Abstract
Production of small-sized peptides is significant because of their health benefits. Ultrafiltration (UF) membrane provides an effective fractionation of small-sized peptides on a large scale. Thus, the present study was aimed to evaluate the performance of multilayer UF membrane in fractionating tilapia fish by-product (TB) protein hydrolysate by observing the permeate flux, peptide transmission, and peptide distribution under different stirring speed, pH of feed solution, and salt concentration (NaCl). The fractionation process was carried out using a dead-end UF membrane system that consists of a stack of two membrane sheets with different (10/5 kDa) and similar (5/5 kDa) pore sizes in one device. The highest permeate flux (10/5 kDa–39.5 to 47.3 L/m2.h; 5/5 kDa– 15.8 to 20.3 L/m2.h) and peptide transmission (10/5 kDa–51.8 to 61.0%; 5/5 kDa–18.3 to 23.3%) for both multilayer membrane configurations were obtained at 3.0 bar, 600 rpm, pH 8, and without the addition of salt. It was also found that the permeates were enriched with small-size peptides (<500 Da) with a concentration of 0.58 g/L (10/5 kDa) and 0.65 g/L (5/5 kDa) as compared to large-sized peptides (500–1500 Da) with concentration of 0.56 g/L (10/5 kDa) and 0.36 g/L (5/5 kDa). This might indicate the enrichment of small-size peptides through the multilayer membrane which could potentially enhance the biological activity of the protein hydrolysate fraction.
1. Introduction
During fish processing, significant amounts of by-products comprised of frames, bones, skins, and tails are generated. Fish by-products contain valuable substances that can be used to generate a high-value product such as fish protein hydrolysate (FPH) that is rich in essential nutrients and bioactive peptides, which is useful for various physiological functions. Conversion of fish by-products to FPH is commonly achieved through enzymatic hydrolysis. Nevertheless, FPH produced through an enzymatic process often comprises a complex mixture of peptides with different amino acid sequences that require subsequent fractionation to produce peptides with a specific size.
Small-size peptides (<10 kDa) recovered from FPH have been reported to exhibit high biological activities and functionalities including antioxidant [1,2], Angiotensin I-converting enzyme (ACE) inhibition [3,4], anti-microbial [5], and anti-allergic [6]. However, it is a challenging process to enrich small-size peptides. Although such peptides size could be obtained by manipulating the degree of hydrolysis (DH) during enzymatic reaction, production of these specific peptides in a large scale remains a major problem. It would be more challenging to achieve if selectivity becomes a priority in this stage. Good selectivity could be achieved through chromatographic processes, making them the top choice for peptide fractionation. However, this method is always associated with low productivity and high costs for industrialization. Therefore, the design of effective fractionation approach is of fundamental importance for peptides separation, especially when the process must be applied on an industrial scale.
Owing to its low cost, high throughput, scale-up ability, and flexibility, membrane technology, particularly ultrafiltration (UF) membrane, seems to be well suited for peptides fractionation [7]. Ultrafiltration (UF) membranes have been extensively used for separating and obtaining peptides with specific sizes [8,9,10]. Ultrafiltration membranes usually operate in a dead-end or cross-flow mode, which differ in the number of streams and the feed flow direction. The dead-end mode has two streams where one stream is for the feed which flows vertically toward the membrane surface while the other stream is for the permeate. The cross-flow mode is designed with three streams for the feed, permeate and retentate. In contrast to the dead-end mode, the feed flows tangentially to the membrane surface in the cross-flow mode [11]. Nevertheless, membrane fouling continues as a main constraint in the UF process, which results in a poor separation [12,13]. The membrane fouling would be more severe in dead-end mode because of the high possibility of protein aggregation that contributed to poor filtration efficiency and low peptide yield [14].
The success of peptides fractionation is not merely caused by the membrane pore size. Other factors such as pH and salt concentration (ionic strength) could affect protein–protein and protein–membrane interaction in the bulk solution that tends to cause aggregation and accumulation on the membrane surface, and eventually caused membrane fouling (Wan et al. 2005). Therefore, proper selection of operating conditions is crucial to achieving effective separation [14,15,16].
Another problem that commonly arises from the application of UF membranes in the fractionation of proteins is low selectivity of the membrane. Poor selectivity in protein fractionation is usually related to the imperfect pore size distribution of the available commercial membranes, limiting the resolving power of the membranes significantly [17]. Multilayer UF membrane was developed to overcome the wide range of membrane pore size distribution as well as to fractionate proteins that were close in size [18]. By stacking membranes in one device with a ‘sandwich’ arrangement, the rejection of undesired protein was intensified with the additional membrane and allowed protein with the desired size to pass through the membrane. In this way, the membrane selectivity could be enhanced, and proteins with relatively close molecular sizes could be separated more effectively than a single layer membrane [17,19]. A multilayer UF membrane’s potential to completely reject unwanted species has been demonstrated in the literature [17,18,19,20,21], but limited to the fractionation of binary protein mixture.
Feins and Sirkar [19] have used a multilayer membrane system to fractionate haemoglobin and bovine serum albumin (BSA), which were closer in molecular weight (64,677 Da and 66,430 Da, respectively). The system consists of a stack of three different membrane layers (1, 2, and 3 membrane layers) made from regenerated cellulose and polyethersulfone membranes with a similar pore size (100 kDa). Complete rejection of BSA was achieved using three layers of polyethersulfone membranes, one on top of the other. The results clearly demonstrate that multi-membrane stacks can be used to fractionate proteins that are relatively close in molecular weight [19]. Md. Yunos and Field [20] studied the effect of multilayer membrane configurations on fractionation of lysozyme, myoglobin, and BSA with a molecular weight between 10 and 70 kDa for single and binary mixtures. The membranes with MWCO combination of 30, 50, and 100 kDa were stacked together in various sandwich (multilayer) arrangements. They aimed to improve the selectivity from the separation by attaining pure protein product in the permeate from binary protein mixture.
It was found that the transmission of proteins using multilayer membranes is comparable to the transmission of a single membrane. Md. Yunos and Field [17] have also studied the transmission and selectivity of lysozyme and myoglobin mixtures using single and multilayer configurations. The results demonstrated that multilayer configurations have higher selectivity of proteins than single membranes with reduced membrane fouling.
While the effect of multilayer membrane configurations to improve the selectivity and reduce the membrane fouling during protein fractionation has been reported in several studies, the effect of process parameters on multilayer membrane performance for peptides fractionation was rarely addressed. Therefore, in the present study, we explore the application of multilayer membrane for separation of peptides from tilapia FPH. The effect of process parameters, including stirring speed, pH, and salt concentration to the peptide’s fractionation was investigated to better understand the peptide transmission due to applying multilayer membrane. The best membrane configuration that could give a higher amount (concentration) of small-size peptides was also examined. Since tilapia (Oreochromis niloticus) is the second most cultured freshwater fish species after carp, the protein hydrolysate from tilapia by-products was selected for this study [22].
2. Materials and Methods
2.1. Preparation of Tilapia By-Product Mince
Tilapia fish (Oreochromis niloticus) was supplied by a local fish farm in Rawang, Selangor, Malaysia. The fish was kept in ice during transportation to the laboratory and directly processed. The tilapia by-products (TB), including head, frames, and tail, obtained after filleting, eviscerating, and hand filleting, were then minced using a high-speed grinder, packed in polyethylene plastic bags, and stored at −20 °C until further use.
2.2. Preparation of Tilapia Protein Hydrolysate Using Alcalase
Protein hydrolysate was prepared from the TB mince by enzymatic hydrolysis following the method described by Roslan et al. [23] with a slight modification. Prior to hydrolysis, TB mince was thawed overnight in a refrigerator (4 ± 1°C). 15% w/v of TB mince mixture was prepared by adding 50 mL of 50 mM phosphate buffer solution (pH 7.5) to the pre-weighed TB mince. The mixture was incubated at 60 °C for 20 min followed by the addition of 2.5% (w/w) alcalase enzyme (Novo Nordisk, Denmark) to start the hydrolysis. After 60 min, the mixture was placed in a water bath at 90 °C for 15 min to stop the enzymatic reaction. The mixture was then cooled on ice before subjected to high-speed centrifugation at 10,000 rpm for 20 min. The supernatant (TB protein hydrolysate) was collected and ready to use for further analysis. The chemical compositions analyses of TB protein hydrolysate were conducted from our previous study [24]. The molecular weight analysis found that TB protein hydrolysates have low molecular weight peptides ranging between 3.5–26.6 kDa. This data would become the basis for the fractionation of TB protein hydrolysate using ultrafiltration membrane.
2.3. Membrane Type and Module
A dead-end UF membrane system Amicon model 8200 stirred ultrafiltration cell (Amicon Corp., Danvers, MA, USA) was used to filter the TB protein hydrolysate. The stirred UF cell is equipped with an external compressed gas source (oxygen), a 4.9 cm bar impeller that was magnetically driven by a stirring hot plate, positioned about 1.5 mm above the membrane, as shown schematically in Figure 1. Phototachometer was used to monitor the stirring speed. Flat-sheet hydrophilic regenerated cellulose (RC) membranes with molecular weight cut-off (MWCO) of 10 kDa (Millipore, PLGC 06210) and 5 kDa (Millipore, PLCC 06210) were used as membrane [23] with 28.7 cm2 filtration active membrane area, and filter diameter of 63.5 mm. The membranes were placed on the membrane holder, which connected with filtrate tubing to collect the permeate. Multilayer membrane technique was employed with two membrane configurations with the skin side on the top for both membranes; 10/5 (top: 10 kDa, bottom: 5 kDa) and 5/5 (5 kDa for both top and bottom membrane).
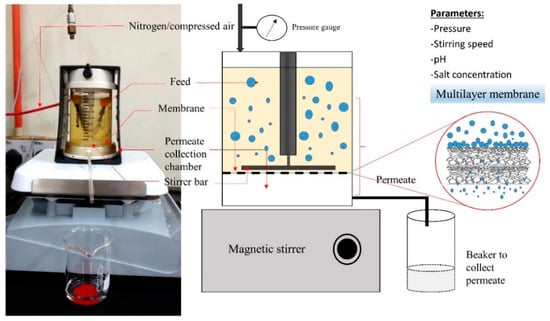
Figure 1.
Schematic diagram of membrane system for fractionation of tilapia by-products (TB) hydrolysate using multilayer membranes.
2.4. Preparation of Membranes and Procedure for Cleaning
Regenerated cellulose (RC) membranes were prepared according to the following procedure. The membrane was soaked overnight in deionized water (DI) and later subjected to membrane compaction at a pressure of 3.5 bar [14]. Then, DI water was filtered using the clean membrane, and water flux was measured at a pressure of 1, 1.5, and 2 bar, which during the cleaning process was used as a reference. The DI water was then replaced by the TB protein hydrolysate, and membrane performance was evaluated. Used RC membrane was cleaned according to the manufacturer recommendations. Firstly, the membrane was placed in a beaker and rinsed with deionized water. DI water was then replaced by 0.1% of NaOH solution and sonicated in an ultrasonic bath for 15 min. The membrane was transferred again into a beaker with deionized water followed by sonication for 15 min. Afterwards, the membrane was rinsed with deionized water until reached a neutral pH, and the water flux was measured. The same cleaning procedure was repeated if the water flux obtained did not reach the initial value. The cleaned membrane was kept in a chiller at 4 °C until further use.
2.5. Experiments of Membrane Filtration
TB protein hydrolysate (200 mL) was used for each experiment. Three different parameters namely stirring speed (0, 300, and 600 rpm), solution pH (3.0, 5.0, 7.0, 8.0, and 9.0), and salt concentration (NaCl; 0, 0.2, 0.4, and 0.6 M) were selected for the fractionation of TB protein hydrolysate which were studied under different pressure (2.0, 2.5, and 3.0 bars). The permeate was collected at every 10 min interval for a total of 70 min, and the flux value for each of the fractions collected was analyzed separately. The supporting information to understand the evolution of the permeate flux over 70 min is described from the Supplementary Materials provided (Figures S1 and S2). The permeates collected were then pooled and further used for the peptide transmission analysis. All experiments were conducted in duplicate.
2.5.1. Permeate Flux
Permeate flux is used as a measure of productivity of the membrane separation process, which represents the rate of mass transport across the membrane [11]. The permeate flux can be expressed according to the following equation [25]:
2.5.2. Peptide Transmission
Peptide transmission can be measured based on the ratio of peptide concentration in the permeate (Cp) to that in the feed (Cf), which can be expressed as below [17]:
where, represent the peptide concentration in the permeate and represent the concentration of peptide in feed solution.
2.6. Peptides Content Measurement
All peptides content in the feed solution, permeate, and retentate were analyzed following to the procedure of Church et al. [26] and Nielsen et al. [27], with some adjustments using the O-phthaldialdehyde (OPA) reagent.
2.7. Determination of Peptides Distribution
The highest peptide transmission obtained through fractionation using 10/5 and 5/5 multilayer membranes were selected for further purification using a gel permeation chromatography on a Pharmacia Superdex Peptide® GL 10/300 column using AKTA Fast Pressure Liquid Chromatography (FPLC) system (AKTA FPLC System, GE Healthcare, USA). Peptide samples (20 µL) were injected and eluted at 25 °C and a constant flow rate of 0.4 mL.min−1. 0.05 M sodium phosphate buffer (pH 7) containing 0.1% of NaCl was used as the mobile phase. Peptides of with molar mass of cytochrome C (12,384 g.mol−1), neurotensin (1678.9 g.mol−1), and leucine enkaphaline (686.8 g mol−1) (Sigma Aldrich, St. Louis, MO, USA) were selected as standards. The peptide concentration was calculated based on the peak area of peptide standard at the wavelength 280 nm.
2.8. Statistical Analysis
Data were analyzed using the Statistical Analysis System (SAS) [28] with ANOVA and Duncan’s multiple range test were used for multiple comparison. By using the same software, the standard deviations were also calculated.
3. Results and Discussion
In this study, a multilayer membrane technique was implemented by employing two different membrane pore sizes (regenerated cellulose membrane, 10 kDa and 5 kDa). Two types of membrane configurations were used: (1) The first arrangement was with the top 10 kDa and bottom 5 kDa, and (2) the second arrangement was using 5 kDa for both top and bottom membrane, while the skin layer of membrane was always on the top (skin layer-backing—skin layer-backing). The selection of the 5 kDa membrane at the bottom of the multilayer membrane arrangement was based on the high ACE-inhibition activity obtained from the previous study [29], aiming for more peptide with sizes less than 1000 kDa to be obtained. The fractionation of TB hydrolysate using a multilayer membrane (10/5 and 5/5 kDa) was performed under three different pressures (2.0, 2.5, and 3.0 bar). The multilayer membrane’s performance was assessed by evaluating the effects of stirring speed, pH of the feed solution, and salt concentration (NaCl) on permeate flux and peptide transmission. In our previous study [30], the effect of membrane pore size (10 kDa and 5 kDa) and operating parameters on the fractionation of TB protein hydrolysate using a single membrane were investigated. The best operating parameters for both membranes were obtained at pressure, stirring speed, and pH of 2.5 bar, 600 rpm, pH 8, respectively, and without the addition of salt (NaCl). A comparison in permeate flux reveals that 10 kDa membrane has 49% higher permeate flux (53 L/m2.h) than 5 kDa membrane (27 L/m2.h). As for the peptide transmission, the percentage of peptide in the permeate for 10 kDa and 5 kDa membranes were 87.33% and 36.11%, respectively.
3.1. Effects of Stirring Speed on Fractionation of TB Protein Hydrolysate Using Multilayer Membranes
The UF membrane system was equipped with an impeller, positioned just above the membrane surface [31]. This impeller served a function to control membrane fouling by inducing a shear force through a rotation to avoid solute accumulation on the membrane surface, thus reducing concentration polarization [32]. The effects of stirring speed on the flux of TB hydrolysate using 10/5 and 5/5 kDa multilayer membranes were shown in Figure 2. It was found that increasing the stirring speed and pressure will increase the permeation flux for both multilayer membranes. The highest permeate flux was observed at 600 rpm for both configurations with the value in the range of 35.9–39.8 L/m2.h for 10/5 membrane and 12.8–19.4 L/m2.h for 5/5 kDa membrane. Permeate flux decreased as the stirring speed decreased to 300 rpm for both multilayer membranes with the values ranging from 31.5–33.5 L/m2.h (10/5 kDa) and 12.1–18.5 L/m2.h (5/5 kDa), respectively. In an unstirred condition (0 rpm), lowest permeate flux was obtained regardless of the membrane configuration.
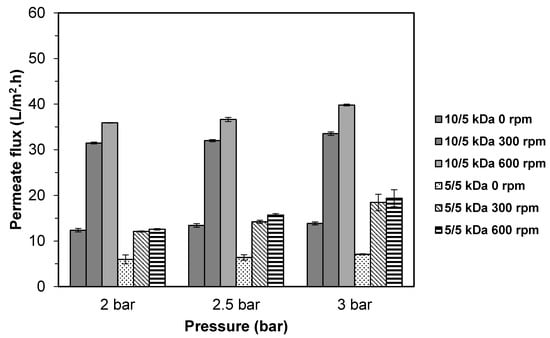
Figure 2.
Permeate flux of TB protein hydrolysate using multilayer ultrafiltration (UF) membranes at different stirring speed.
The results demonstrated that high stirring speed reduced the concentration polarization significantly (p < 0.05). This might be due to high turbulence developed by the stirrer inside the UF system that could sweep away a solute on the membrane’s surface, thus reducing the concentration polarization effect [31,33,34]. Under the unstirred condition, there will be more chances for protein to aggregate and deposit on the membrane surface, promoting membrane fouling and leading to a reduction in the permeate flux. Sarkar et al. [31,34], and Datta et al. [33] also reported that the higher the stirring speed applied, the higher permeate flux could be achieved.
Figure 3 shows the peptide transmission using 10/5 and 5/5 kDa multilayer membranes at different stirring speeds. As expected, the lowest peptide transmission was obtained at 0 rpm with the values of 18.0–20.9% (10/5 kDa) and 8.6–11.5% (kDa). A significant increase (p < 0.05) in peptide transmission could be observed at stirring speeds of 300 rpm and 600 rpm for 10/5 kDa multilayer membrane, with values ranging from 37.3–46.8% and 49.0–55.7%, respectively. For the 5/5 kDa membrane, there was no significant difference (p < 0.05) in peptide transmission observed between stirring speeds of 300 and 600 rpm, although the peptide transmission value increased as pressure increased (2.0 to 3.0 bar).
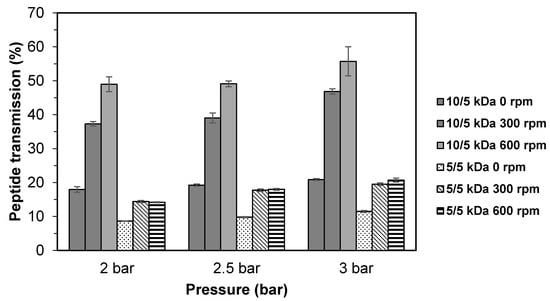
Figure 3.
Peptide transmission of TB protein hydrolysate through multilayer UF membranes at different stirring speeds.
The accumulation of large solute molecules on the membrane’s surface was prominent at unstirred condition or lower stirring speed. This situation will lead to the formation of a solute layer on the membrane surface that eventually contributes to lower peptide transmission. Increasing the stirring speed could overcome this problem by allowing more peptide molecules to permit through the membrane and increase the peptide transmission.
For the 10/5 kDa configuration, the turbulence created at high stirring speed may have blocked the accumulation of solute by sweeping the solute away from the membrane surface, which minimized diffusion in the boundary layer, thus reducing the concentration polarization [33]. As for the 5/5 kDa configuration, the effect of stirring speed on peptide transmission was less pronounced, which could be due to severe membrane fouling caused by protein adsorption [32]. Even at high stirring speed used (600 rpm), it could not increase the permeate flux. The findings of this study suggest that the stirring speed and membrane configuration have a significant effect on the fractionation of TB protein hydrolysate using UF and it is possible to reduce the concentration polarization and the mass transfer coefficient of the peptides [29]. Based on the highest permeate flux and peptide transmission for both multilayer membrane configurations (10/5 and 5/5 kDa), the stirring speed at 600 rpm was chosen for the following experiments.
3.2. Effects of Solution pH on the Fractionation of TB Protein Hydrolysate Using Multilayer Membranes
To understand the role of peptide charge on mass transfer during fractionations of TB protein hydrolysate through multilayer membranes (at pressure 2.0, 2.5, and 3.0 bar), different values of pH (3, 5, 7, 8, and 9) were tested which represent acidic, neutral, and alkaline conditions. As the isoelectric point (pI) of TB protein hydrolysate is unknown, evaluating the flux and peptide transmission at different pH conditions is crucial. Changes in pH will likely affect the electrostatic interactions between peptide–peptide and peptide–membrane, thus affecting the mass transfer coefficient of peptides through the membrane [15]. This is due to the fact that peptides have both positive and negative charges at N-terminal and C-terminal residues of their amino acids side chains [29].
Figure 4 shows the permeate flux obtained from the fractionation of TB protein hydrolysate using multilayer membrane at different pH. For 10/5 kDa membrane, the highest permeate flux was obtained at pH 8 with the values of 37.5–46.1 L/m2.h, followed by pH 7 (36.3–44.1 L/m2.h) and pH 9 (35.8–43.1 L/m2.h). A significant decreased (p < 0.05) in permeate flux was observed when fractionation was conducted in acidic (pH 3 and 5) condition, with the values ranging from 34.6–41.2 L/m2.h and 34.7–40.7 L/m2.h, respectively. However, there was no significant difference in permeate fluxes (p > 0.05) between pH 3 and pH 5. In comparison with 5/5 kDa membrane, the highest permeate flux was found at pH 3 (16.7–24.3 L/m2.h) followed by pH 5 (16.0–20.7 L/m2.h). The permeate flux values declined significantly (p < 0.05) when fractionation was conducted in neutral and alkaline conditions (pH 7, 8, and 9). It was clearly shown that there is a difference in fractionation behavior between the 10/5 kDa and 5/5 kDa membrane at different pH. Permeability of hydrolysate solution through the 10/5 kDa membranes was favorable at more basic conditions, while the 5/5 kDa membrane was at an acidic condition.
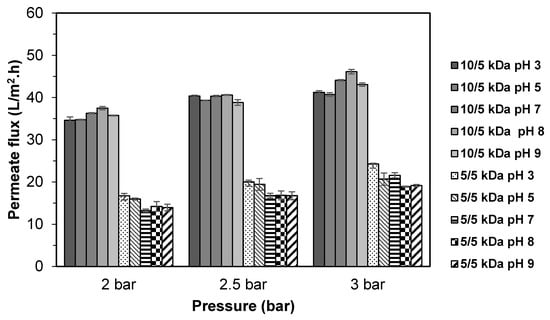
Figure 4.
Permeate flux of TB protein hydrolysate using multilayer UF membranes at different pH of feed solutions.
Basically, protein in the solution showed a net electrostatic charge depending on the solution pH. The change in solution pH can alter the electrical charge on both the protein and the membrane due to the ionization or deionization of various acidic/basic groups on the protein and the membrane surface, which can cause either attractive or repulsive interactions [35]. At pH that is below the isoelectric point (pI), the protein molecules are predominantly positively charged, and vice versa. If the membrane carried a charge with respect to the protein’s charge, there would be an electrostatic repulsion effect. In the case where the membrane had an opposite charge, a surface layer was formed due to the adsorption of the protein molecules [25]. RC membrane used in this study possessed a negatively charged surface. As the solution pH was increased to 7 and 8, the peptide would become negatively charged and increase the electrostatic repulsion effect between the like-charged membrane and peptides. Consequently, accumulation of peptide on the membrane surface was minimized, leading to a better flux performance. This could explain the higher permeate flux at pH 8 for the 10/5 kDa multilayer membrane. Saidi et al. [15] also found that the membrane permeability was more prominent at alkaline condition for the fractionation of tuna dark muscle hydrolysate. While at pH 3 and 5 for 10/5 kDa membrane, lower permeate flux values were obtained. This condition might be due to the interaction between oppositely charged peptides and the membrane that promoted peptide buildup on the membrane surface [31]. On the contrary, higher permeate flux was obtained at pH 3 for the 5/5 kDa multilayer membrane.
Generally, the protein carries a positive charge at a lower pH, and the membrane itself carries a negative charge. It could be expected that electrostatic repulsion would be minimal at this condition and reduces permeate flux. Surprisingly, an opposite observation was obtained. This might be attributed to the high content of acidic side chain (negatively charged) in their peptide’s profiles such as glutamic and aspartic acids [24]. The presence of more negative charge in the feed solution, which was a similar charge with the membrane could result in a great electrostatic repulsion effect. Permeability of feed solution would be favorable at this condition, thus lowering the accumulation of peptides on the membrane surface. Ghosh and Cui [36] reported a similar finding in which a slightly higher flux value was obtained at lower pH due to the greater electrostatic repulsion effect between proteins and membrane which carry the same charge.
Figure 5 shows the peptide transmission during the fractionation of TB hydrolysate using multilayer membranes at various range of pH solution. For the 10/5 kDa membrane, the peptide transmission showed similar behavior with the permeate flux, where at pH 8, the highest peptide transmission was found with the values ranging from 52.01–61.48, followed by pH 7 (49.09–60.52) and pH 9 (42.35–60.38). Low peptide transmission was obtained at pH 3 and pH 5 (p > 0.05). At pH 8 and 7 (basic condition), higher peptide transmission was obtained. This could be due to the increase of negative charges in peptide molecules which limit the membrane fouling (low peptide accumulation on the membrane surface) as a result of electrostatic repulsion between peptide and the membrane surface, thus allowing the peptide to pass through the membrane [15]. At lower pH, slightly lower peptide transmission might be due to high tendency of interaction between the hydrophobic peptides that carry positive charge and the hydrophilic surface of the negatively charged membrane as explained previously, which hindered the peptides to pass through the membrane [14,29].
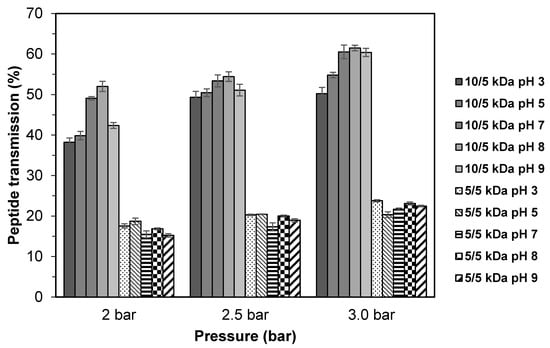
Figure 5.
Peptide transmission of TB protein hydrolysate through multilayer UF membranes at different pH of feed solution.
Peptide transmission behavior for the 5/5 kDa membrane has a slight difference compared to the 10/5 kDa membrane. There was no significant difference (p > 0.05) in peptide transmission at pH 3, 5, and 8, although the highest permeate flux was found at pH 3 (16.7–24.3 L/m2.h). This could be related to the peptide rejection, owing to the peptide–membrane interaction, which is electrostatically favorable due to the identical charge between solute and membrane [35], thus lowering the peptide transmission. Contrary to the flux, it became more evenly distributed as membrane fouling proceeded [37]. Several researchers [14,34,38,39] have reported that the different values of peptide transmission with different pH could be linked to (i) the effect of pH on protein–membrane repulsion, (ii) the effect of pH on the electrostatic double layer, and (iii) the pH-dependent conformation of the protein. Based on the results obtained, pH 8 was the best pH condition for fractionation of TB hydrolysate using multilayer membrane.
3.3. Effects of Salt Concentration (NaCl) on the Fractionation of TB Protein Hydrolysate Using Multilayer Membranes
Another important factor that should be investigated in the fractionation of peptides is the salt concentration (ionic strength). The presence of salt in the solution would give an electrostatic double layer effect known as the Debye layer and change the protein–protein and protein–membrane interactions [38], affecting the flux protein transmission. Based on the previous findings [16,32,40], the selectivity of certain proteins can be improved by adding salt into feed solution. Salt could strengthen the ionic interactions between the membrane and the peptides, leading to better transmission of peptides [40].
The effect of different salt concentration (0 M, 0.2 M, 0.4 M, and 0.6 M of NaCl) on the fractionation of TB protein hydrolysate using multilayer membranes (10/5 and 5/5 kDa) were investigated using NaCl (sodium chloride) at conditions of 600 rpm of rotation speed, pH 8 for feed solution and different pressure (2.0, 2.5 and 3.0 bar). As shown in Figure 6, the highest flux values for 10/5 kDa membranes were obtained at 0 M of NaCl (39.5–47.3 L/m2.h). There is a clear trend of decreasing in permeate flux values when salt is added at concentration from 0.2 M to 0.6 M with values of 39.3–46.4 L/m2.h, 36.9–45.2 L/m2.h, 34.0–44.7 L/m2.h, respectively. However, for 5/5 kDa membrane, the permeate flux was not significantly affected by the addition of salt. This result is in accordance with Prata-Vidal et al. [16], who observed a reduction in permeate flux when salt was added to caseinomacropeptide hydrolysate due to the reduction of electrostatic repulsive force brought about by the electrostatic double layer effect of the counterions present in the salt, thus promoting the occurrence of fouling and consequently reducing the flux [41,42].
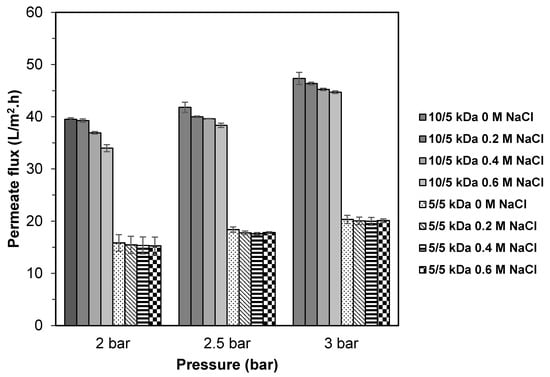
Figure 6.
Permeate flux of TB protein hydrolysate using multilayer UF membranes at different salt concentration (NaCl).
The results of peptide transmission of TB protein hydrolysate obtained through UF multilayer membranes at different salt (NaCl) concentration are presented in Figure 7. Without addition of salt (0 M NaCl), the highest transmission was achieved, and this was followed by 0.2 M, 0.4 M, and 0.6 M with the values ranging of 51.8–61.9%, 48.2–60.7%, 47.3–54.3%, and 47.2–52.5%, respectively. There was a significant reduction (p < 0.05) in peptide transmission when salt is added into the solution, which indicates that the addition of salt (NaCl) would give an adverse effect. The 5/5 kDa membrane configuration also shows a similar trend where the highest peptide transmission (18.3–23.3%) was obtained at 0 M of salt and reduced significantly (p < 0.05) as salt is added.
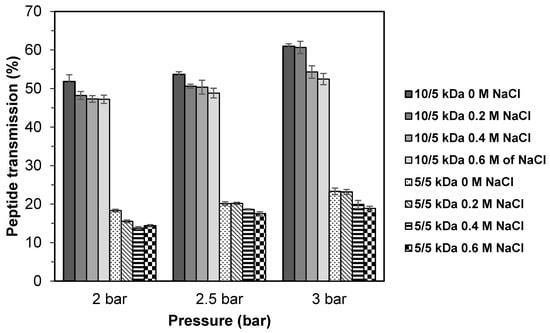
Figure 7.
Peptide transmission of TB protein hydrolysate through multilayer UF membranes at different salt concentration (NaCl).
Comparing to our earlier study using a single membrane [30], it was demonstrated that adding one layer of membrane led to the decline in the permeate flux (11–25%) as well as peptide transmission (30–35%), possibly due to additional mass transfer resistance, which reduces transportation of peptides through the membrane and means rejection would be more favorable. It had been expected that the permeate flux and peptide transmission for multilayer membranes would be slightly lower than those of the single membrane configuration.
Although these results differ from some published studies [43,44], they are in agreement with recent studies reported by Prata-Vidal et al. [16] and Wang and Tang [37]. Prata-Vidal et al. [41] who found that the retention of small peptides is more evident with salt’s addition at 0.4 M concentration. Wang and Tang [37] observed that BSA fouling was promoted by the addition of NaCl from 1–100 mM. It appears that the electrostatic repulsion and protein–membrane interactions were minimized in the presence of salt. A compact layer may have formed on the membrane surface as the fouling becomes severe, which causes low protein transmission [38,44]. It was not feasible to fractionate TB protein hydrolysate using UF membrane in the presence of salt (NaCl). Based on the findings of fractionation of TB hydrolysate using multilayer membrane, the best conditions selected for determining peptides distributions were at stirring speed of 600 rpm, pH 8, without salt, and a pressure of 3 bar for both 10/5 and 5/5 kDa membranes.
3.4. Peptides Concentration
The permeate fractions obtained through multilayer membranes (10/5 and 5/5) at 3.0 bar, 600 rpm, and pH 8 were analyzed for their peptide’s concentration. The FPLC chromatography profile of permeate fractions from both multilayer membrane’s configuration is shown in Figure 8, and the peptide concentration is presented in Table 1. Based on the profile in Figure 8, all the peptides in the permeates were lower than 1500 Da. This means only small-size peptides could pass through the membrane and proved that the multilayer configuration effectively blocked the peptides with larger size (>1500 Da). This situation could be due to polarization and fouling that could form a dynamic layer on the membrane surface [33] and allowed small-sized peptides to pass through the membrane.
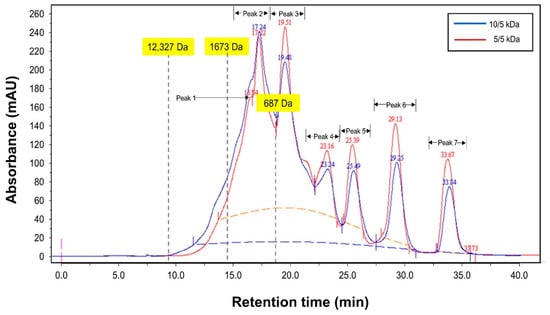
Figure 8.
FPLC (Fast Pressure Liquid Chromatography) chromatogram profiles of TB protein hydrolysate that fractionated using ultrafiltration membranes of 10/5 and 5/5 kDa at 3 bar, 300 rpm, and pH 8.

Table 1.
Peptide concentration.
Fractionation through 10/5 kDa membrane managed to obtain an almost similar concentration between large-size peptides (>500 Da) with values of 0.56 g/L and small-size peptides (<500 Da) with values of 0.58 g/L. Only one peak (peak 2—895 Da) of peptide with less than 1500 Da was detected for 10/5 kDa membrane. For the 5/5 kDa membrane, two peaks of peptides with less than 1500 Da (peak 1 for 1088 with 0.14 g/L and peak 2 for 895 Da with 0.22 g/L); with a total concentration of 0.36 g/L; were detected. These peptides have shown a reduction in amount (0.36 g/L) as compared to 10/5 kDa membrane. Surprisingly, the total concentration of small-size peptides (<500 Da) was higher for the 5/5 kDa membrane (0.65 g/L) as compared to 10/5 kDa membrane (0.58 g/L). The smaller pore size of the 5/5 kDa membrane might allow peptides with a smaller size to pass through the membrane. Saidi et al. [15] stated that the membrane selectivity is related to the pore size, shape, and solute charge. When a comparison is made on each peptide’s composition derived from both membranes (10/5 vs. 5/5), there is some enrichment detected in peptide composition. It was discovered that from a peptide fraction of less than 500 Da, fractionation using 5/5 kDa managed to increase the peptide concentration at peak 4, 6, and 7 with an increment of 700%, 33.3%, and 42.9%, respectively, but a reduction in peptide concentration at peak 3 and 5 with percentage values of 15.2% and 12.5%, respectively. The definite reason for these findings is uncertain. However, it might be related to the complete rejection of large size peptides on the retentate side as a result of membrane stacking and allowing smaller peptides to pass through the membrane. This finding is consistent with those reported by Feins and Sirkar [18], who conducted protein fractionation of β-lactoglobulin (35.5 kDa) and myoglobin (17.6 kDa) using multilayer membrane. They found out that with the addition of each additional membrane, the concentration of β-lactoglobulin in the permeate stream was reduced, ultimately resulting in a pure myoglobin product. Another possible reason for the higher concentration of small-size peptides in the permeate could be related to the different composition of peptides profiles and considering solute–solute and membrane–solute interactions that are highly affected by environmental factors such as pH and ionic strength. Although ultrafiltration membrane is considered a size-based separation technique, there is significant evidence that protein retention and transmission are also determined by solution pH, ionic strength, and membrane charge [15].
4. Conclusions
The multilayer membranes’ performance in fractionating TB protein hydrolysate was greatly influenced by membrane pore size, multilayer configuration, and operating parameters (pressure, stirring speed, pH of feed solution, and salt concentration). It was found that for both 10/5 kDa and 5/5 kDa multilayer membranes, the best fractionation conditions were achieved at a pressure of 3.0 bar, stirring speed of 600 rpm, pH 8 of feed solution, and with no addition of salt (NaCl). A significant reduction in permeate flux and peptide transmission were observed for the fractionation of TB protein hydrolysate using multilayer membrane, which indicates that rejection characteristics can be enhanced by stacking membranes together. In addition, better control of operating parameters may result in selective peptide migration in the permeate solution, where the concentration of peptides with size < 500 Da was found to be higher (0.58 g/L for 10/5 kDa and 0.65 g/L for 5/5 kDa) as compared to peptides with size > 500 Da (0.56 g/L for 10/5 kDa and 0.36 g/L for 5/5 kDa). A high quantity of small-sized peptides in the permeate solution is highly desirable due to their significant health benefits and potential application in pharmaceuticals, nutraceuticals, and food industries.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9717/9/3/446/s1, Figure S1: Effect of stirring speed on fractionation of TB protein hydrolysate using 10/5 kDa multilayer membranes, Figure S2: Effect of stirring speed on fractionation of TB protein hydrolysate using 5/5 kDa multilayer membranes.
Author Contributions
Conceptualization, J.R. and S.M.M.K.; methodology, S.M.M.K., K.F.M.Y., and N.A.; investigation, J.R.; writing—original draft preparation, J.R.; writing—review & editing, S.M.M.K., K.F.M.Y., and N.A.; supervision, S.M.M.K., K.F.M.Y., and N.A.; funding acquisition, S.M.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research University Grant Scheme (RUGS) under the Universiti Putra Malaysia (Vote no.: 9321500) and Science Fund Research Grant (Note no.: 5450692) from Ministry of Science, Technology, and Innovation (MOSTI), Malaysia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
Special thanks to Universiti Malaysia Sabah for providing funding to submit this manuscript. Acknowledgement also to UPM for the research grant funding (RUGS) and MOSTI that given the grant funding through Science Fund Research Grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zamorano-Apodaca, J.C.; García-Sifuentes, C.O.; Carvajal-Millán, E.; Vallejo-Galland, B.; Scheuren-Acevedo, S.M.; Elena, L.S.M. Biological and functional properties of peptide fractions obtained from collagen hydrolysate derived from mixed by-products of different fish species. Food Chem. 2020, in press. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Belleville, M.P.; Amar, R.B. Production and fractionation of tuna by-product protein hydrolysate by ultrafiltration and nanofiltration: Impact on interesting peptides fractions and nutritional properties. Food Res. Int. 2014, 65, 453–461. [Google Scholar] [CrossRef]
- Bougatef, A.; Nedjar-Arroume, N.; Ravallec-Plé, R.; Leroy, Y.; Guillochon, D.; Barkia, A.; Nasri, M. Angiotensin I-converting enzyme (ACE) inhibitory activities of sardinelle (Sardinella aurita) by-products protein hydrolysates obtained by treatment with microbial and visceral fish serine proteases. Food Chem. 2008, 111, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.N.A.M.; Sarbon, N.M. Angiotensin converting enzyme (ACE), antioxidant activity and functional properties of shortfin scad (Decapterus macrosoma) muscle protein hydrolysate at different molecular weight variations. Biocatal. Agric. Biotechnol. 2019, 20, 101254. [Google Scholar] [CrossRef]
- Robert, M.; Zatylny-Gaudina, C.; Fournierd, V.; Corree, E.; Le Corguillée, G.; Bernayc, B.; Henry, J. Molecular characterization of peptide fractions of a Tilapia (Oreochromis niloticus) by-product hydrolysate and in vitro evaluation of antibacterial activity. Process Biochem. 2015, 50, 487–492. [Google Scholar] [CrossRef]
- Wang, K.; Siddanakoppalub, P.N.; Ahmed, I.; Pavase, T.R.; Lin, H.; Li, Z. Purification and identification of anti-allergic peptide from Atlantic Salmon (Salmo salar) byproduct enzymatic hydrolysates. J. Funct. Foods. 2020, 72, 104084. [Google Scholar] [CrossRef]
- Khairkar, S.R.; Amol, V.; Pansare, A.V.; Shedge, A.A.; Chhatre, S.Y.; Suresh, A.K.; Chakrabarti, S.; Patil, V.R.; Nagarkar, A.A. Hydrophobic interpenetrating polyamide-PDMS membranes for desalination, pesticides removal and enhanced chlorine tolerance. Chemosphere 2020, 258, 127179. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Kristinsson, H.G. ACE-inhibitory activity of tilapia protein hydrolysates. Food Chem. 2009, 117, 582–588. [Google Scholar] [CrossRef]
- Kim, S.; Je, J.; Kim, S. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ranathunga, S.; Rajapakse, N.; Kim, S.K. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur. Food Res. Technol. 2006, 222, 310–315. [Google Scholar] [CrossRef]
- Cui, Z.F.; Jiang, Y.; Field, R.W. Chapter 1—Fundamentals of Pressure-Driven membrane separation processes. In Membrane Technology; Cui, Z.F., Muralidhara, H.S., Eds.; Elsevier: Burlington, MA, USA, 2010; pp. 1–18. [Google Scholar]
- Atkinson, A.J.; Wang, J.; Grzebyk, K.; Zhang, Z.; Jung, D.; Zeng, D.; Pollard, A.; Gold, A.; Coronell, O. Scalable fabrication of anti-biofouling membranes through 2-aminoimidazole incorporation during polyamide casting. J. Membr. Sci. 2019, 579, 151–161. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Seyedpour, S.F.; Aktij, S.A.; Giagnorio, M.; Bazrafshan, N.; Mollahosseini, A.; Samadi, F.; Ahmadalipour, S.; Firouzjaei, F.D.; Esfahani, M.R.; et al. Recent advances in functionalized polymer membranes for biofouling control and mitigation in forward osmosis. J. Membr. Sci. 2020, 596, 117604. [Google Scholar] [CrossRef]
- Das, R.; Bhattacherjee, C.; Ghosh, S. Effects of operating parameters and nature of fouling behavior in ultrafiltration of sesame protein hydrolysate. Desalination 2009, 237, 268–276. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Amar, R.B.; Belleville, M.P. Fractionation of a tuna dark muscle hydrolysate by a two-step membrane process. Sep. Purif. Technol. 2013, 108, 28–36. [Google Scholar] [CrossRef]
- Prata-Vidal, M.; Bouhallab, S.; Henry, G.; Aimar, P. An experimental study of caseinomacropeptide hydrolysis by trypsin in a continuous membrane reactor. Biochem. Eng. J. 2001, 8, 195–202. [Google Scholar] [CrossRef]
- Md. Yunos, K.F.; Field, R.W. Rejection amplification in the ultrafiltration of binary protein mixtures using sandwich configurations. Chem. Eng. Process. 2008, 47, 1053–1060. [Google Scholar] [CrossRef]
- Feins, M.; Sirkar, K. Highly selective membranes in protein ultrafiltration. Biotechnol. Bioeng. 2004, 86, 603–611. [Google Scholar] [CrossRef]
- Feins, M.; Sirkar, K. Novel internally staged ultrafiltration for protein purification. J. Membr. Sci. 2005, 248, 137–148. [Google Scholar] [CrossRef]
- Md Yunos, K.F.; Field, R.W. Effect of sandwich configuration of ultrafiltration membranes on protein fractionation. Desalination 2006, 199, 222–224. [Google Scholar] [CrossRef]
- Field, R.W.; Md Yunos, K.F.; Cui, Z. Separation of proteins using sandwich membranes. Desalination 2009, 245, 597–605. [Google Scholar] [CrossRef]
- El-Sayed, A.M. Tilapia Culture; CABI Publishing: Oxfordshire, UK, 2006. [Google Scholar]
- Roslan, J.; Mustapa Kamal, S.M.; Yunos, K.F.; Abdullah, N. Optimization of enzymatic hydrolysis of tilapia (Oreochromis niloticus) by-product using response surface methodology. Int. Food Res. J. 2015, 22, 1117–1123. [Google Scholar]
- Roslan, J.; Mustapa Kamal, S.M.; Yunos, K.F.; Abdullah, N. Characterization of Fish Protein Hydrolysate from Tilapia (Oreochromis niloticus) by-Product. Agric. Agric. Sci. Procedia 2014, 2, 312–319. [Google Scholar] [CrossRef]
- Ghosh, R. Protein Bioseparation using Ultrafiltration; Imperial College Press: London, UK, 2003; p. 166. [Google Scholar]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT User’s Guide, Version 6, 4th ed.; SAS Institute Inc.: Cary, NC, USA, 1989; Volume 2. [Google Scholar]
- Roslan, J.; Mustapa Kamal, S.M.; Yunos, K.F.; Abdullah, N. Assessment on multilayer ultrafiltration membrane for fractionation of tilapia by-product protein hydrolysate with angiotensin I-converting enzyme (ACE) inhibitory activity. Sep. Purif. Technol. 2017, 173, 250–257. [Google Scholar] [CrossRef]
- Roslan, J.; Mustapa Kamal, S.M.; Yunos, K.F.; Abdullah, N. Evaluation on performance of dead-end ultrafiltration membrane in fractionating tilapia by-product protein hydrolysate. Sep. Purif. Technol. 2018, 195, 21–29. [Google Scholar] [CrossRef]
- Sarkar, D.; Bhattacharya, A.; Bhattacharjee, C. Modelling the performances of a standard single stirred ultrafiltration cell using variable velocity back transport flux. Desalination 2010, 261, 89–98. [Google Scholar] [CrossRef]
- Wan, Y.; Ghosh, R.; Cui, Z. Fractionation of Proteins Using Ultrafiltration: Developments and Challenges. Dev. Chem. Eng. Mineral. Process. 2005, 13, 121–136. [Google Scholar] [CrossRef]
- Datta, D.; Bhattacharjee, S.; Nath, A.; Das, R.; Bhattacharjee, C.; Datta, S. Separation of ovalbumin from chicken egg white using two-stage ultrafiltration technique. Sep. Purif. Technol. 2009, 66, 353–361. [Google Scholar] [CrossRef]
- Sarkar, P.; Ghosh, S.; Dutta, S.; Sen, D.; Bhattrjee, C. Effect of different operating parameters on the recovery of proteins from casein whey using a rotating disc membrane ultrafiltration cell. Desalination 2009, 249, 5–11. [Google Scholar] [CrossRef]
- Aravind, U.K.; Mathew, J.; Aravindakumar, C.T. Transport studies of BSA, lysozyme and ovalbumin through chitosan/polystyrene sulfonate multilayer membrane. J. Membr. Sci. 2007, 299, 146–155. [Google Scholar] [CrossRef]
- Ghosh, R.; Cui, Z.F. Fractionation of BSA and lysozyme using ultrafiltration: Effect of pH and membrane pretreatment. J. Membr. Sci. 1998, 139, 17–28. [Google Scholar] [CrossRef]
- Wang, Y.N.; Tang, C.Y. Protein fouling of nanofiltration, reverse osmosis, and ultrafiltration membranes-The role of hydrodynamic conditions, solution chemistry, and membrane properties. J. Membr. Sci. 2011, 376, 275–282. [Google Scholar] [CrossRef]
- Wan, Y.; Lu, J.; Cui, Z. Separation of lysozyme from chicken egg white using ultrafiltration. Sep. Purif. Technol. 2006, 48, 133–142. [Google Scholar] [CrossRef]
- Groleau, P.E.; Morin, P.; Gauthier, S.F.; Pouliot, Y. Effect of physicochemical conditions on peptide-peptide interactions in a tryptic hydrolysate of β-lactoglobulin and identification of aggregating peptides. J. Agric. Food Chem. 2003, 51, 4370–4375. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Riera, F.A. Influence of ionic strength on peptide membrane fractionation. Sep. Purif. Technol. 2013, 119, 129–135. [Google Scholar] [CrossRef]
- Lin, S.H.; Hung, C.L.; Juang, R.S. Effect of operating parameters on the separations of proteins in aqueous solutions by dead-end ultrafiltration. Desalination 2008, 234, 116–125. [Google Scholar] [CrossRef]
- She, Q.; Tang, C.Y.; Wang, Y.N.; Zhang, Z. The role of hydrodynamic conditions and solution chemistry on fouling during ultrafiltration. Desalination 2009, 249, 1079–1087. [Google Scholar] [CrossRef]
- Pouliot, Y.; Wijers, M.C.; Gauthier, S.F.; Nadeau, L. Fractionation of whey protein hydrolysates using charged UF/NF membranes. J. Membr. Sci. 1999, 158, 105–114. [Google Scholar] [CrossRef]
- Nau, F.; Kerhervé, F.L.; Leonil, J.; Daufin, G. Selective separation of tryptic beta-casein peptides through ultrafiltration membranes: Influence of ionic interactions. Biotechnol. Bioeng. 1995, 46, 246–253. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).