Hydroxy-tyrosol as a Free Radical Scavenging Molecule in Polymeric Hydrogels Subjected to Gamma-Ray Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrogels Preparation
2.2. Gamma-Ray Irradiation
2.3. Rheological Analysis
2.4. IR Chemical Analysis
2.5. HT Extraction and HPLC Quantification
2.6. Statistical Analysis
3. Results
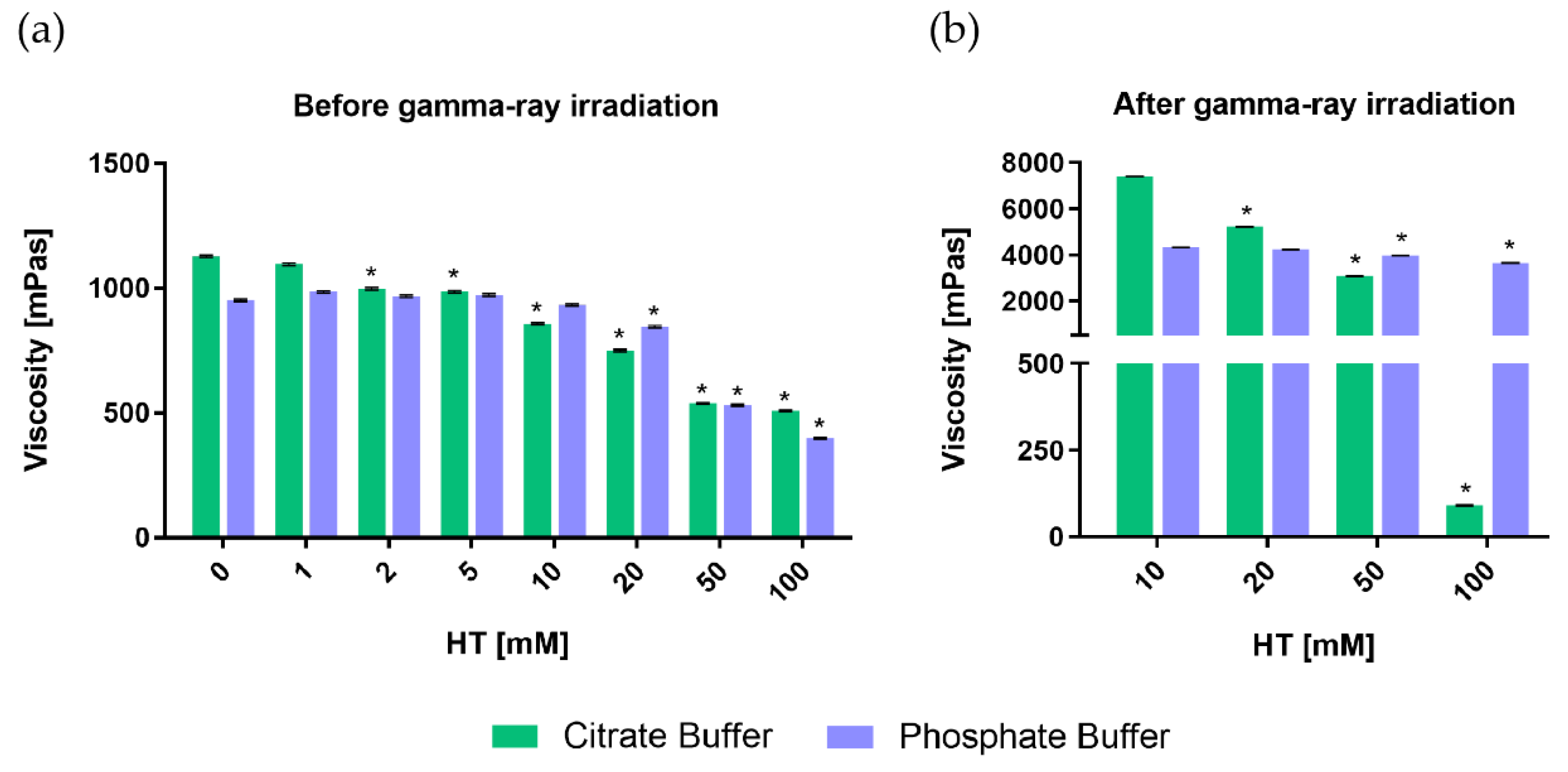
3.1. Effects of Hydroxytyrosol on Rheological Properties of PEO Hydrogels
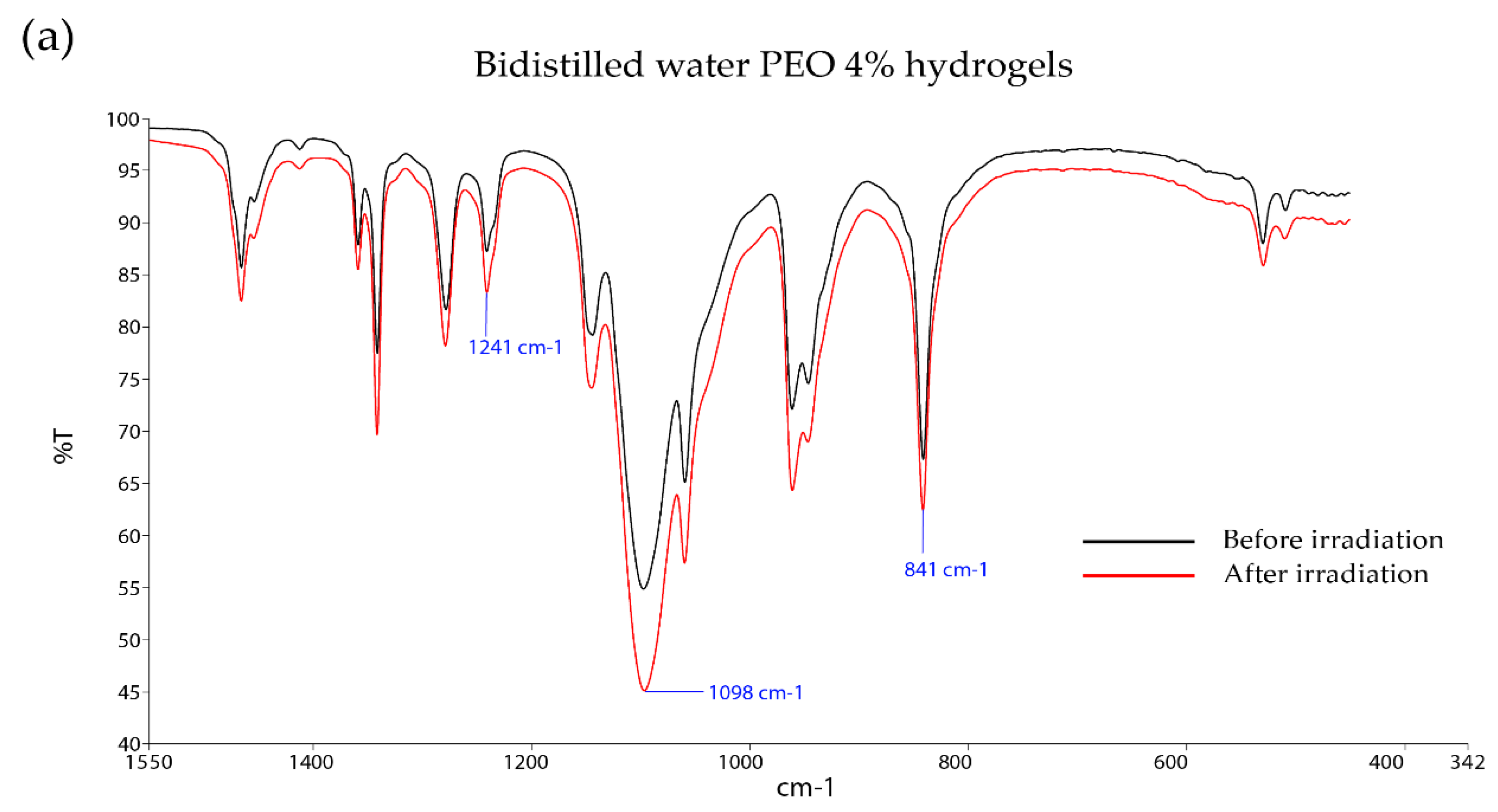
3.2. Effects of Citrate and Phosphate Buffer Solutions on the Chemical Structure of Irradiated PEO Hydrogels
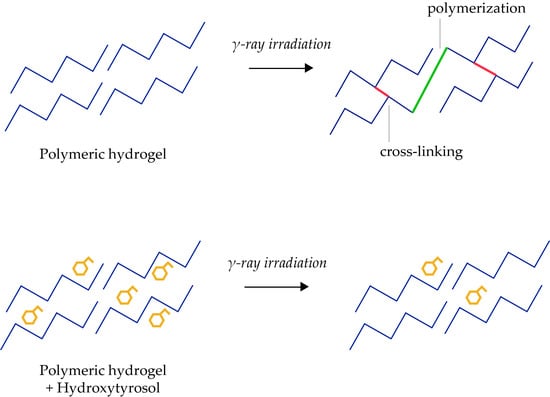
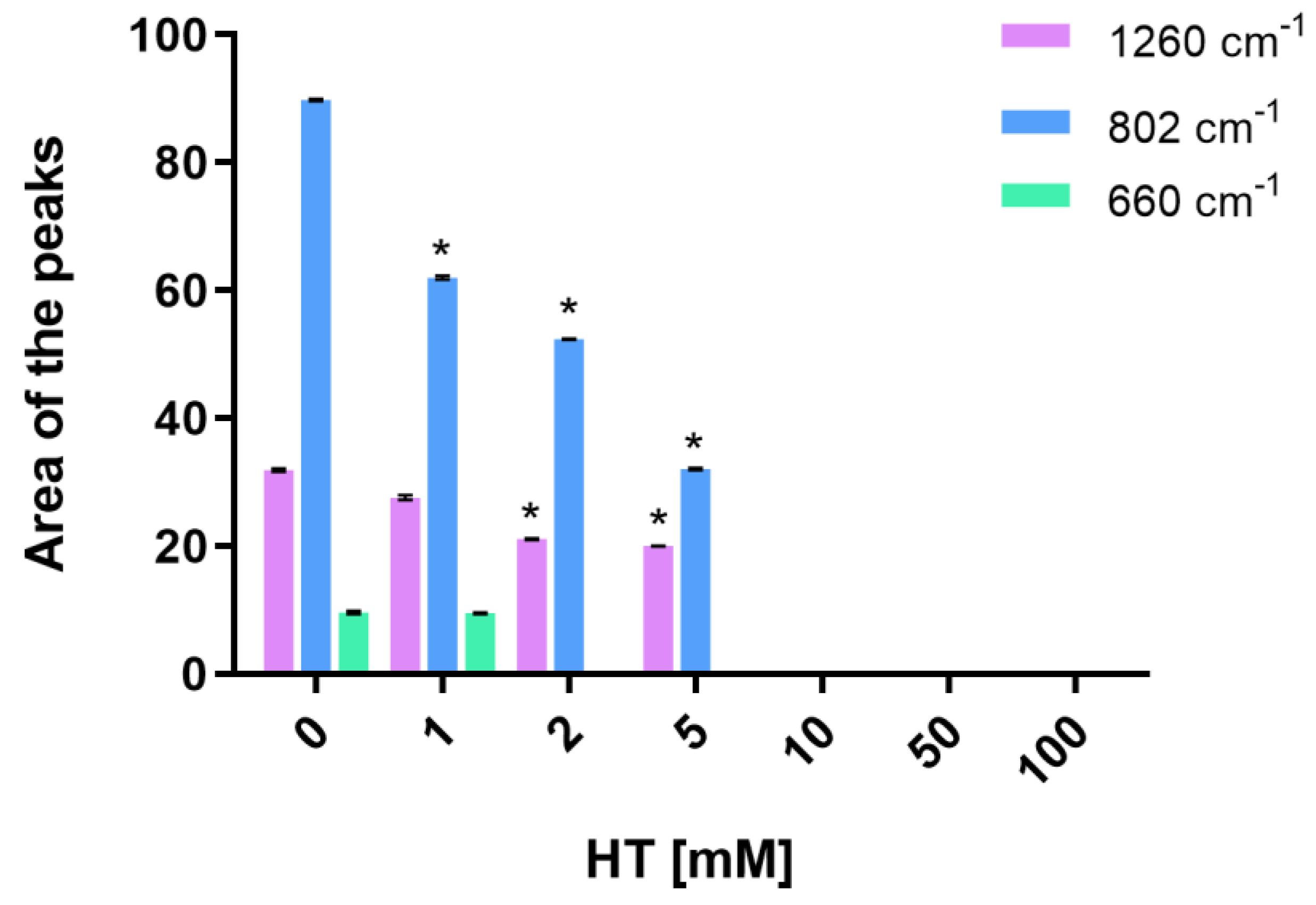
3.3. Hydroxytyrosol Protects PEO Hydrogels from Gamma Ray Irradiation-Induced Polymerization
3.4. Hydroxytyrosol Protects PEO Hydrogels from Gamma Ray Irradiation-Induced Cross-Linking
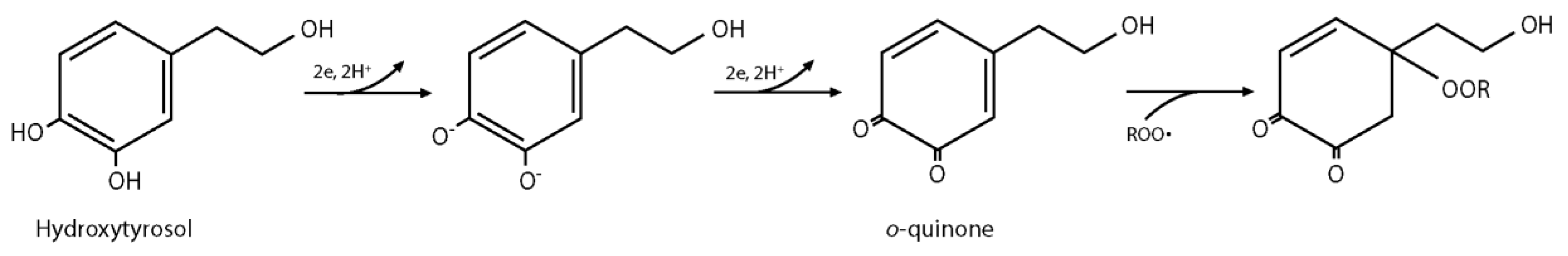
3.5. Quantification of Residual Hydroxytyrosol in PEO Hydrogels after Sterilization by Gamma Irradiation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Polymeric hydrogels: Characterization and biomedical applications. Des. Monomers Polym. 2009, 12, 197–220. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Bodugoz-Senturk, H.; Macias, C.; Muratoglu, O.K. Vitamin C hinders radiation cross-linking in aqueous poly(vinyl alcohol) solutions. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 265, 92–97. [Google Scholar] [CrossRef]
- Oral, E.; Ghali, B.W.; Rowell, S.L.; Micheli, B.R.; Lozynsky, A.J.; Muratoglu, O.K. A surface crosslinked UHMWPE stabilized by vitamin E with low wear and high fatigue strength. Biomaterials 2010, 31, 7051–7060. [Google Scholar] [CrossRef] [PubMed]
- Fruhwirth, G.O.; Wagner, F.S.; Hermetter, A. The αpROX assay: Fluorescence screening of the inhibitory effects of hydrophilic antioxidants on protein oxidation. Anal. Bioanal. Chem. 2006, 384, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bolaños, J.G.; López, Ó.; López-García, M.Á.; Marset, A. Biological Properties of Hydroxytyrosol and Its Derivatives. In Olive Oil-Constituents, Quality, Health Properties and Bioconversions; InTech: London, UK, 2012; pp. 375–396. ISBN 978-953-307-921-9. [Google Scholar]
- Umeno, A.; Takashima, M.; Murotomi, K.; Nakajima, Y.; Koike, T.; Matsuo, T.; Yoshida, Y. Radical-scavenging activity and antioxidative effects of olive leaf components oleuropein and hydroxytyrosol in comparison with homovanillic alcohol. J. Oleo Sci. 2015, 64, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Yüksel Aydar, A.; Öncü Öner, T.; Üçok, E.F.; Aydar, A.Y.; Assistant, R. Effects of Hydroxytyrosol on Human Health. EC Nutr. 2017, 11, 147–157. [Google Scholar]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From laboratory investigations to future clinical trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Davidson, G. Spectroscopic Properties of Inorganic and Organometallic Compounds: Volume 26; Royal Society of Chemistry: Cambridge, UK, 1993; ISBN 978-0-85186-474-7. [Google Scholar]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, G. Periodontal Regeneration Using Gelatin Hydrogels Incorporating Basic Fibroblast Growth Factor. Biomed. J. Sci. Tech. Res. 2018, 4, 3820–3824. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Hydrogel-Based Strategies to Advance Therapies for Chronic Skin Wounds. Annu. Rev. Biomed. Eng. 2019, 21, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Etzler, F.M.; Wang, Z. Use of texture analysis to study hydrophilic solvent effects on the mechanical properties of hard gelatin capsules. Int. J. Pharm. 2006, 324, 128–135. [Google Scholar] [CrossRef] [PubMed]



| HT Starting Concentration | Citrate Buffer-Based Polyethylen Oxide (PEO) Hydrogel | Phosphate Buffer-Based PEO Hydrogel | ||
|---|---|---|---|---|
| (HT) Before Irradiation | (HT) After Irradiation | (HT) Before Irradiation | [HT] After Irradiation | |
| 1 mM | 0.999 | / | 0.849 | / |
| 2 mM | 1.823 | / | 1.734 | / |
| 5 mM | 4.835 | 4.301 | 4.785 | / |
| 10 mM | 9.867 | 9.411 | 8.913 | 5.761 |
| 20 mM | 19.852 | 19.432 | 17.881 | 11.327 |
| 50 mM | 49.926 | 48.966 | 42.859 | 28.059 |
| 100 mM | 99.918 | 98.998 | 91.875 | 56.107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorini, M.; Crognaletti, V.; Sabry, O.; Scalise, L.; Fattori, P. Hydroxy-tyrosol as a Free Radical Scavenging Molecule in Polymeric Hydrogels Subjected to Gamma-Ray Irradiation. Processes 2021, 9, 433. https://doi.org/10.3390/pr9030433
Fiorini M, Crognaletti V, Sabry O, Scalise L, Fattori P. Hydroxy-tyrosol as a Free Radical Scavenging Molecule in Polymeric Hydrogels Subjected to Gamma-Ray Irradiation. Processes. 2021; 9(3):433. https://doi.org/10.3390/pr9030433
Chicago/Turabian StyleFiorini, Mauro, Veronica Crognaletti, Omar Sabry, Lorenzo Scalise, and Paolo Fattori. 2021. "Hydroxy-tyrosol as a Free Radical Scavenging Molecule in Polymeric Hydrogels Subjected to Gamma-Ray Irradiation" Processes 9, no. 3: 433. https://doi.org/10.3390/pr9030433
APA StyleFiorini, M., Crognaletti, V., Sabry, O., Scalise, L., & Fattori, P. (2021). Hydroxy-tyrosol as a Free Radical Scavenging Molecule in Polymeric Hydrogels Subjected to Gamma-Ray Irradiation. Processes, 9(3), 433. https://doi.org/10.3390/pr9030433