Vermicomposting Process to Endosulfan Lactone Removal in Solid Substrate Using Eisenia fetida
Abstract
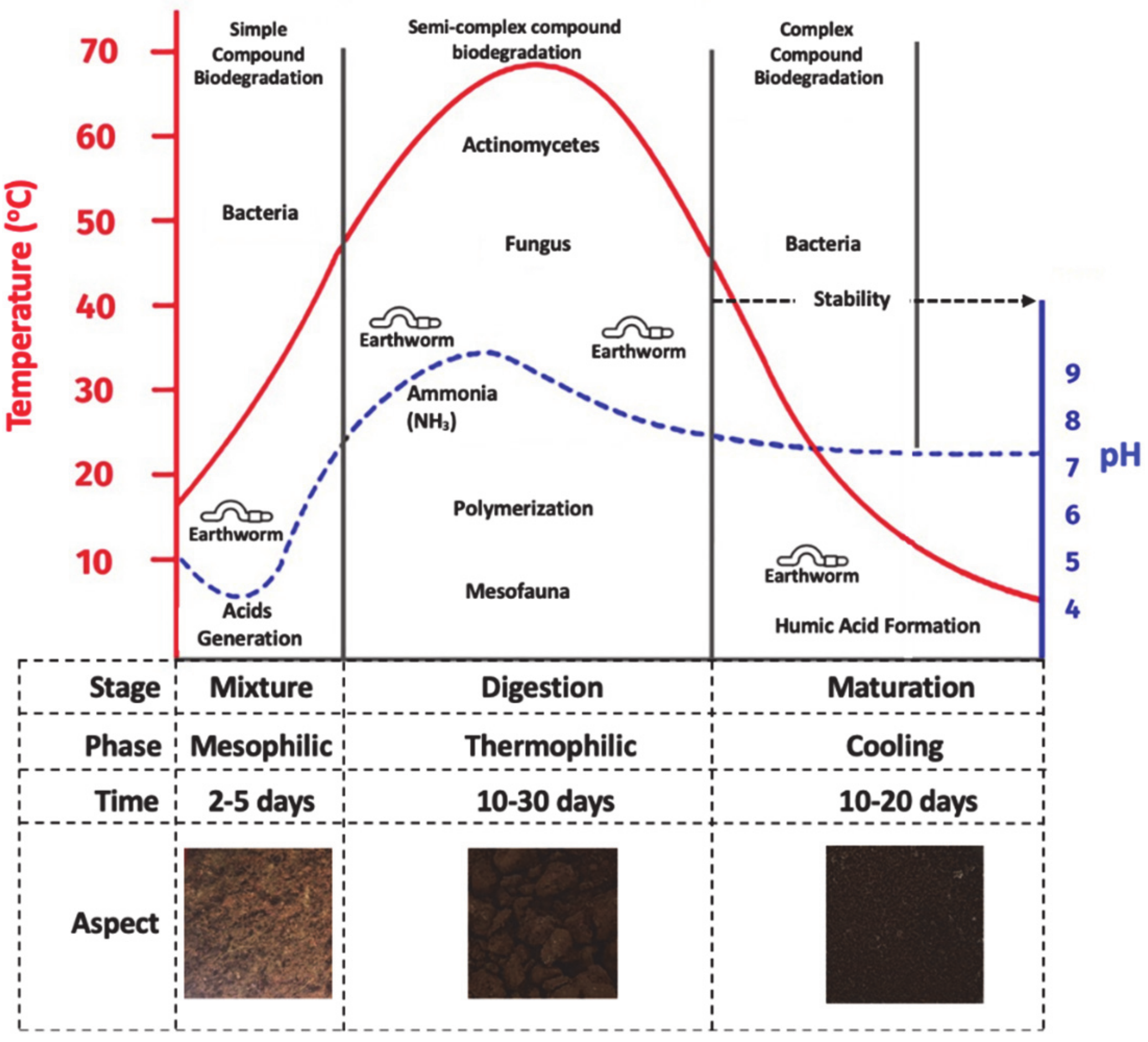
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Raw Material and Solid Substrate
2.3. Physicochemical Characteristics of the Solid Substrate and Vermicompost
2.4. Removal Process
2.5. Optimization to EL Removal
2.6. Enzyme Activity Measurement
2.7. SDS-PAGE Electrophoresis
3. Results and Discussions
3.1. Physicochemical Characteristics of Solid Substrate and Vermicompost
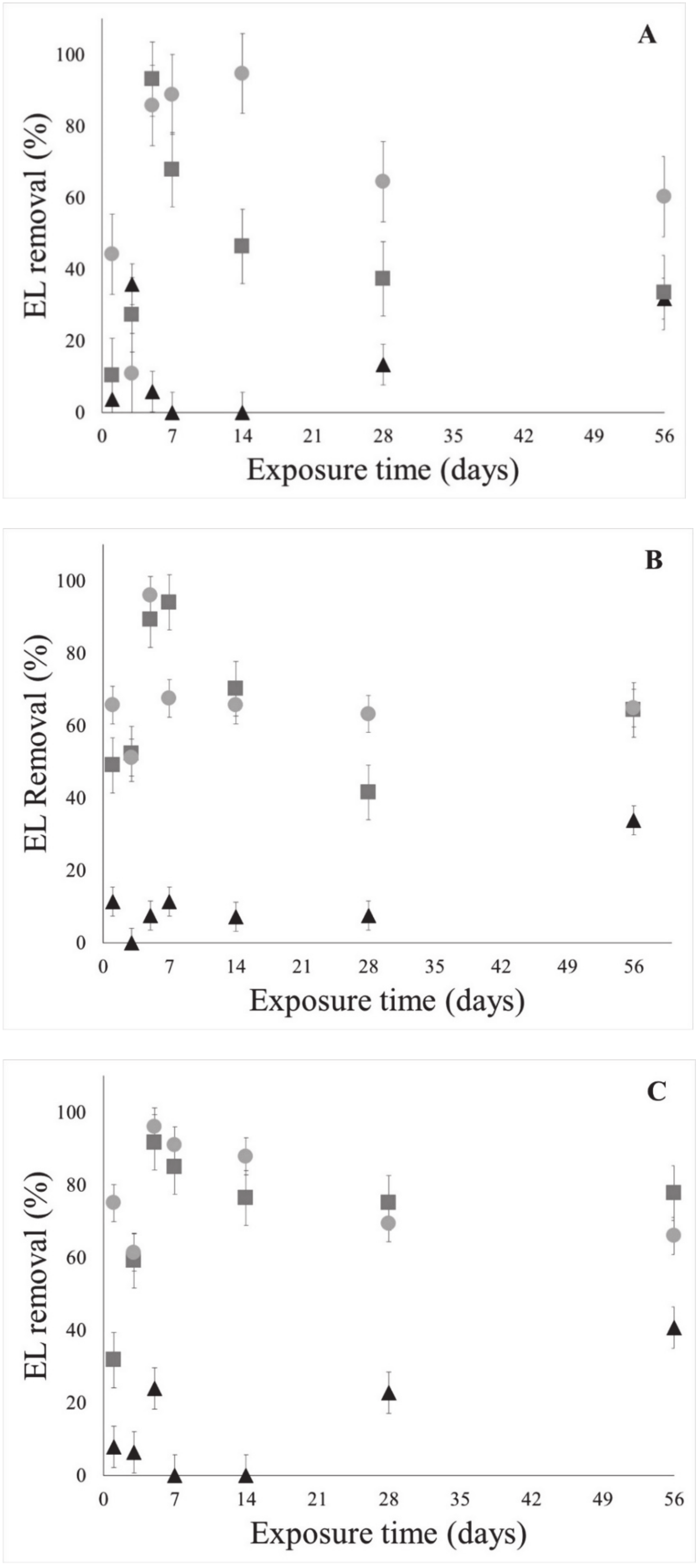
3.2. Non-Sterile Solid Substrate
3.2.1. Endosulfan Lactone Removal on Non-Sterile Solid Substrate
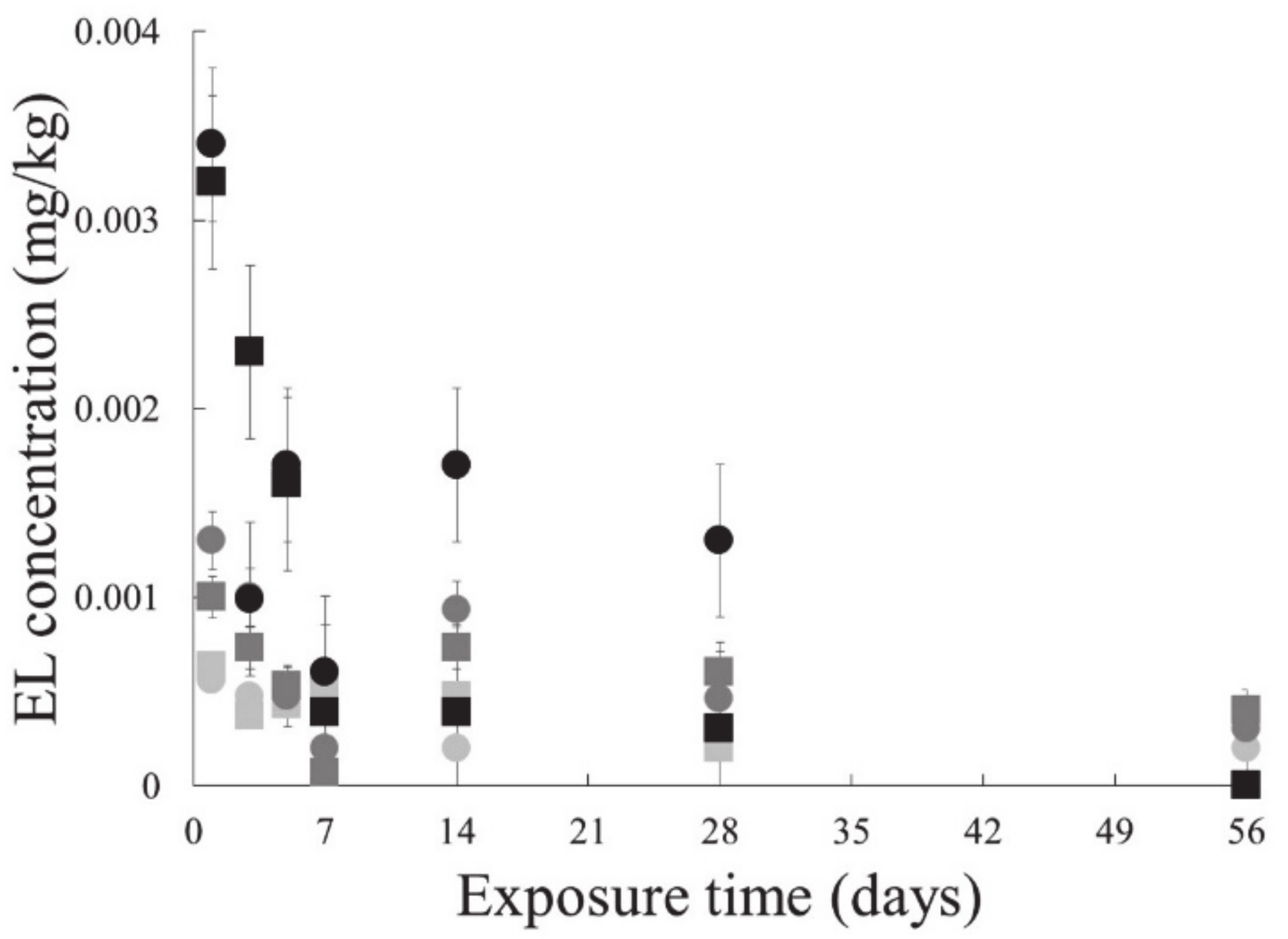
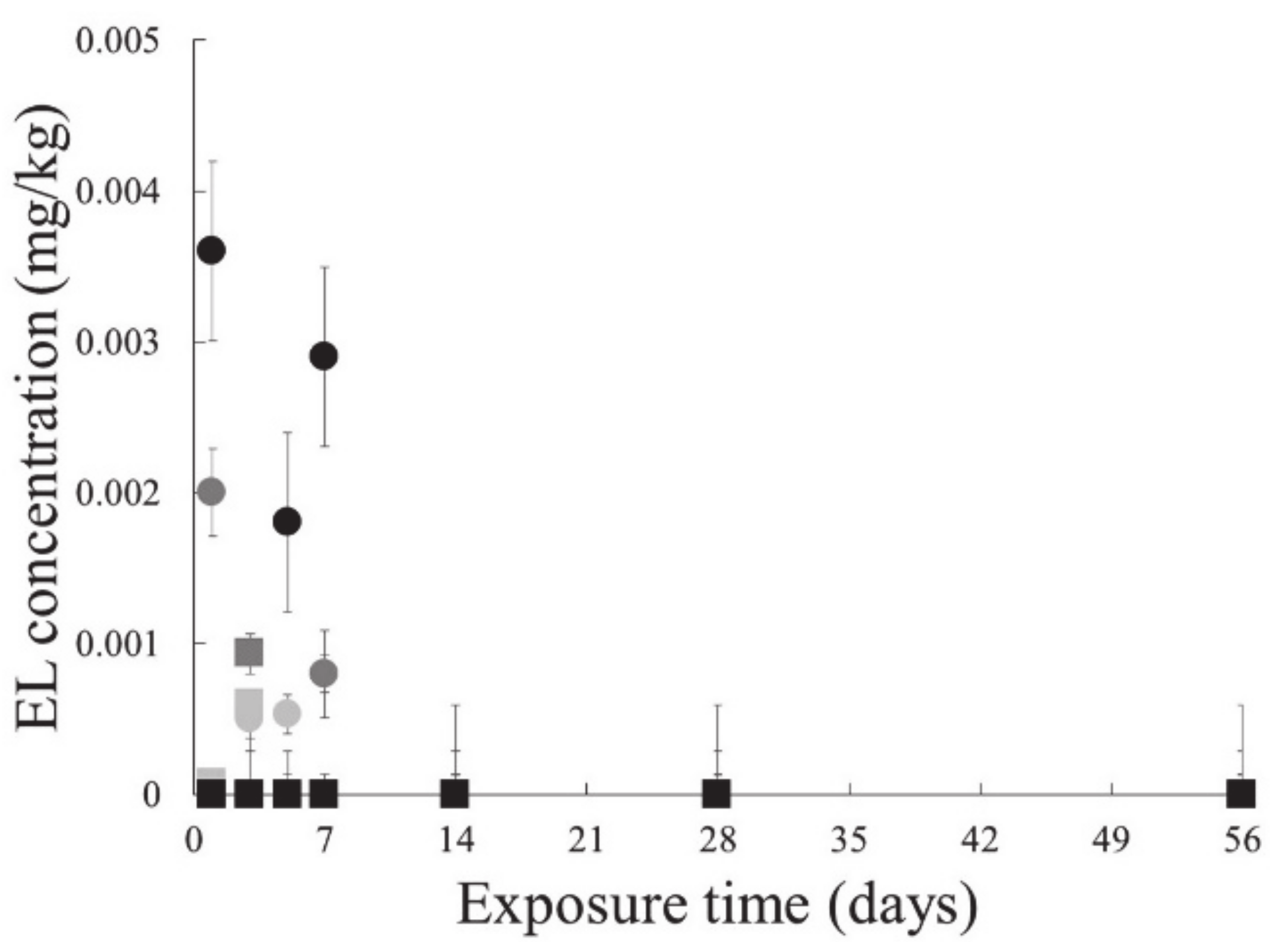
3.2.2. Endosulfan Lactone in Earthworm
3.3. Sterile Solid Substrate
3.3.1. Endosulfan Lactone Removal on Sterile Solid Substrate
3.3.2. Endosulfan Lactone in Earthworm
3.4. Optimization of Endosulfan Lactone Removal
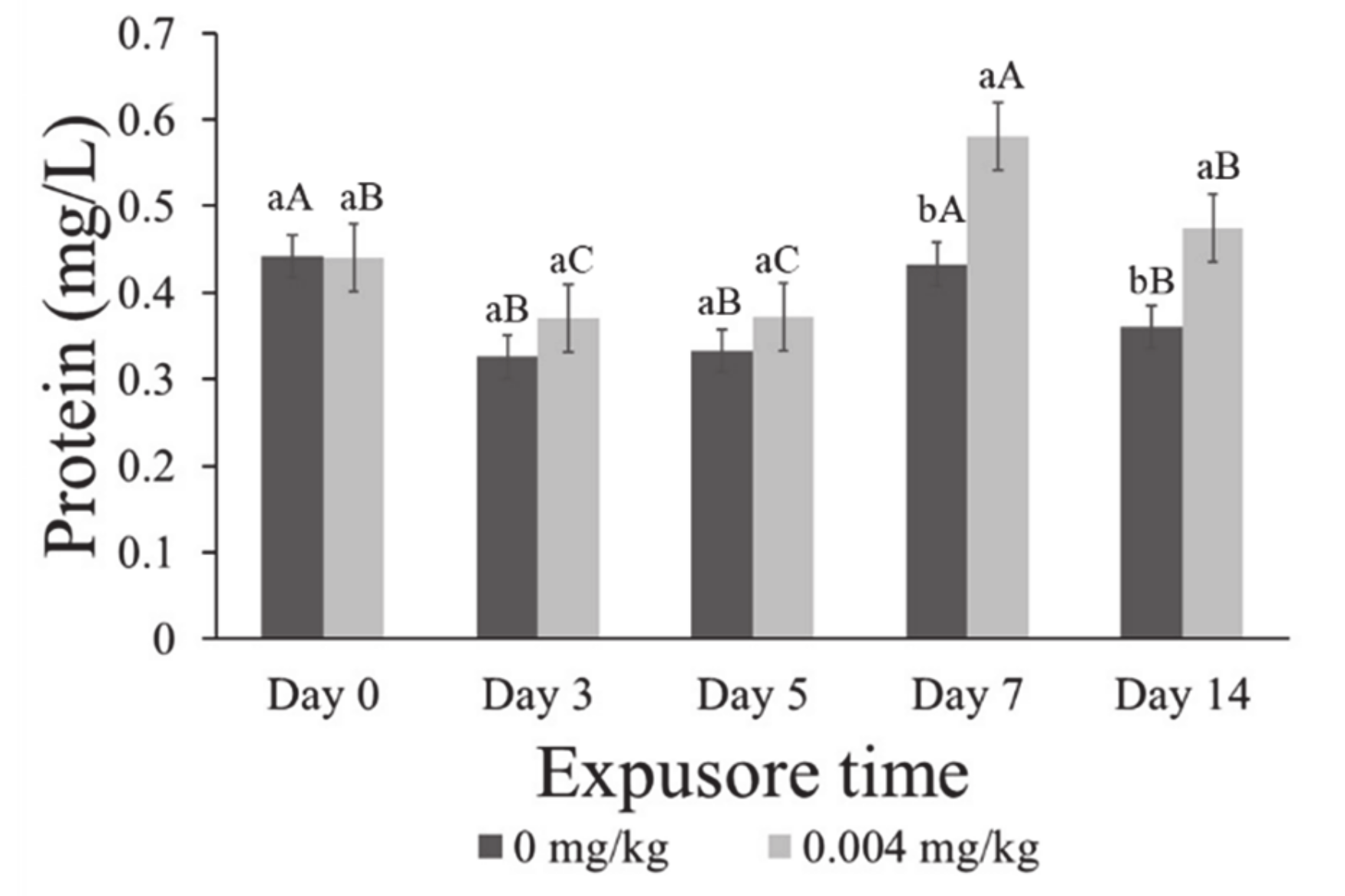
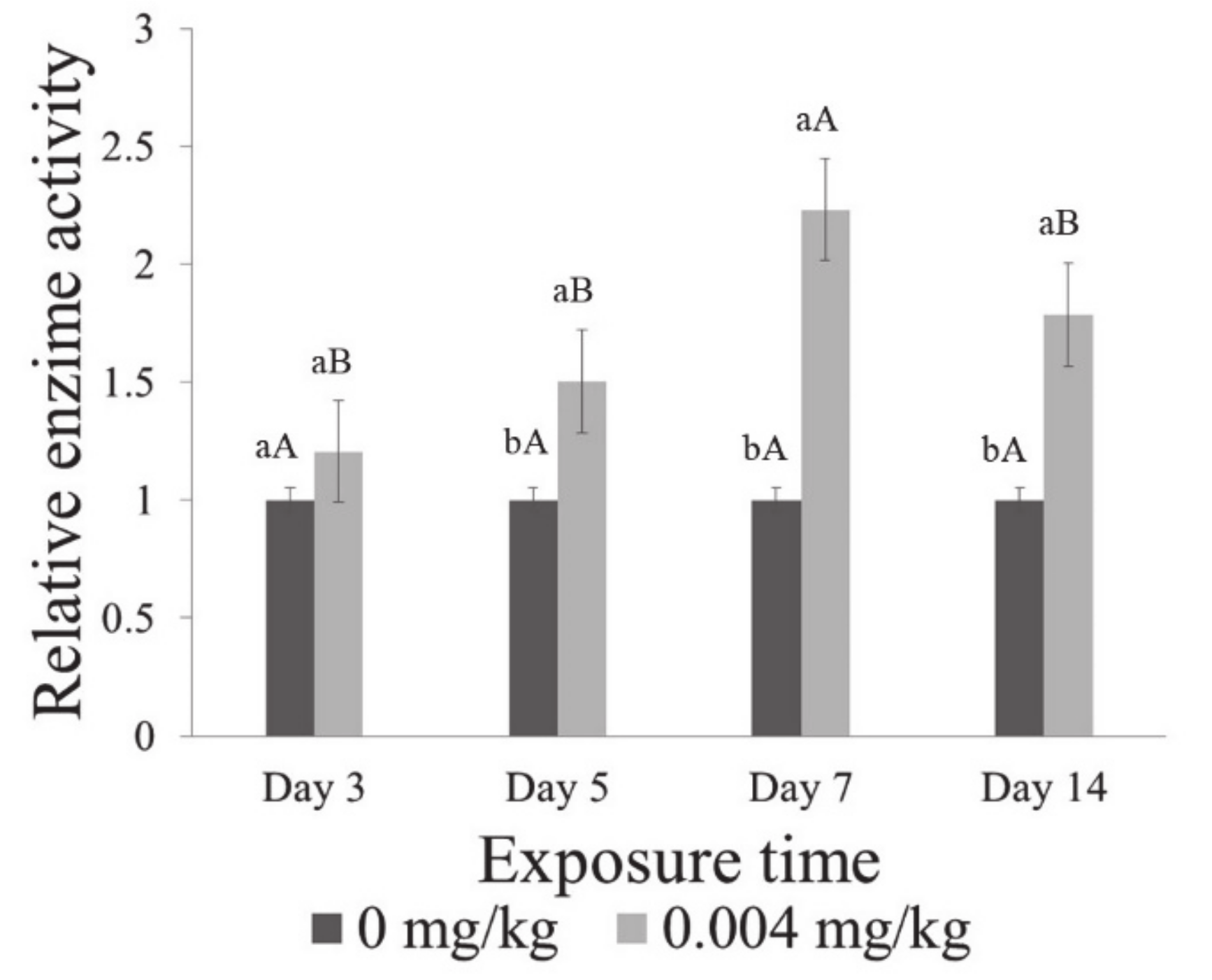
3.5. Enzymatic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Odukkathil, G.; Vasudevan, N. Residues of endosulfan in surface and subsurface agricultural soil and its bioremediation. J. Environ. Manag. 2016, 165, 72–80. [Google Scholar] [CrossRef]
- Bejarano, F.; Souza, J.; Weber, J.M.; Guadarrama, C.; Escamilla, E.; Beristain, B.; Ramirez, F. El Endosulfán y sus Alternativas en América Latina; Red de Acción en Plaguicidas y Alternativas de América Latina: Mexico City, Mexico, 2009. [Google Scholar]
- Stockholm Convention on Persistent Organic Pollutants Review Committee. Report of the Persistent Organic Pollutants Review Committee on the Work of Its Fifth Meeting; UNEP/POPS/POPRC.5/10/Add.2; UNEP: Geneva, Switzerland, 2009. [Google Scholar]
- Betancurt, L.A.; Ocampo, R.; Ríos, L.A. La problemática del endosulfán: Aspectos químicos, analíticos y ambientales. Luna Azul 2015, 40, 293–313. [Google Scholar]
- Stockholm Convention on Persistent Organic Pollutants Review Committee. Report of the Persistent Organic Pollutants Review Committee on the Work of Its First Meeting; UNEP/POPS/POPRC; UNEP: Geneva, Switzerland, 2010. [Google Scholar]
- Park, B.-S.; Yoo, J.-H.; Kim, J.-H.; Kim, J.-E.; Lee, S.-E. Biotransformation of endosulfan by the tiger worm, Eisenia fetida. J. Agric. Chem. Environ. 2012, 1, 20–27. [Google Scholar] [CrossRef][Green Version]
- Contreras-Ramos, S.M.; Alvarez-Bernal, D.; Dendooven, L. Characteristics of earthworms (Eisenia fetida) in PAHs contami-nated soil amended with sewage sludge or vermicompost. Appl. Soil Ecol. 2009, 41, 269–276. [Google Scholar] [CrossRef]
- Villalobos-Maldonado, J.J.; Meza-Gordillo, R.; Mancilla-Margalli, N.A.; Ayora-Talavera, T.R.; Rodríguez-Mendiola, M.A.; Arias-Castro, C.; Vázquez-Villegas, P.T.; Gutiérrez-Miceli, F.A.; Ruíz-Valdiviezo, V.M. Removal of Decachlorobiphenyl in Vermicomposting Process Amended with Rabbit Manure and Peat Moss. Water Air Soil Pollut. 2015, 226, 1–11. [Google Scholar] [CrossRef]
- Dendooven, L.; Alvarez-Bernal, D.; Contreras-Ramos, S.M. Earthworms, a means to accelerate removal of hydrocarbons (PAHs) from soil? A mini-review. Pedobiologia 2011, 54, S187–S192. [Google Scholar] [CrossRef]
- Villalobos-Moldonado, J.J.; Meza-Gordillo, R.; Enciso-Sáenz, S.; Castañon-González, J.H.; Rosales-Quintero, A.; Lagu-nas-Rivera, S.; Rincón-Rosales, R. Identificación molecular de bacterias en Eisenia fetida Savigny cultivadas, con potencial de remoción de contaminantes orgánicos persistentes. Agroproductividad 2017, 10, 51–56. [Google Scholar]
- NOM-021-RECNAT-2000. Establece las especificaciones de fertilidad, salinidad y clasificación de suelos. Estudios, muestreos y análisis. Diario Of. Fed. 2000, 14, 17. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Durán, L.; Henríquez, C. Caracterización química, física y microbiológica de vermicompostes producidos a partir de cinco sustratos orgánicos. Agron. Costarric. 2007, 31, 41–51. [Google Scholar]
- Organization for Economic Cooperation and Development OECD. Earthworm, acute toxicity test-207. In OECD Guideline for Testing of Chemicals; OECD Publishing: Paris, France, 1984. [Google Scholar] [CrossRef]
- Vazquez-Villegas, P.T.; Meza-Gordillo, R.; Gutiérrez-Miceli, F.A.; Ruiz-Valdiviezo, V.M.; Villalobos-Maldonado, J.J.; Montes-Molina, J.A.; Fernadez-Toledo, A.A.J. Determinación de CL50 y CE50 de endosulfán lactona y diazinón en lombriz de tierra (Eisenia foetida). Agroproductividad 2018, 11, 105–111. [Google Scholar]
- Peñaloza, G.S.A.; Rincon, D.J.P.; Restrepo, L.G.C. Evaluation of wet sterilization and microwave sterilization of two types of soil. Rev. Científica 2013, 1, 87–93. [Google Scholar]
- Li, W.; Dai, Y.; Xue, B.; Li, Y.; Peng, X.; Zhang, J.; Yan, Y. Biodegradation and detoxification of endosulfan in aqueous me-dium and soil by Achromobacter xylosoxidans strain CS5. J. Hazard. Mater. 2009, 167, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Villegas, P.T. Toxicological and Physiological Responses of Eisenia fetida Exposed to Endosulfan Lactone. Ph.D. Thesis, Tecnológico Nacional de México/IT Tuxtla Gutiérrez, Chiapas, Mexico, 2019. [Google Scholar]
- Vázquez-Villegas, P.T.; Meza-Gordillo, R.; Luján-Hidalgo, M.C.; Cruz-Salomón, A.; Ruíz-Valdiviezo, V.M.; Gutiérrez-Miceli, F.A.; Montes-Molina, J.A. Optimization and validation of an extraction method for endosulfan lactone on a solid substrate. Processes 2021, 9, 284. [Google Scholar] [CrossRef]
- Mosleh, Y.Y.; Couderchet, M.; Vernet, G. Acute and sublethal effects of two insecticides on earthworms (Lumbricus terrestris L.) under laboratory conditions. Environ. Toxicol. 2003, 18, 1–8. [Google Scholar] [CrossRef]
- Aguilar-Vázquez, J. Bioacumulación y Eliminación de Endosulfán Lactona en Eisenia fetida. Master’s Thesis, Tecnológico Nacio-nal de México/IT Tuxtla Gutiérrez, Chiapas, Mexico, 2019. [Google Scholar]
- Islam, M.A.; Sakkas, V.; Albanis, T.A. Application of statistical design of experiment with desirability function for the removal of organophosphorus pesticide from aqueous solution by low-cost material. J. Hazard. Mater. 2009, 170, 230–238. [Google Scholar] [CrossRef]
- Layne, E. Spectrophotometric and turbidimetric methods for measuring proteins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1957; Volume 3, pp. 447–454. [Google Scholar]
- Zhang, T.; Zhu, Y.; Gunaratna, C. Rapid and quantitative determination of metabolites from multiple cytochrome P450 probe substrates by gradient liquid chromatography-electrospray ionization-ion trap mass spectrometry. J. Chromatogr. B 2002, 780, 371–379. [Google Scholar] [CrossRef]
- Cao, X.; Song, Y.; Kai, J.; Yang, X.; Ji, P. Evaluation of EROD and CYP3A4 activities in earthworm Eisenia fetida as biomarkers for soil heavy metal contamination. J. Hazard. Mater. 2012, 243, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Li, W.; Xiao, N.W.; Liu, X.H.; Ge, F. Effect of herbicide acetochlor on cytochrome P450 monooxygenases and GST of earthworms Eisenia fetida. J. Environ. Sci. China 2006, 18, 135–140. [Google Scholar]
- Perdomo, C.; Barbazán, M.; Durán, J. Área de Suelos y Aguas. CÁTEDRA de Fertilidad; Universidad de la Republica: Motevideo, Uruguay, 2003. [Google Scholar]
- Castillo-Cerma, C.M. Selección y Calibración de Indicadores Locales y Técnicos para Evaluar la Degradación de los Suelos Lade-ras, enla Microcuenca Cuscamá el Tuma. Bachelor’s Thesis, Universidad Nacional Agraria, Managua, Nicaragua, 2005. [Google Scholar]
- Vanegas, A.F.C. Relación del Carbono y Nitrógeno del Suelo con Usos y Coberturas del Terreno en Alcalá, Valle del Cauca. Ph.D. Thesis, Universidad Tecnológica de Pereira, Pereira, Risaralda, 2008. [Google Scholar]
- EPA. Ecological Effects Test Guidelines; OPPTS 850.4225 Seedling Emergence, Tier II; National Service Center for Environmental Publications: Washington, DC, USA, 1996. [Google Scholar]
- Vijver, M.; Wolterbeek, H.; Vink, J.P.; Vangestel, C. Surface adsorption of metals onto the earthworm Lumbricus rubellus and the isopod Porcellio scaber is negligible compared to absorption in the body. Sci. Total Environ. 2005, 340, 271–280. [Google Scholar] [CrossRef]
- Coutiño-González, E.; Hernández-Carlos, B.; Gutiérrez-Ortiz, R.; Dendooven, L. The earthworm Eisenia fetida accelerates the removal of anthracene and 9, 10-anthraquinone, the most abundant degradation product, in soil. Int. Biodeterior. Biodegrad. 2010, 64, 525–529. [Google Scholar] [CrossRef]
- Ali, M.; Kazmi, A.; Ahmed, N. Study on effects of temperature, moisture and pH in degradation and degradation kinetics of aldrin, endosulfan, lindane pesticides during full-scale continuous rotary drum composting. Chemosphere 2014, 102, 68–75. [Google Scholar] [CrossRef]
- Verma, K.; Agrawal, N.; Farooq, M.; Misra, R.; Hans, R. Endosulfan degradation by a Rhodococcus strain isolated from earthworm gut. Ecotoxicol. Environ. Saf. 2006, 64, 377–381. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Y.; Zhu, L.; Wang, J.; Wang, J.; Du, Z.; Zhang, C. Influence of isolated bacterial strains on the in situ bio-degradation of endosulfan and the reduction of endosulfán-contaminated soil toxicity. Ecotoxicol. Environ. Saf. 2018, 160, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Viñas Canals, M. Biorremediación de Suelos Contaminados por Hidrocarburos: Caracterización Microbiológica, Química y Ecotoxicológica. Ph.D. Thesis, Universidad de Barcelona, Barcelona, Spain, 2005. [Google Scholar]
- Adhikary, S. Vermicompost, the story of organic gold: A review. Agric. Sci. 2012, 3, 905–917. [Google Scholar] [CrossRef]
- Kauschke, E.; Mohrig, W.; Cooper, E.L. Coelomic fluid proteins as basic components of innate immunity in earthworms. Eur. J. Soil Biol. 2007, 43, S110–S115. [Google Scholar] [CrossRef]
- Bilej, M.; Procházková, P.; Šilerová, M.; Josková, R. Earthworm Immunity. Adv. Exp. Med. Biol. 2010, 708, 66–79. [Google Scholar] [CrossRef]
- Qiu, X.; Li, W.; Tian, Y.; Leng, X. Cytochrome P450 monooxygenases in the cotton bollworm (Lepidoptera: Noctuidae): Tissue difference and induction. J. Econ. Entomol. 2003, 96, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Wu, H.; Wei, L.; Zhao, J.; Lu, H.; Yu, J. Proteomic and metabolomic analysis of earthworm Eisenia fetida exposed to different concentrations of 2,2′,4,4′-tetrabromodiphenyl ether. J. Proteom. 2013, 91, 405–416. [Google Scholar] [CrossRef]
- Wang, X.; Chang, L.; Sun, Z.; Zhang, Y. Comparative proteomic analysis of differentially expressed proteins in the earth-worm Eisenia fetida during Escherichia coli O157: H7 stress. J. Proteome Res. 2010, 9, 6547–6560. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, Y.; Thunders, M.; Cavanagh, J.; Matthew, C.; Wang, X.; Zhou, X.; Qiu, J. Differential protein expression and localization of CYP450 enzymes in three species of earthworm; is this a reflection of environmental adaptation? Chemosphere 2017, 171, 485–490. [Google Scholar] [CrossRef]
- Achazi, R.K.; Flenner, C.; Livingstone, D.R.; Peters, L.D.; Schaub, K.; Scheiwe, E. Cytochrome P450 and dependent activities in unexposed and PAH-exposed terrestrial annelids. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 121, 339–350. [Google Scholar] [CrossRef]
- Cao, X.; Bi, R.; Song, Y. Toxic responses of cytochrome P450 sub-enzyme activities to heavy metals exposure in soil and correlation with their bioaccumulation in Eisenia fetida. Ecotoxicol. Environ. Saf. 2017, 144, 158–165. [Google Scholar] [CrossRef] [PubMed]





| Endosulfan Lactone (mg/kg) | Earthworm (Number) |
|---|---|
| 0.001 | 0 |
| 0.004 | 5 |
| 0.009 | 10 |
| Parameter | Solid Substrate | Vermicompost | Reference Values | References |
|---|---|---|---|---|
| Moisture (%) | 36 ± 5.6 | 92 ± 2.6 | - | [11] |
| pH | 6.9 ± 0.08 | 7.3 ± 0.02 | Strongly acidic (<5.0) Moderately acidic (5.1–6.5) Neutral (6.6–7.3) Moderately alkaline (7.4–8.5) Strongly alkaline (>8.5) | |
| NInorganic (mg/Kg) | 48 ± 1.9 | 56 ± 5.8 | Very low (0–10 mg/Kg) Low (10–20 mg/Kg) Medium (20–40 mg/Kg) High (40–60 mg/Kg) Very high (>60 mg/Kg) | |
| NTotal (%) | 0.25 ± 0.002 | 0.30 ± 0.008 | Very low (<0.05%) Low (0.05–0.10%) Medium (0.10–0.15%) High (0.15–0.25%) Very high (>0.25%) | |
| Real density (g/cm3) | 1.05 ± 0.006 | 1.19 ± 0.006 | Very low (<2.4) Low (2.4–2.60) Medium (2.60–2.80) High (>2.80) | [29] |
| Organic matter (%) a | 7.3 ± 0.34 | 10.9 ± 0.26 | Very low (<0.5%) Low (0.6–1.5%) Medium (1.6–3.5%) High (3.6–6.0%) Very high (>6.0%) | [11] |
| TOC (%) | 7.48 ± 0.26 | 14.15 ± 0.69 | - | |
| C/N | 29.92 | 47.17 | C/N low (<10) C/N high (>25) | [13,30] |
| Sample (Days) | Vmax (μmol/min) | Km (μmol) | Enzymatic Activity (mmol/mg Protein Per min) |
|---|---|---|---|
| 0 | 0.0155 e | 0.7274 e | 0.2087 e |
| 3 | 0.0187 d | 3.0458 a | 0.2518 d |
| 5 | 0.0233 c | 2.7540 b | 0.3138 c |
| 7 | 0.0346 b | 2.5489 c | 0.4659 a |
| 14 | 0.0502 a | 2.0157 d | 0.3730 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Villegas, P.T.; Meza-Gordillo, R.; Cruz-Salomón, A.; Ruíz-Valdiviezo, V.M.; Gutiérrez-Miceli, F.A.; Villalobos-Maldonado, J.J.; Montes-Molina, J.A.; Aguilar-Vázquez, J.; Domínguez, Z. Vermicomposting Process to Endosulfan Lactone Removal in Solid Substrate Using Eisenia fetida. Processes 2021, 9, 396. https://doi.org/10.3390/pr9020396
Vázquez-Villegas PT, Meza-Gordillo R, Cruz-Salomón A, Ruíz-Valdiviezo VM, Gutiérrez-Miceli FA, Villalobos-Maldonado JJ, Montes-Molina JA, Aguilar-Vázquez J, Domínguez Z. Vermicomposting Process to Endosulfan Lactone Removal in Solid Substrate Using Eisenia fetida. Processes. 2021; 9(2):396. https://doi.org/10.3390/pr9020396
Chicago/Turabian StyleVázquez-Villegas, Paola T., Rocío Meza-Gordillo, Abumalé Cruz-Salomón, Víctor M. Ruíz-Valdiviezo, Federico A. Gutiérrez-Miceli, Juan J. Villalobos-Maldonado, Joaquín A. Montes-Molina, Janet Aguilar-Vázquez, and Zaira Domínguez. 2021. "Vermicomposting Process to Endosulfan Lactone Removal in Solid Substrate Using Eisenia fetida" Processes 9, no. 2: 396. https://doi.org/10.3390/pr9020396
APA StyleVázquez-Villegas, P. T., Meza-Gordillo, R., Cruz-Salomón, A., Ruíz-Valdiviezo, V. M., Gutiérrez-Miceli, F. A., Villalobos-Maldonado, J. J., Montes-Molina, J. A., Aguilar-Vázquez, J., & Domínguez, Z. (2021). Vermicomposting Process to Endosulfan Lactone Removal in Solid Substrate Using Eisenia fetida. Processes, 9(2), 396. https://doi.org/10.3390/pr9020396









