Academic Press: Boston, MA, USA, 2015; Chapter 20; pp. 339–359. [Google Scholar]
- Chai, M.; Tu, Q.; Lu, M.; Yang, Y.J. Esterification pretreatment of free fatty acid in biodiesel production, from laboratory to industry. Fuel Process. Technol. 2014, 125, 106–113. [Google Scholar] [CrossRef]
- Park, J.Y.; Wang, Z.M.; Kim, D.K.; Lee, J.S. Effects of water on the esterification of free fatty acids by acid catalysts. Renew. Energy 2010, 35, 614–618. [Google Scholar] [CrossRef]
- Gole, V.L.; Gogate, P.R. Intensification of synthesis of biodiesel from non-edible oil using sequential combination of microwave and ultrasound. Fuel Process. Technol. 2013, 106, 62–69. [Google Scholar] [CrossRef]
- Worapun, I.; Pianthong, K.; Thaiyasuit, P. Optimization of biodiesel production from crude palm oil using ultrasonic irradiation assistance and response surface methodology. J. Chem. Technol. Biotechnol. 2011, 87, 189–197. [Google Scholar] [CrossRef]
- Vanichseni, T.; Intaravichai, S.; Saitthiti, B.; Kiatiwat, T. Potential biodiesel production from palm oil for Thailand. Kasetsart J. 2002, 36, 83–97. [Google Scholar]
- Okoro, L.N.; Okwuanalu, D.; Nwaeburu, C. Calorimetric determination of energy content of alcohol fuels and blends with kerosene. Int. J. Res. Chem. Environ. 2012, 2, 102–105. [Google Scholar]
- Hanh, H.D.; Dong, N.T.; Okitsu, K.; Nishimura, R.; Maeda, Y. Biodiesel production by esterification of oleic acid with short-chain alcohols under ultrasonic irradiation condition. Renew. Energy 2009, 34, 780–783. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, G.; Singh, C.P. Fast, easy ethanolysis of coconut oil for biodiesel production assisted by ultrasonication. Ultrason. Sonochem. 2010, 17, 555–559. [Google Scholar] [CrossRef]
- Oliveira, P.A.; Baesso, R.M.; Moraes, G.C.; Alvarenga, A.V.; Costa-Félix, R.P.B. Ultrasound methods for biodiesel production and analysis. In Biofuels-State of Development; IntechOpen: London, UK, 2018; pp. 121–148. [Google Scholar] [CrossRef]
- Somnuk, K.; Soysuwan, N.; Prateepchaikul, G. Continuous process for biodiesel production from palm fatty acid distillate (PFAD) using helical static mixers as reactors. Renew. Energy 2018, 131, 100–110. [Google Scholar] [CrossRef]
- Somnuk, K.; Soysuwan, N.; Prateepchaikul, G. Optimizing three-step production of methyl ester from palm fatty acid distillate: A response surface methodology approach. Biofuels 2017, 351–360. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, D.; Parajuli, K.; Upadhyay, S.; Jiang, Y.; Duan, Z. Comparison of four quantitative techniques for monitoring microalgae disruption by low-frequency ultrasound and acoustic energy efficiency. Environ. Sci. Technol. 2018, 52, 3295–3303. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Z.; Sun, D.W. Kinetic modeling of ultrasonic-assisted extraction of phenolic compounds from grape marc: Influence of acoustic energy density and temperature. Ultrason. Sonochem. 2014, 21, 1461–1469. [Google Scholar] [CrossRef]
- Somnuk, K.; Prasit, T.; Prateepchaikul, G. Effects of mixing technologies on continuous methyl ester production: Comparison of using plug flow, static mixer, and ultrasound clamp. Energy Convers. Manag. 2017, 140, 91–97. [Google Scholar] [CrossRef]
- Jansri, S.; Prateepchaikul, G. Comparison of biodiesel production from high free fatty acid, crude coconut oil via saponification followed by transesterification or a two-stage process. Kasetsart 2011, 45, 110–119. [Google Scholar]
- Somnuk, K.; Prasit, T.; Phanyusoh, D.; Prateepchaikul, G. Continuous methyl ester production with low frequency ultrasound clamps on a tubular reactor. Biofuels 2018, 1–7. [Google Scholar] [CrossRef]
- Somnuk, K.; Smithmaitrie, P.; Prateepchaikul, G. Two-Stage continuous process of methyl ester from high free fatty acid mixed crude palm oil using static mixer coupled with high-intensity of ultrasound. Energy Convers. Manag. 2013, 75, 302–310. [Google Scholar] [CrossRef]
- Noipin, K.; Kumar, S. Optimization of ethyl ester production assisted by ultrasonic irradiation. Ultrason. Sonochem. 2015, 22, 548–558. [Google Scholar] [CrossRef]
- Das, A.K.; Dewanjee, S. Optimization of extraction using mathematical models and computation. Comput. Phytochem. 2018, 75–106. [Google Scholar]
- Dharma, S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Silitonga, A.S.; Kusumo, F.; Mahlia, T.M.I. Optimization of biodiesel production process for mixed Jatropha curcas–Ceiba pentandra biodiesel using response surface methodology. Energy Convers. Manag. 2016, 115, 178–190. [Google Scholar] [CrossRef]
- Gaurav, A.; Dumas, S.; Mai-Chau, T.Q.; Ng, F.T.T. A Kinetic model for a Single Step Biodiesel Production from a High Free Fatty Acid (FFA) Biodiesel Feedstock over a Solid Heteropolyacid Catalyst. Green Energy Environ. 2019, 4, 328–341. [Google Scholar] [CrossRef]
- Gupta, R.B.; Demirbas, A. Gasoline, Diesel, and Ethanol Biofuels from Grasses and Plants; Cambridge University Press: Cambridge, UK, 2010; pp. 110–111. [Google Scholar]
- Cheng, J. Biomass to Renewable Energy Processes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Bart, J.C.J.; Palmeri, N.; Cavallaro, S. Biodiesel Science and Technology; Woodhead Publishing Limited: Cambridge, UK, 2010; p. 219. [Google Scholar]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of vegetable oils with ethanol and characterization of the key fuel properties of ethyl esters. Energies. 2009, 2, 362–376. [Google Scholar] [CrossRef]
- The Department of Alternative Energy Development and Efficiency (DEDE). Characteristics and Quality of Fatty Acid Methyl Ester Biodiesel. 2013. Available online: http://www.ratchakitcha.soc.go.th/DATA/PDF/2556/E/158/15.PDF (accessed on 4 April 2020).
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Alternative Fuels Data Center: Methanol. Available online: https://afdc.energy.gov/fuels/emerging_methanol.html (accessed on 8 February 2021).
- Ning, L.; Duan, Q.; Chen, Z.; Kou, H.; Liu, B.; Yang, B.; Zeng, K. A comparative study on the combustion and emissions of a non-road common rail diesel engine fueled with primary alcohol fuels (methanol, ethanol, and n-butanol)/diesel dual fuel. Fuel 2020, 266, 117034. [Google Scholar] [CrossRef]
- Zabaruddin, N.H.; Abdullah, L.C.; Mohamed, N.H.; Choong, T.S. Optimization using response surface methodology (RSM) for biodiesel synthesis catalyzed by radiation-induced kenaf catalyst in packed-bed reactor. Processes 2020, 8, 1289. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Downing, R.S. Heterogeneous catalytic transformations for environmentally friendly production. Appl. Catal. A-Gen 1999, 189, 163–183. [Google Scholar] [CrossRef]


| Process | Independent Variable | Coded Level | |||||
|---|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | +1 | +1.682 | |||
| First step | E1 | Ethanol (vol.%) | 19.8 | 30.0 | 45.0 | 60.0 | 70..2 |
| C1 | Sulfuric acid (vol.%) | 0.6 | 2.0 | 4.0 | 6.0 | 7.4 | |
| L1 | Length of US reactor (mm) | 100 | 200 | 400 | 600 | 700 | |
| Second step | E2 | Ethanol (vol.%) | 26.4 | 40.0 | 60.0 | 80.0 | 93.6 |
| C2 | Sulfuric acid (vol.%) | 0.6 | 2.0 | 4.0 | 6.0 | 7.4 | |
| L2 | Length of US reactor (mm) | 100 | 200 | 400 | 600 | 700 | |
| −1.414 | −1 | 0 | +1 | +1.414 | |||
| Third step | E3 | Ethanol vol (%) | 2.9 | 5.0 | 10.0 | 15.0 | 17.1 |
| C3 | KOH (g L−1) | 1.2 | 2.0 | 4.0 | 6.0 | 6.8 | |
| Run | First Step | Second Step | Third Step | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 | C1 | L1 | EE1 | E2 | C2 | L2 | EE2 | E3 | C3 | EE3 | |
| (vol.%) | (vol.%) | (mm) | (wt.%) | (vol.%) | (vol.%) | (mm) | (wt.%) | (vol.%) | (g.L−1) | (wt.%) | |
| 1 | 19.8 | 4 | 400 | 17.52 | 26.4 | 4 | 400 | 90.4 | 2.9 | 4 | 97.15 |
| 2 | 30 | 2 | 200 | 46.49 | 40 | 2 | 200 | 89.81 | 5 | 2 | 97.72 |
| 3 | 30 | 2 | 600 | 41.08 | 40 | 2 | 600 | 91.43 | 5 | 6 | 97.76 |
| 4 | 30 | 6 | 200 | 29.28 | 40 | 6 | 200 | 92.01 | 10 | 1.2 | 98.88 |
| 5 | 30 | 6 | 600 | 24.95 | 40 | 6 | 600 | 93.62 | 10 | 4 | 99.41 |
| 6 | 45 | 0.6 | 400 | 60.54 | 60 | 0.6 | 400 | 95.34 | 10 | 4 | 99.48 |
| 7 | 45 | 4 | 100 | 57 | 60 | 4 | 100 | 96.21 | 10 | 4 | 99.47 |
| 8 | 45 | 4 | 400 | 57.63 | 60 | 4 | 400 | 96.96 | 10 | 4 | 99.46 |
| 9 | 45 | 4 | 400 | 57.28 | 60 | 4 | 400 | 96.21 | 10 | 6.8 | 98.67 |
| 10 | 45 | 4 | 400 | 57.22 | 60 | 4 | 400 | 96.96 | 15 | 2 | 99.61 |
| 11 | 45 | 4 | 400 | 57.68 | 60 | 4 | 400 | 96.21 | 15 | 6 | 99.67 |
| 12 | 45 | 4 | 700 | 57.88 | 60 | 4 | 700 | 95.84 | 17.1 | 4 | 99.65 |
| 13 | 45 | 7.4 | 400 | 48.26 | 60 | 7.4 | 400 | 95.3 | |||
| 14 | 60 | 2 | 200 | 73.72 | 80 | 2 | 200 | 97.72 | |||
| 15 | 60 | 2 | 600 | 69.57 | 80 | 2 | 600 | 96.25 | |||
| 16 | 60 | 6 | 200 | 67.57 | 80 | 6 | 200 | 95.91 | |||
| 17 | 60 | 6 | 600 | 62.67 | 80 | 6 | 600 | 95.44 | |||
| 18 | 70.2 | 4 | 400 | 70.21 | 93.6 | 4 | 400 | 97.41 | |||
| Coefficient | First Step | Second Step | Third Step | |||
|---|---|---|---|---|---|---|
| Value | p-Value | Value | p-Value | Value | p-Value | |
| β0 | −5.215 | 0.465266941 | 65.48228 | 0.000000003 | 94.39872 | 0.000000000 |
| β1 | 2.568 | 0.000000286 | 0.60754 | 0.000005625 | 0.58638 | 0.000000124 |
| β2 | −6.245 | 0.000152343 | 2.63245 | 0.000549458 | 0.61564 | 0.000043540 |
| β3 | −0.007 | 0.036638878 | 0.02101 | 0.004638713 | −0.02014 | 0.000001383 |
| β4 | −0.020 | 0.000003644 | −0.00016 | 0.020544591 | −0.07847 | 0.000032700 |
| β5 | 0.085 | 0.005142023 | −0.02191 | 0.004196180 | ||
| β6 | −0.00287 | 0.000138645 | ||||
| β7 | −0.15704 | 0.006491940 | ||||
| β8 | −0.000014 | 0.031592333 | ||||
| R2 | 0.994 | 0.962 | 0.994 | |||
| R2adjusted | 0.988 | 0.928 | 0.991 | |||
| Source | SS | MS | F0 | Fcrit | DOF 1 |
|---|---|---|---|---|---|
| First-step continuous esterification | |||||
| Regression | 4243.8 | 848.77 | 192.16 | 3.106 (F0.05,5,12) | 5 |
| Residual | 53 | 4.417 | 12 | ||
| Lack-of-fit error | 52.84 | 5.871 | 105.4165 | 9 | |
| Pure error | 0.167 | 0.05569 | 3 | ||
| Total | 4296.8 | 17 | |||
| Second-step continuous esterification | |||||
| Regression | 96.589 | 12.07 | 28.43 | 3.230 (F0.05,8,9) | 8 |
| Residual | 3.822 | 0.425 | 9 | ||
| Lack-of-fit error | 3.259 | 0.543 | 2.898 | 6 | |
| Pure Error | 0.562 | 0.188 | 3 | ||
| Total | 100.41 | 17 | |||
| Third-step continuous transesterification | |||||
| Regression | 8.67 | 2.168 | 302.2 | 4.120 (F0.05,4,7) | 4 |
| Residual | 0.05 | 0.007 | 7 | ||
| Lack-of-fit error | 0.048 | 0.012 | 14.86 | 4 | |
| Pure error | 0.0024 | 0.0008 | 3 | ||
| Total | 8.721 | 11 | |||
| Processing Step | Condition | |
|---|---|---|
| Optimized | Recommended | |
| First step | ||
| Condition | ||
| Ethanol (vol.%) | 64.4 | 46.1 |
| Sulfuric acid (vol.%) | 0.6 | 1.4 |
| Length of US reactor (mm) | 100 | 400 |
| Residence time in US reactor (s) | ≈ 2.6 | ≈ 10.4 |
| Ethyl ester yield | ||
| Predictive model (wt.%) | 74.65 | 64 |
| Actual experiment (wt.%) | 74.21 | 66.68 |
| Second step | ||
| Condition | ||
| Ethanol (vol.%) | 92.1 | 57 |
| Sulfuric acid (vol.%) | 2 | 2.1 |
| Length of US reactor (mm) | 200 | 400 |
| Residence time in US reactor (s) | ≈ 5.2 | ≈ 10.4 |
| Ethyl ester yield | ||
| Predictive model (wt.%) | 98.37 | 95.5 |
| Actual experiment (wt.%) | 97.88 | 95.32 |
| Third step | ||
| Condition | ||
| Ethanol (vol.%) | 14.6 | 14.6 |
| Potassium hydroxide (g L−1 of oil) | 3.9 | 3.9 |
| Residence time in US reactor (s) | ≈ 19 | ≈ 19 |
| Ethyl ester yield | ||
| Predictive model (wt.%) | 99.9 | 99.9 |
| Actual experiment (wt.%) | 99.87 | 99.87 |
| Total | ||
| Ethanol consumption (vol.%) | 171.1 | 117.7 |
| Sulfuric acid consumption (vol.%) | 2.6 | 3.5 |
| Potassium hydroxide consumption (g L−1 of oil) | 3.9 | 3.9 |
| Total length of US reactor (mm) | 400 | 800 |
| Total residence time in US reactor (s) | ≈ 26.8 | ≈ 39.8 |
| Composition 1, Yield 2 and Residual Ethanol | wt.% |
|---|---|
| First step esterification | |
| Composition of first step-esterified oil 1 | |
| Free fatty acids | 30.88 |
| Ethyl esters | 66.68 |
| Triglycerides | 0 |
| Diglycerides | 2.07 |
| Monoglycerides | 0.37 |
| Yield of first-step esterified oil 2 | 132.91 |
| Yield of first-step generated wastewater | 11.89 |
| Residual ethanol in the first step-esterified oil | 18.14 |
| Residual ethanol in the first step-wastewater | 7.7 |
| Second step esterification | |
| Composition of second-step esterified oil 1 | |
| Free fatty acids | 3.11 |
| Ethyl esters | 95.32 |
| Triglycerides | 0 |
| Diglycerides | 1.24 |
| Monoglycerides | 0.33 |
| Yield of second-step esterified oil 2 | 131.16 |
| Yield of second-step generated wastewater | 79.37 |
| Residual ethanol in the second step-esterified oil | 27.87 |
| Residual ethanol in the second-step wastewater | 44.65 |
| Third step transesterification | |
| Composition of purified biodiesel 1 | |
| Free fatty acids | - |
| Ethyl esters | 99.87 |
| Triglycerides | - |
| Diglycerides | 0.03 |
| Monoglycerides | 0.11 |
| Yield of crude biodiesel 2 | 61.85 |
| Residual ethanol in the mixture | 19.09 |
| Purification | |
| Yield of purified biodiesel 2 | 56.16 |
| Property | Results | Method | Biodiesel Standard | ||
|---|---|---|---|---|---|
| THA [42] | US [43] | Europe [43] | |||
| (ASTM, EN) | (ASTM) | (EN) | |||
| Ethyl esters (wt.%) | 98.15 | EN 14103 | 96.5 min | 96.5 min | |
| Linolenic acid esters (wt.%) | - | EN 14103 | 12.0 max | - | 12.0 max |
| Density at 15 °C (kg m−3) | 870 | ASTM D1298 | 860–900 | - | 860–900 |
| Viscosity at 40 °C (cSt) | 4.9 | ASTM D445 | 3.5–5.0 | 1.9–6.0 | 3.5–5.0 |
| Flash point (°C) | 139 | ASTM D93 | 120 min | 93 min | 101 min |
| Carbon residue (wt.%) | <0.1 | ASTM D4530 | 0.3 max | 0.05 max | 0.3 max |
| Water (mg kg−1) | 45.6 | EN ISO 12937 | 500 max | - | - |
| Copper strip corrosion | No. 1a | ASTM D130 | No.1 max | No.3 max | No.1 max |
| Acid value (mgKOH.g−1) | 0.39 | ASTM D664 | 0.50 max | 0.50 max | 0.50 max |
| Iodine value (g Iodine 100 g−1) | 45.3 | EN 14111 | 120 max | - | 120 max |
| Ethanol (wt.%) | <0.01 | EN 14110 | 0.2 max | 0.2 max | 0.2 max |
| Monoglyceride (wt.%) | 0.88 | EN 14105 | 0.7 max | - | 0.8 max |
| Diglyceride (wt.%) | 0.03 | EN 14105 | 0.2 max | - | 0.2 max |
| Triglyceride (wt.%) | 0.06 | EN 14105 | 0.2 max | - | 0.2 max |
| Free glycerin (wt.%) | - | EN 14105 | 0.02 max | 0.02 max | 0.02 max |
| Total glycerin (wt.%) | 0.24 | EN 14105 | 0.25 max | 0.24 max | 0.25 max |
| Phosphorus (wt.%) | <0.00182 | EN 14107 | 0.001 max | 0.001 max | 0.0004 max |
| Sulfated ash (wt.%) | 0.0335 | ASTM D 874 | 0.02 max | 0.02 max | 0.02 max |
| Cloud point (°C) | 10 | ASTM D2500 | Report 1 | ||
| Pour point (°C) | 3 | ASTM D97 | Report 1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somnuk, K.; Phanyusoh, D.; Thawornprasert, J.; Oo, Y.M.; Prateepchaikul, G. Continuous Ultrasound-Assisted Esterification and Transesterification of Palm Fatty Acid Distillate for Ethyl Ester Production. Processes 2021, 9, 449. https://doi.org/10.3390/pr9030449
Somnuk K, Phanyusoh D, Thawornprasert J, Oo YM, Prateepchaikul G. Continuous Ultrasound-Assisted Esterification and Transesterification of Palm Fatty Acid Distillate for Ethyl Ester Production. Processes. 2021; 9(3):449. https://doi.org/10.3390/pr9030449
Chicago/Turabian StyleSomnuk, Krit, Dunyawat Phanyusoh, Jarernporn Thawornprasert, Ye Min Oo, and Gumpon Prateepchaikul. 2021. "Continuous Ultrasound-Assisted Esterification and Transesterification of Palm Fatty Acid Distillate for Ethyl Ester Production" Processes 9, no. 3: 449. https://doi.org/10.3390/pr9030449
APA StyleSomnuk, K., Phanyusoh, D., Thawornprasert, J., Oo, Y. M., & Prateepchaikul, G. (2021). Continuous Ultrasound-Assisted Esterification and Transesterification of Palm Fatty Acid Distillate for Ethyl Ester Production. Processes, 9(3), 449. https://doi.org/10.3390/pr9030449







