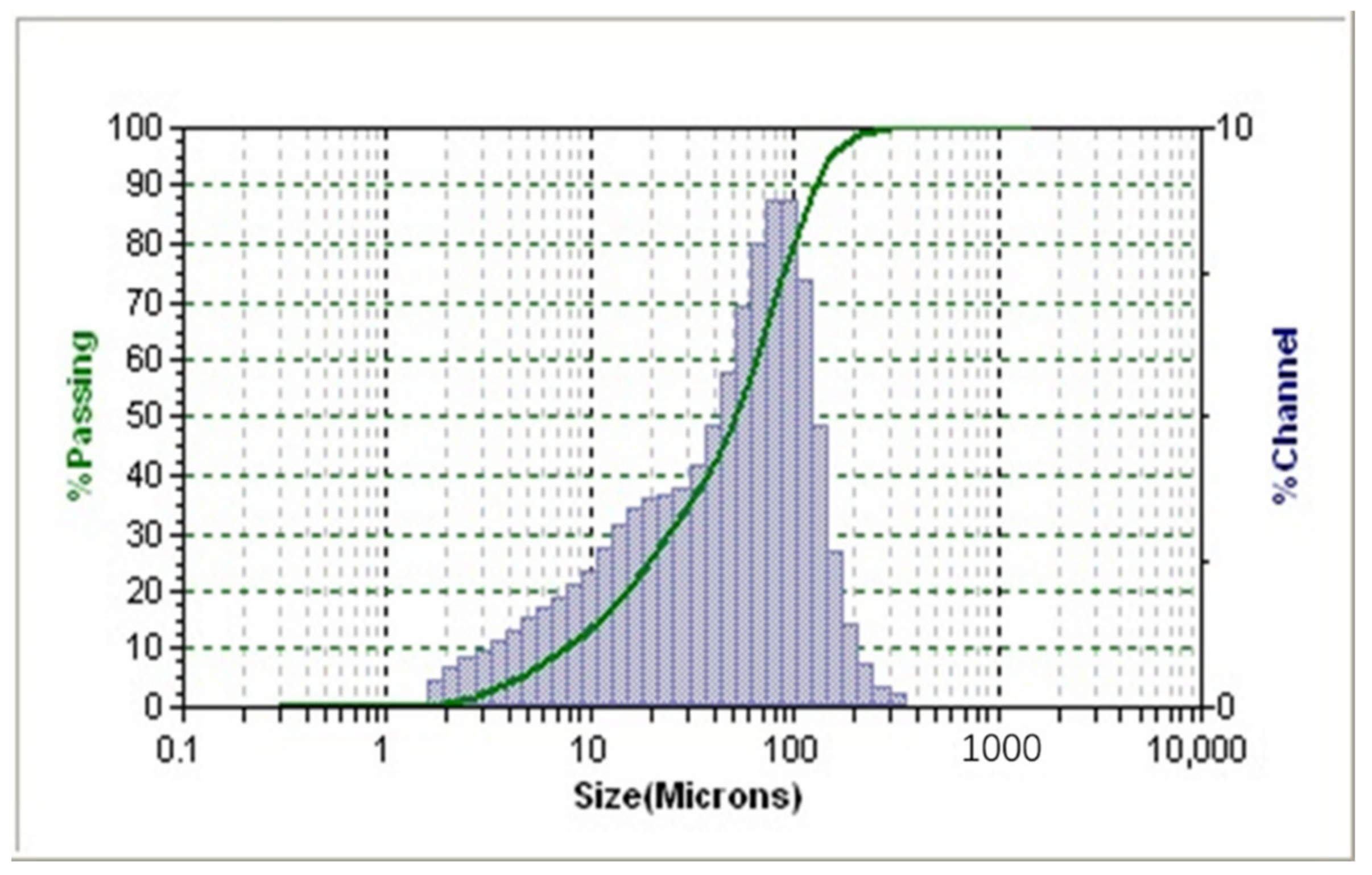
1. Introduction
Coal, as a main energy source, makes an important contribution to the national economy with its consumption accounting for 58% of China’s total energy consumption in 2018 [
1]. However, the fine low rank coal often was discarded or stored in yard near the mine due to the difficulty of effective utilization, which caused severe environmental pollution and waste of resources. In 2017, the National Energy Administration of China clearly proposed the separation and utilization of low rank coal with late coal forming period, high volatile content, and low reactivity [
2].
Froth flotation is a very useful method to facilitate the quality of coal. Unfortunately, fuel oil/kerosene, a conventional collector, is very inefficient to facilitate low rank coal flotation, and a considerable amount of collector is required to attain a satisfying recovery [
3]. The high oxygen content, especially the abundance of polar groups (phenolic/alcoholic hydroxyl, carbonyl, carboxyl, ether, and methoxyl), results in the poor floatability of coal [
4,
5,
6,
7]. These hydrophilic functional groups weaken the surface hydrophobicity of low rank coal via absorbing water molecules to form hydration film [
8]. Moreover, some weakly acidic groups (phenolic OH and COOH) ionize in solution making the coal surface negatively charged [
9]. Low flotation efficiency, large consumption of collector, and high cost are the main problems that restrict the efficient utilization of fine low rank coal.
Electrokinetic properties and contact angle of coal-water are used to characterize the wettability of coal that is essential to the bubble-particle attachment and mineralization process. Numbers of studies have been done to change the wettability of low rank coal. Heteropolar collectors, such as lubricating oil, vegetable oils, amine, carboxylic acid, α-furanacrylic acid, and tetrahydrofuran ester series, were used to promote hydrophobicity of coal surface with the rapid development of a relevant interaction mechanism [
10,
11,
12,
13,
14,
15,
16]. Jia et al. [
14] pioneered the low rank/oxidized coal flotation with a series of tetrahydrofuran (THF) esters as collectors. It was revealed that a higher flotation efficiency can be obtained thanks to the hydrogen bonds between THF series and coal surface. Gui et al. [
15] proposed that α-furanacrylic acid as collectors interacting with the oxidized coal surface must be connected by water molecules in hydration film instead of directly interacting with the hydrophilic functional groups of the coal surface. The tendency of the collector to spread over the coal surface was due to the high interfacial interaction free energy between the collectors and the coal surface. In general, hydrophobic interaction, π-bonding, hydrogen bonding, and electrostatic force are major components of interaction forces of coal surface-heteropolar collectors [
14,
15,
17]. For poor floatability of coal, it was accepted that the polar head of the collector interacted with its polar functional groups on the surface. Compared to the single heteropolar/nonpolar reagent, mixtures of heteropolar reagents and nonpolar materials, namely compound/mixed collectors, including oxidized diesel, biodiesel, waste vegetable oil, and polar/nonpolar mixture [
18,
19,
20,
21], have all been suggested to be more effective in facilitating the recovery of low rank/oxidized coal. It was indicated that the compositions of some collectors were more complex with polar groups, and the low rank/oxidized coal recovery was increased by the synergistic effect of different polar oil molecules [
22]. Furthermore, surfactants have often been employed to combine with oil to promote floatability of low rank coal [
13,
23,
24,
25,
26,
27,
28,
29,
30,
31]. When surfactants were used as emulsifiers, oily collectors were dispersed into a large number of droplets [
32]. The added numbers of oil droplets increased the collision probability between coal particles and oil droplets to assist flotation kinetics [
33]. Furthermore, when surfactants were absorbed on the solid/water interface and solid/oil interface, the required energy of the oily collector spreading over the solid surface was reduced. Above methods markedly raise the low-rank coal flotation efficiency, but the related mechanism of collector-coal is not well understood [
9,
34].
Previous studies rarely considered the price and the source of reagents. The cheap and efficient mixed collector became our research object. Its flotation properties and related mechanism need to be explored. Herein, oleic acid-dodecane (OA-D), oleic acid (OA), and dodecane (D) was used as collectors and the surface free energy of low rank coal treated with different collectors at coal-gas, coal-water and coal-water-coal interfaces was calculated. The synergistic mechanism of OA-D absorbed on the coal surface was predicted by combining the law of wetting heat and the results of FTIR and X-ray Photoelectron Spectroscopy (XPS). Furthermore, the improvement of the floatability of the low rank coal was investigated by flotation tests. Finally, the synergetic mechanism of OA-D in low rank coal flotation was proposed, that is, oleic acid and dodecane molecules formed relatively ordered “supramolecular structure” on the low rank coal surface.
3. Results and Discussion
3.1. Surface/Interface Free Energy Analysis
3.1.1. Wettability of Coal Particles in the Presence of Collectors
The contact angles for water (
), α-bromonaphthalene (
), and formamide (
) on the coal surface treated with D, OA, and OA-D are listed in
Table 2. The results showed that the highest value of contact angle was observed for
and the lowest for
. For water and formamide, the contact angles for coal treated with D, OA, and OA-D were higher than untreated coal particles, and their order was OA-D > OA > D. For α-bromonaphthalene, the contact angles for coal treated with D, OA, and OA-D were lower than untreated coal, and the contact angles decreased in the order of D > OA > OA-D. It was demonstrated that the coal surface changed to more hydrophobic and homogeneous after treated with nonpolar/polar reagent, and the better performance was obtained by the combination of the two.
3.1.2. Surface Free Energy of Coal Surface Treated with Different Reagents
The values used for calculations are presented in
Table 3, which were all taken from literatures [
41,
42,
43]. The calculated results of surface free energy components of coal/coal + reagent are listed in
Table 4.
Many organic components in low rank coal surface contain a multitude of polar groups including -OH, -COOH, >C=O, and -CO-, which can form hydrogen bonds with water molecules, causing the surface free energy to rise. Moreover, the
bond in the aromatic ring of the coal surface plays a greater role in polar interactions with absorbed collectors. The
bond, >C=O, and -CO- groups are the origin of the Lewis base (
), and the -COOH group is the origin of Lewis acid (
). Meanwhile, -OH is both the origin of Lewis acid and Lewis base part of
[
36]. The purpose of introducing collectors into the system was to reduce the contribution of these polar components and the surface free energy of low rank coal.
Table 4 showed that the surface free energies of coal treated with collectors were lower than that of untreated coal. Both
and
values depended on the kind of collectors absorbed on the coal surface. The relative order of
components was D< OA < OA-D, while
components appeared in the opposite trend. No matter whether the collectors worked or not, Lifsbitz-van der Waals (
) of coal surface free energy, was greater than Lewis acid-base components (
), which demonstrated that the nonpolar character of coal was more remarkable than the polar character that determined the wettability and hydrophobicity of the coal surface.
The surface free energy of coal treated with OA-D was the minimum, 40.51 mJ/m2, which was 4.71 and 1.62 mJ/m2 lower than that treated with D and OA, respectively. This indicated that OA-D decreased the surface free energy of coal by collaborative adsorption with D and OA reducing the nonpolar component and polar component, respectively.
3.1.3. Interaction Free Energy on Coal-Water Interface
The calculated values of the free energy change between coal particles and water were listed in
Table 5. The values of interaction free energy between water and coal particle were all negative, which suggested that the interactions were spontaneous and the hydration films can be formed on the particle surface. The greater the negative value was, the stronger the coal surface hydrophilicity was, and the surface was easier to be wetted by water. The negative value of Gibbs free energy between water and coal treated with reagents were reduced, which meant that the hydrophilicity was decreased and the hydration film formed on the coal surface was thinner. The surface hydrophilicity sequence of the coal treated with reagents was: D + coal > OA + coal > OA-D + coal. OA-D can change Gibbs free energy to the greatest extent with the absolute value of interaction energy 74.52 mJ/m
2, which was 27.41, 22.52, and 9.67 mJ/m
2 lower than that treated with no collector, D, and OA, respectively. This was due to the fact that OA and D were together adsorbed on coal surface to induce the strongest hydrophobic surface and the thinnest hydration film. The above results were well in agreement with the results of the coal surface free energy.
3.1.4. Interaction Free Energy between Coal Particles in the Solution Phase
The free energy of interaction between coal particles in the solution phase was calculated and summarized in
Table 6. The free energy between coal particles in water phase was negative, which suggested that there were attractive interactions between coal particles and they could spontaneously flocculate. In the presence of collectors, the increase in the absolute values of free energy resulted from the Lewis acid-base force. The Lewis acid-base free energy increased after the adsorption of collectors, indicating that the Lewis acid-base interactions was strengthened and was the main interaction between coal particles. The coal surface became more homogeneous after treated with collectors, so the interactions between coal particles and the possibility of agglomeration improved. These results may facilitate the appearance of coarse particles enhancing the flotation efficiency. The free energy absolute value of coal particles treated with OA-D was the highest (77.58 mJ/m
2), which can be attributed to that OA and D collaborative adsorbed on coal surface which created a more hydrophobic surface.
3.2. Wetting Heat Analysis
In the flotation system, the collectors replace water molecules on the coal surface which can change wettability of coal surface. The energy released in this process can reflect the intensity of collector’s adsorption.
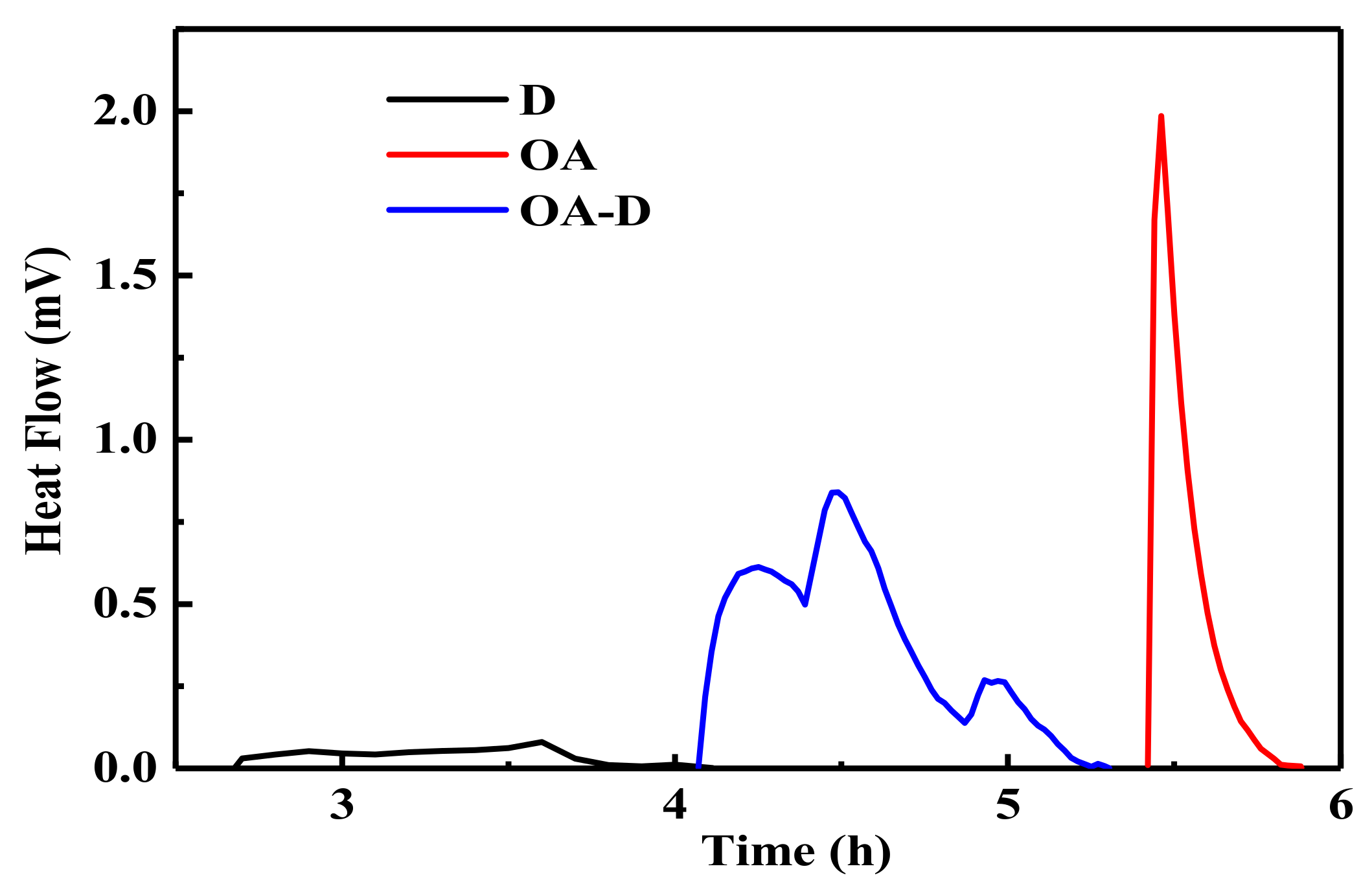
Figure 2 shows the heat flow curves between coal and collectors. No peak was observed for the heat flow of D which indicated that the interaction between D and coal was weak. The peaks for heat flows of OA and OA-D were observed, indicating that absorption strength between D/OA-D and coal increased. The related wetting heat is shown in
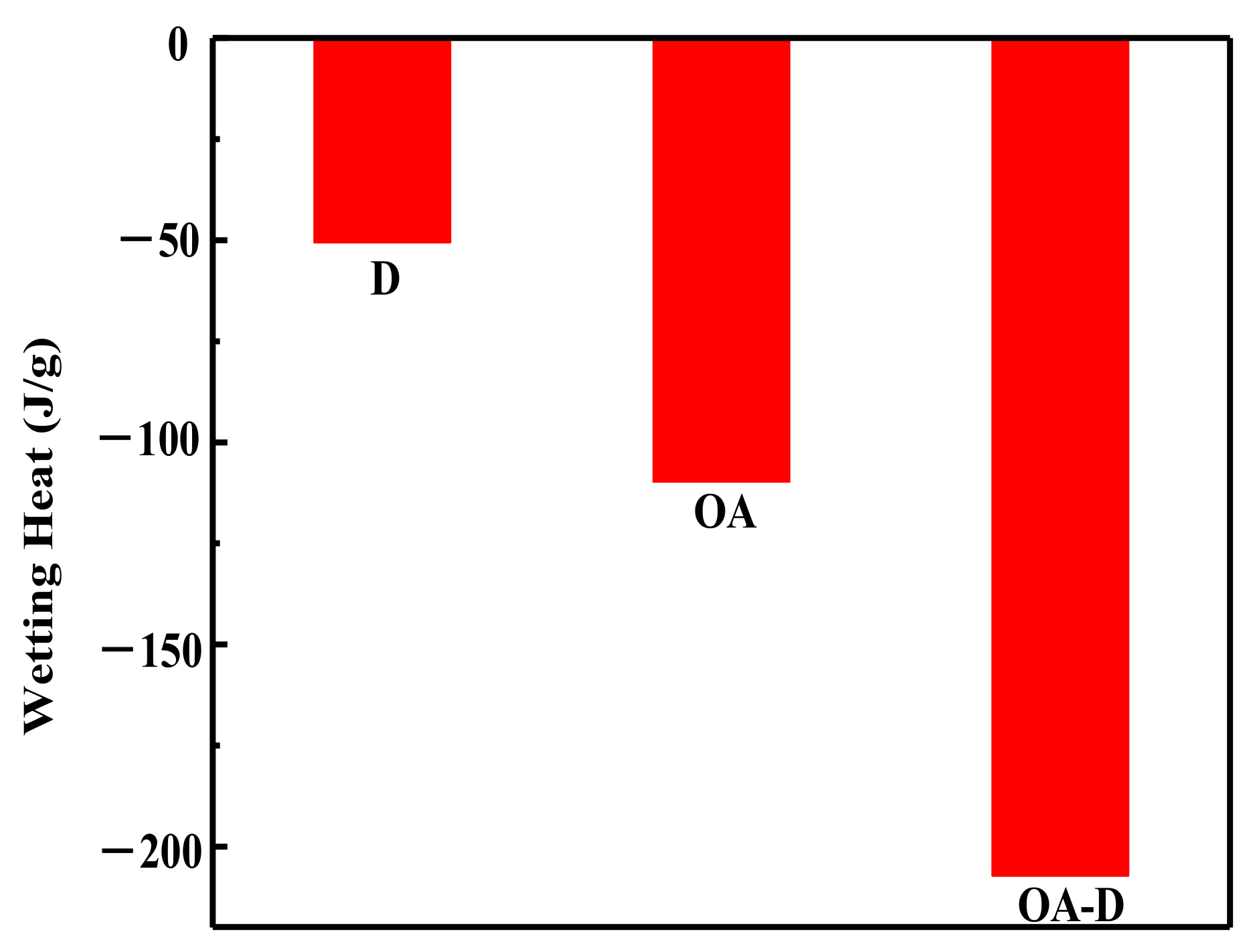
Figure 3. The negative values of the wetting heat revealed that the adsorption process was exothermic and spontaneous. OA-D had the largest absolute value of wetting heat (207.14 g/J), which was about 2 times that of OA and 4 times that of D. This was mainly due to the fact that OA-D wetted the coal surface easier with strong forces including hydrogen bond and van der Waals between OA-D and coal surface. It can be predicted that OA-D was a more effective collector than OA/D due to its superior hydrophobic modification performance.
3.3. FTIR Analysis
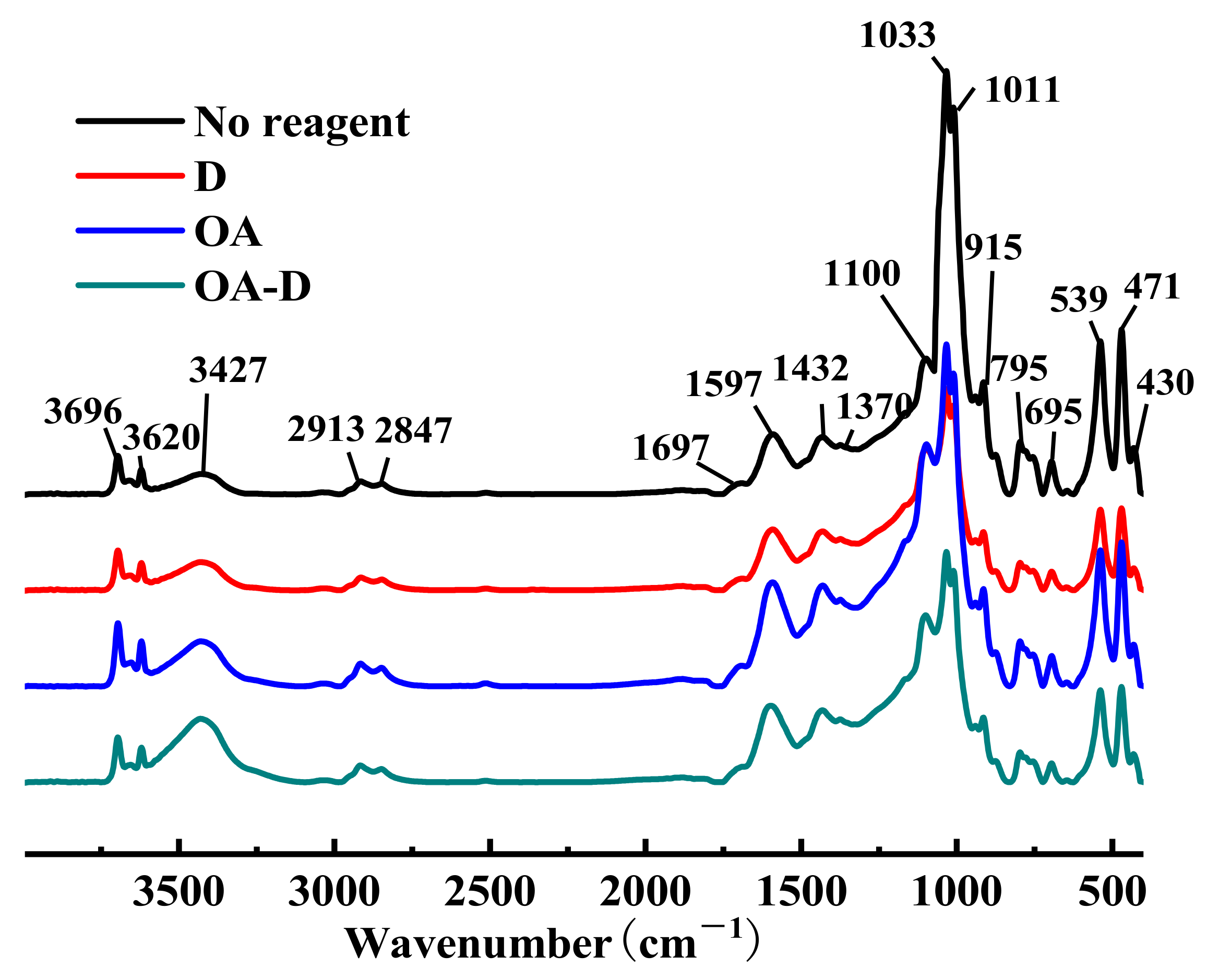
FTIR spectra of low rank coal treated with different collectors are shown in
Figure 4. Whether coal was absorbed by single or combined collector, no new absorption peaks and no shift (shift > 4 cm
−1) of peaks appeared in the FTIR spectra of flotation concentrate, which gave an indication of there was physical adsorption between coal and collectors.
The relative absorbance of peaks at 3427, 2913, 1692, 1597, 1432, 1370, and 1100 cm−1 attributing to organic constituents in coal were strengthened after the adsorption of collectors, which can be caused by the increase of the content of organic components. The main reason was that there were many polar groups in the organic components of the low rank coal, and these organic components were enriched during the flotation process. Meanwhile, characteristic peaks at 1033, 1011, 915, 539, and 471 cm−1 relating to inorganic minerals declined significantly, which may be explained by the decline of ash and sulfur.
The amplitude intensity of the stretching vibration peak of OH at 3427 cm−1 (assigned to alcohol or phenol) was in the order of D < OA < OA-D, indicating that the collecting ability of these three collectors also increased in turn. The peaks at 1033, 1011, 915, 539, and 471 cm−1, relating to inorganic minerals, were reduced as follows: D > OA-D > OA. OA-D, a relatively effective collector in low rank coal flotation, had better collecting capability than a single reagent (OA/D), but its selection ability was in the middle. It integrated the collecting capability of OA and the selection ability of D.
3.4. XPS Analysis
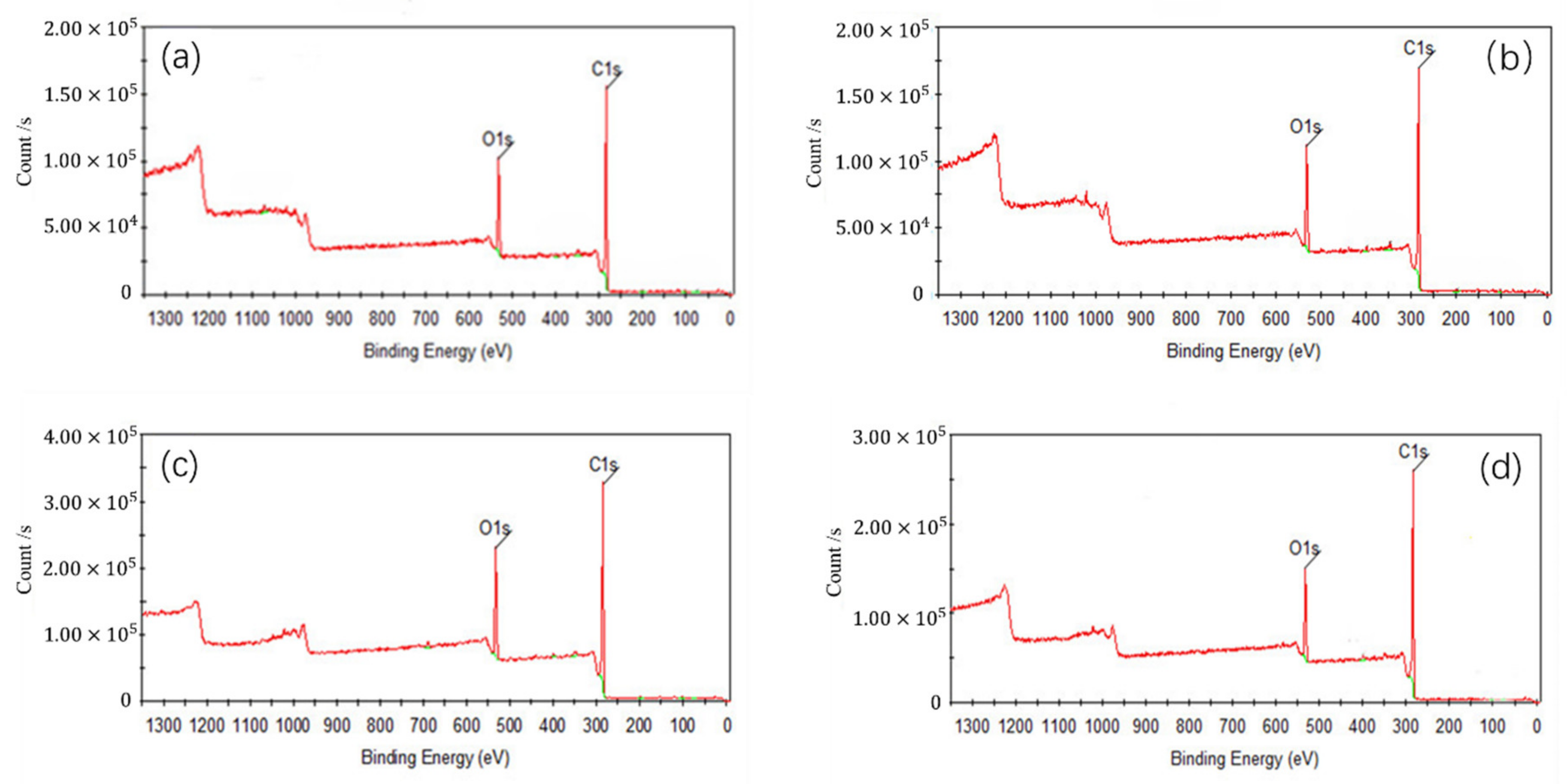
In
Figure 5, C1s peaks of coal surface were enhanced after adsorption of collectors, because some polar groups of the coal surface were covered and the hydrophobicity of coal surface was enhanced. C1s peak of the coal after the adsorption of OA was stronger than that of D, which indicated that OA had a strong modification effect on low rank coal surface. Compared to single collector, C1s peak of the coal after adsorbed OA-D was the strongest due to the synergistic effect of OA and D.
Table 7 listed the contents of carbon and oxygen on low rank coal surface treated with collectors. As can be observed, for all the collectors, the carbon contents of low rank coal surface increased, but the oxygen contents decreased. It was evident that an increase of 0.77~2.41% in carbon content were obtained in the presence of D, OA, and OA-D, which can be attributed to the fact that the hydrophobic sites of coal surface were covered by a nonpolar D and hydrophilic sites of coal surface were masked by heteropolar OA. However, whether D or OA, it cannot be absorbed on the hydrophobic and hydrophilic sites of coal surface at the same time to enhance hydrophobicity to a large extent. Therefore, the combination of OA and D as a collector can completely enhance hydrophobicity of coal surface.
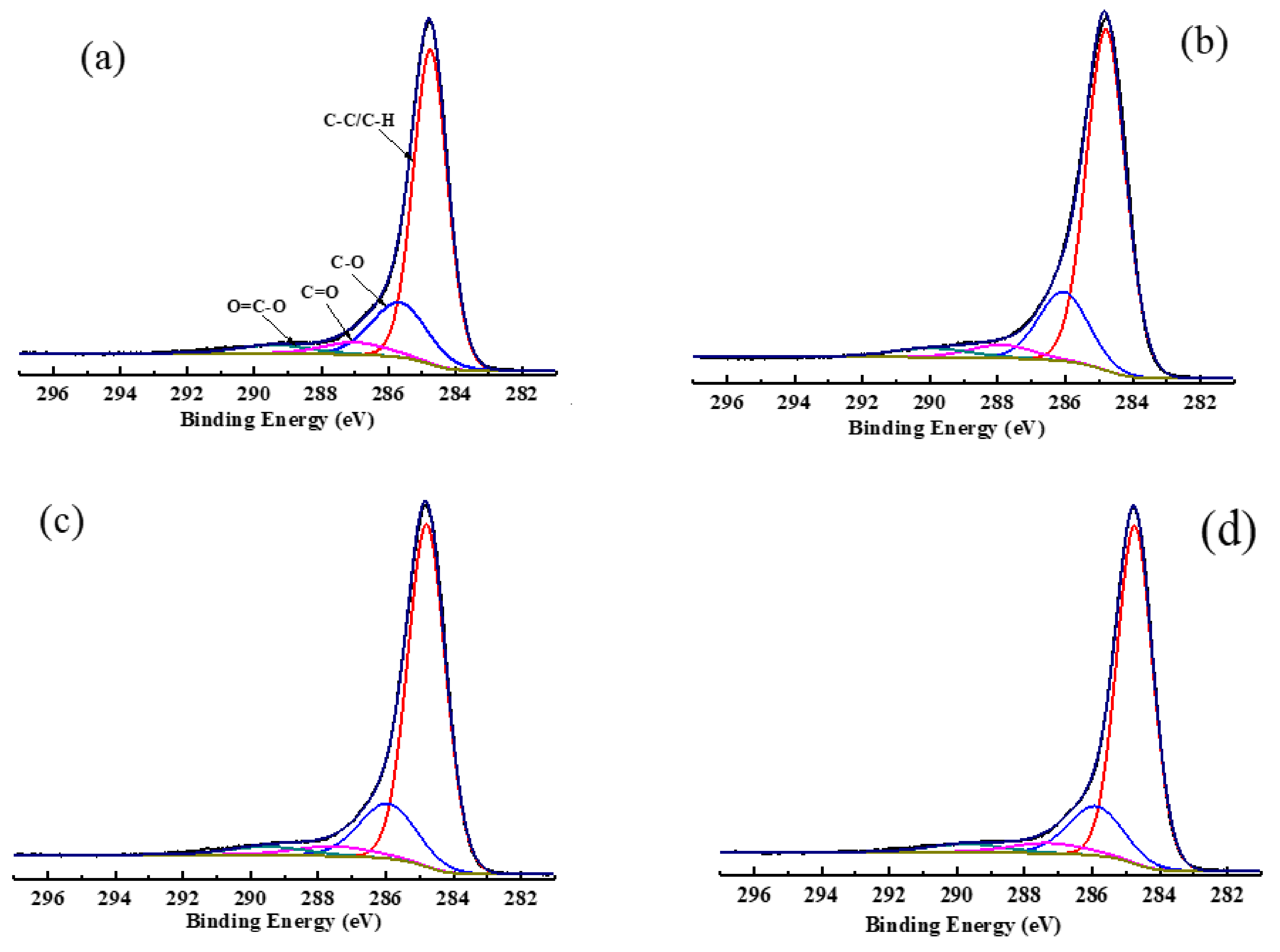
The C1s spectra of low rank coal with and without adsorption of collectors and its peak fitting are shown in
Figure 6, whereas its carbon contents are listed in
Table 8. As can be seen in
Figure 6, there are four forms of carbon that exit in the surface structure of coal: aromatic graphitized carbon/aliphatic carbon (284.8 eV, C-C/C-H), phenolic/ether carbon (285.8~286.1 eV, C-O), carboxide (287.6~287.9 eV, C=O), and carboxyl (289.2~290.0 eV, COOH) [
44].
Table 8 demonstrates that the major combination form of the carbon structure was C-C/C-H, accounting for 70.61%. The contents of C-O, C=O, and COOH were 19.90%, 5.35%, and 4.14%, respectively. With the adsorption of collectors, the order of the contents of C-C/C-H was OA-D > OA> D. When OA-D was used as a collector, the contents of C-C/C-H increased by 3.92% and the contents of C-O, C=O, and COOH fell by 3.3%, 0.42%, and 0.19%, respectively. However, the contents of C-C/C-H were only 1.96% and 3.27% higher than those of raw coal in the case of D and OA, respectively. Therefore, it can be concluded that the polar head of OA, containing COOH that was the hydrogen bond donor and acceptor, and can form a hydrogen bond with the oxygen-containing groups of the coal surface. The polar head of OA was mainly absorbed on the C-O group of coal surface and the nonpolar head extended outward, which facilitated D to absorb on the hydrophobic area of coal surface or the area modified by OA. These findings once again demonstrated the synergistic effect between OA and D.
3.5. Flotation Results
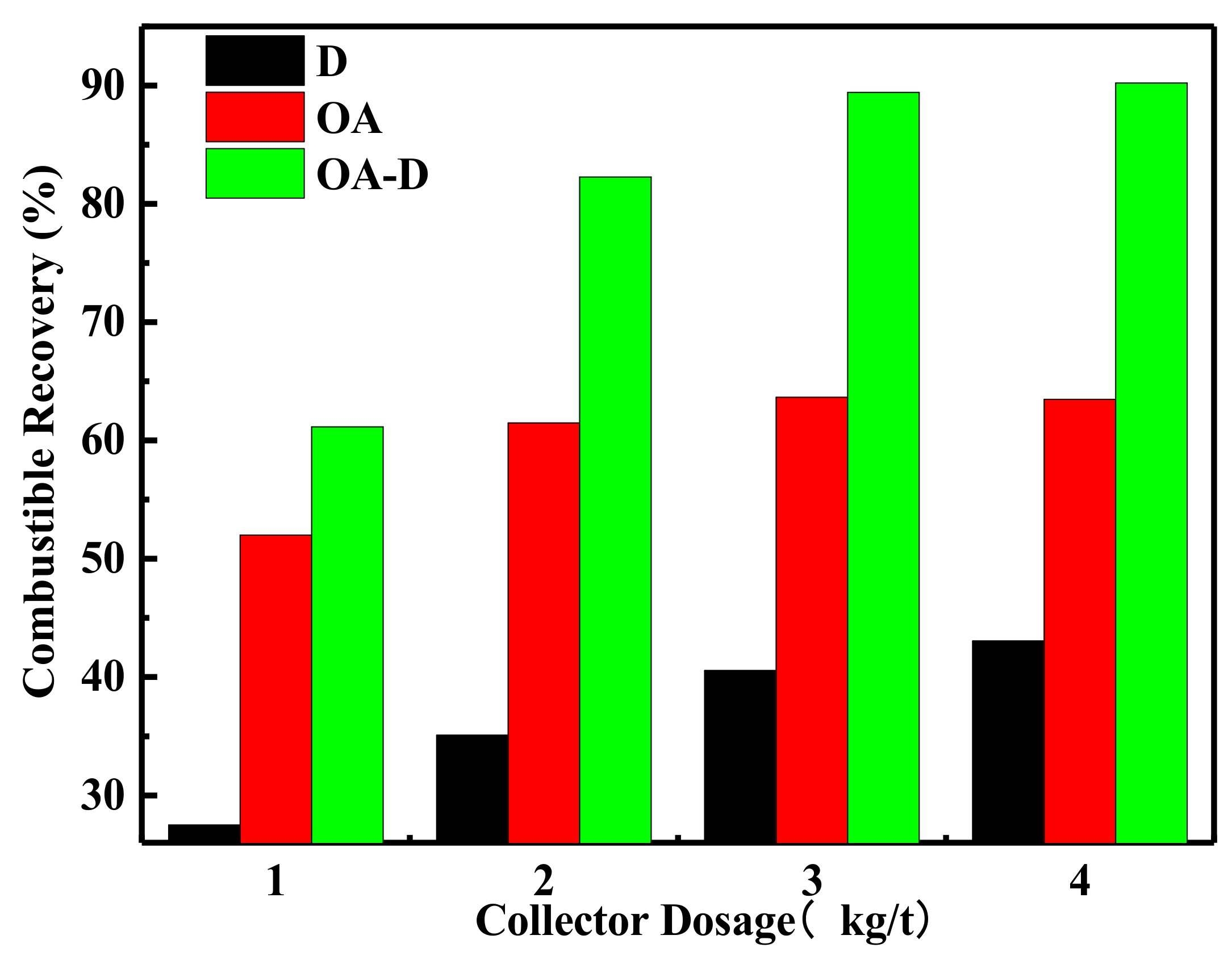
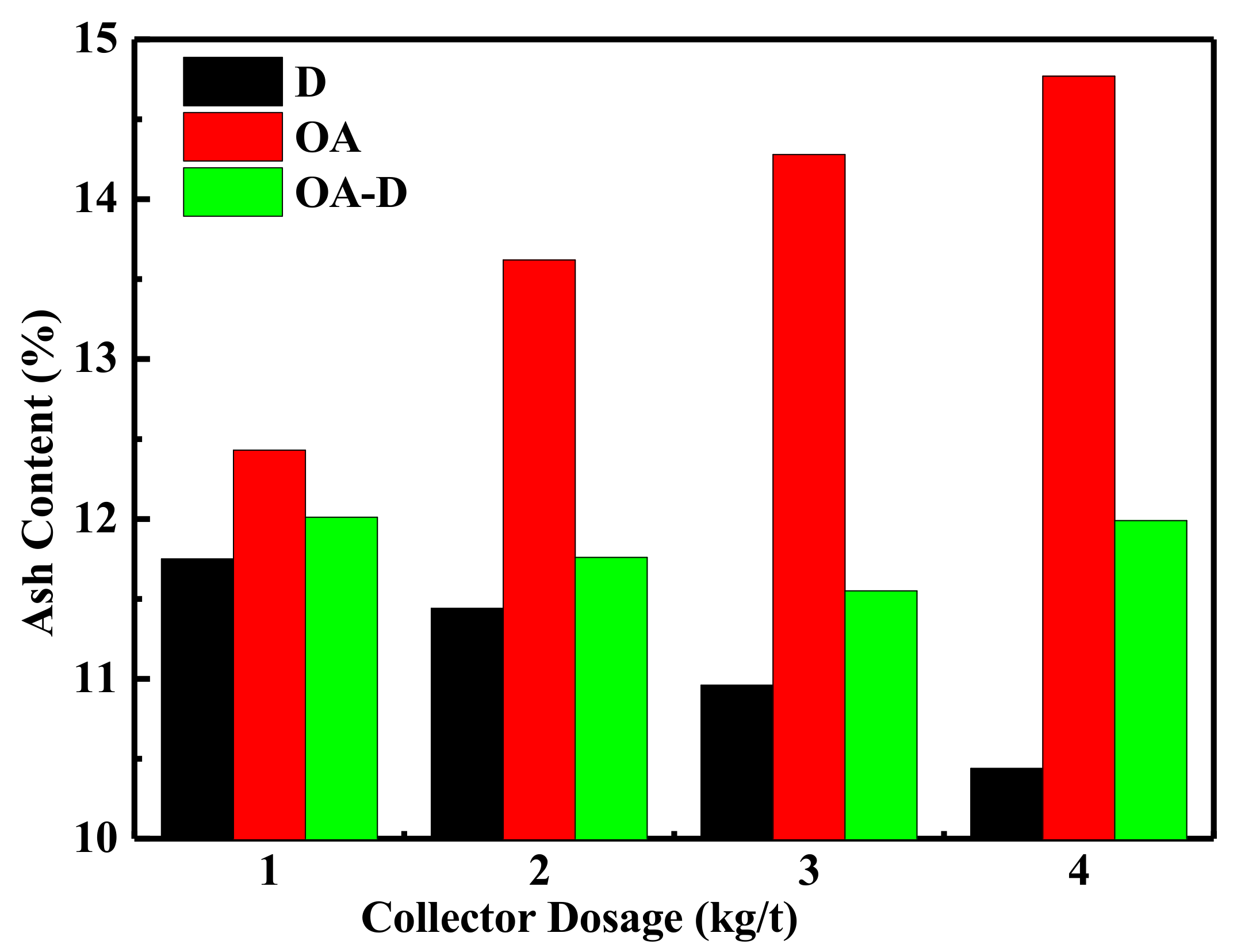
The effect of collector dosage on combustible recovery and concentrate ash content is shown in
Figure 7 and
Figure 8, respectively. In
Figure 7, the combustible recovery increased with the increase of collector dosage. When the collector dosage was more than 3 kg/t, the recovery remained basically unchanged. At the collector dosage 3 kg/t of each collector, the order of combustible recovery under each collector was OA-D > OA > D. The combustible recovery was 89.45% with OA-D, which was 48.86% and 25.77% higher than with D and OA alone. As collector dosage increased, concentrated ash content decreased using D as a collector, while it increased in the case of D/OA-D. At the same collector dosage, OA had the highest concentrated ash content, and the lowest ash content was achieved by D, with OA-D in the middle. This might be due to OA bound with oxygen-containing groups of coal surface as well as gangue mineral. Therefore, the top flotation performance was obtained at OA-D dosage of 3 kg/t, with the highest combustible recovery and an acceptable concentrate ash content. These results were well consistent with the above conclusion about the synergistic effect of OA and D for low rank coal flotation.
3.6. Synergetic Mechanism
Higher combustible recovery can be obtained using OA-D as a collector than a single collector. The thermodynamic properties of coal particles treated with OA-D became more hydrophobic with thinner hydration film. The results of wetting heat, FTIR and XPS revealed that OA-D was the most effective collector making the low rank coal easier to float.
Different from the viewpoints of former scholars [
21,
23,
27,
45], we proposed that there might be a relatively ordered “supramolecular structure” when OA-D molecules were absorbed on the low rank coal surface.
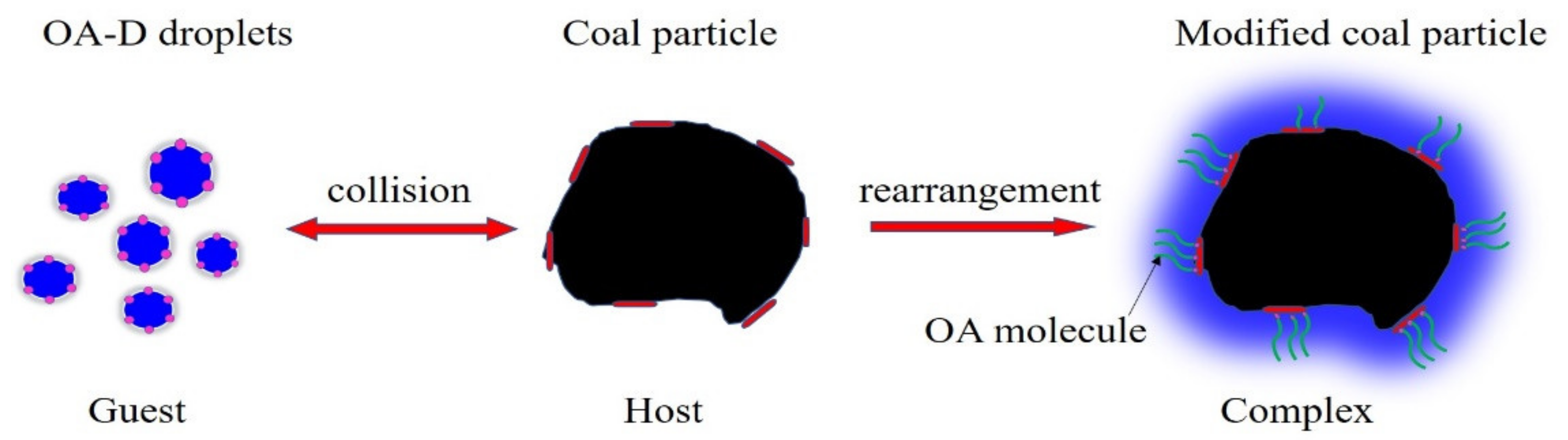
Figure 9 shows the formation process of the supramolecular system. OA-D droplets and low rank coal particles acted as guest and host, respectively. When OA-D droplets collided with the low rank coal particles, the small droplets were deformed and reconstructed in the molecular arrangement. OA molecules and D molecules were respectively adsorbed on the polar and nonpolar regions of coal surface via weak interactions, such as hydrogen bonds, van der Waals forces, and hydrophobic forces. Furthermore, the hydrocarbon chains of OA and D can interact by van der Waals forces and hydrophobic forces. Herein, the assembled complex of OA-D molecules with stable structure called “supramolecular structure”.
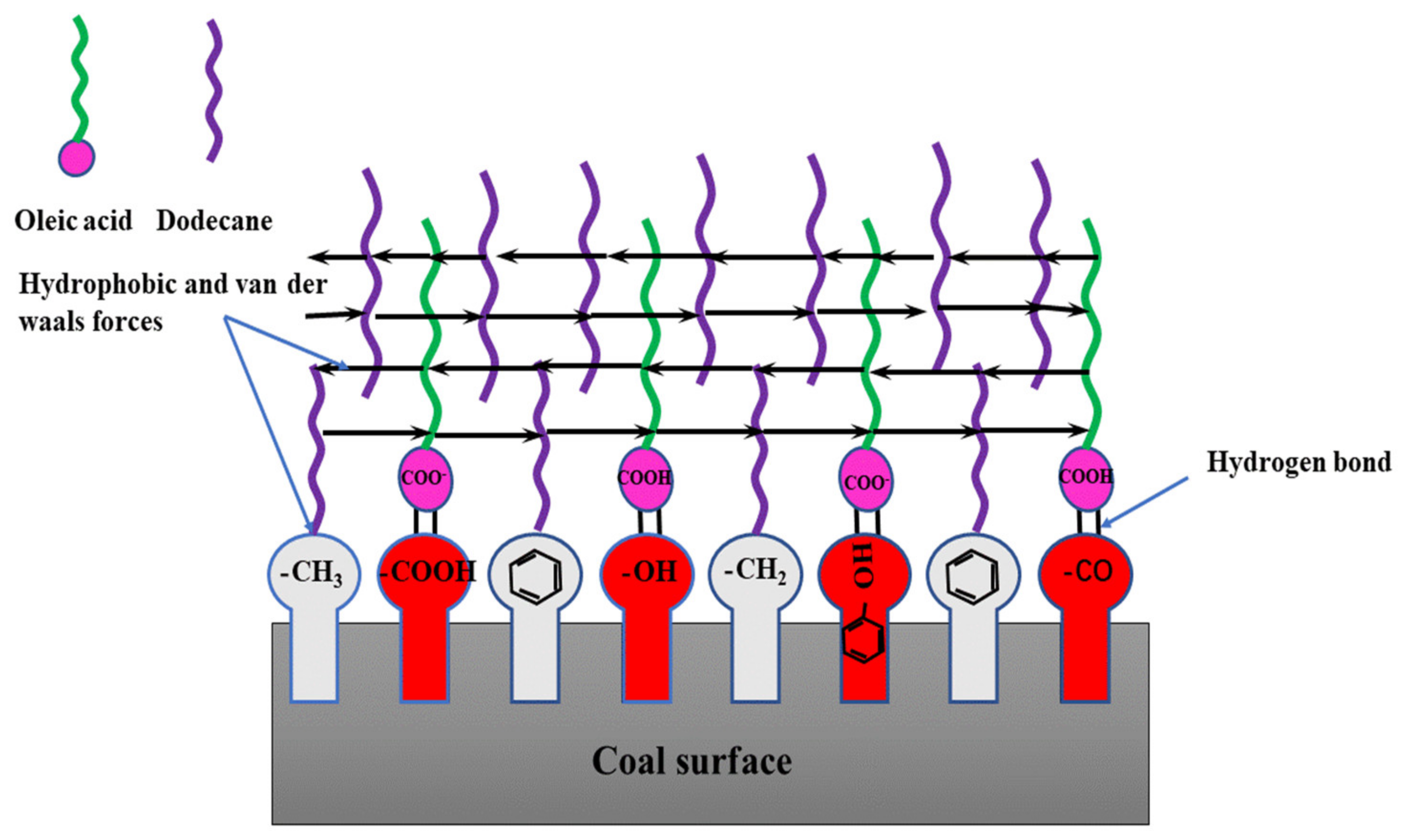
As seen in
Figure 10, the low rank coal surface had hydrophobic sites including benzene, methyl and methylene et al., and hydrophilic sites including -OH, >C=O, -COOH, and -O- et al. When OA-D interacted with coal surface, the OA molecules recognized the hydrophilic sites and D molecules recognized hydrophobic sites. At the same time, the hydrocarbon chains between OA and D molecules interacted with each other, and the energy of the whole system decreased forming a relatively stable molecular layer. Either OA or D interacted with the coal surface alone, there was just weak interaction between them. In case of OA-D, they matched together with the functional groups of low rank coal surface with considerable energy reduction, which was coincident with the results of wetting heat.
The adsorption of OA-D on low rank coal surface was of cooperativity, directivity, and selectivity. The additive and synergetic effect of each part can form strong intermolecular interaction. Although there was no chemical bond between OA-D molecules and functional groups of low rank coal surface, the total binding force between them was relatively strong. OA-D molecules can form compact molecular layers on the low rank coal surface, and the external of whole supramolecular body was all hydrocarbon chains with high hydrophobicity.
4. Conclusions
In this work, a series of experiments were carried out to find a new perspective on the synergetic effect of oleic acid (OA) and dodecane (D) in improving low rank coal flotation. Thermodynamic results showed that coal particles treated with OA-D became more hydrophobic, had thinner hydrate film, and were more likely to condense, which was conducive to promote the floatability of coal. OA-D had the largest absolute value of wetting heat (207.14 g/J), which was about 2 times that of OA and 4 times that of D. According to the results of FTIR, OA-D had a better collecting capability than a single collector (OA or D), and its selection ability was in the middle. It integrated the collecting capability of OA and the selection ability of D. The polar head of OA, containing COOH that was the hydrogen bond donor and acceptor, can form hydrogen bonds with the oxygen-containing groups of the coal surface. The polar head of OA mainly absorbed on the C-O group of coal surface and the nonpolar head extended outward, which facilitated D to absorb on the hydrophobic area of coal surface or the area modified by OA. The optimal flotation results were obtained at an OA-D dosage of 3 kg/t with 89.54% combustible recovery and 11.54% concentrated ash content. Synergetic mechanism of OA-D molecules was that a relatively ordered “supramolecular structure “was formed on the coal surface. There were hydrophobic or van der Waals forces between oleic acid chain and dodecane chain, in addition to the hydrogen bonds, hydrophobic, or van der Waals forces between collector molecules and groups of the coal surface.