Abstract
The solubility in triple water-salt systems containing NdCl3, PrCl3, YCl3, TbCl3 chlorides, and water-soluble fullerenol C60(OH)24 at 25 °C was studied by isothermal saturation in ampoules. The analysis for the content of rare earth elements was carried out by atomic absorption spectroscopy, for the content of fullerenol—by electronic spectrophotometry. The solubility diagrams in all four ternary systems are simple eutonic, both consisting of two branches, corresponding to the crystallization of fullerenol crystal-hydrate and rare earth chloride crystal-hydrates, and containing one nonvariant point corresponding to the saturation of both solid phases. On the long branches of C60(OH)24*18H2O crystallization, a C60(OH)24 decreases by more than 2 orders of magnitude compared to the solubility of fullerenol in pure water (salting-out effect). On very short branches of crystallization of NdCl3*6H2O, PrCl3*7H2O, YCl3*6H2O, and TbCl3*6H2O, the salting-in effect is clearly observed, and the solubility of all four chlorides increases markedly. The four diagrams cannot be correctly approximated by the simple one-term Sechenov equation (SE-1), and very accurately approximated by the three-term modified Sechenov equation (SEM-3). Both equations for the calculation of nonelectrolyte solubility in electrolyte solutions (SE-1 and SEM-3 models) are obtained, using Pitzer model of virial decomposition of excess Gibbs energy of electrolyte solution. It is shown that semi-empirical equations of SE-1 and SEM-3 models may be extended to the systems with crystallization of crystal-solvates.
1. Introduction
This work continues the cycle of studies, devoted to the study of solubility diagrams in systems, containing simultaneously water-soluble nanoclusters—light fullerenols-C60(OH)n or C70(OH)m, tris-malonates C60 and C70, (bis-, tris- and octo-) adducts of light fullerenes and rare earth salts (Sm3+, La3+, Gd3+, Y3+), actinoids (UO22+, and some transition and s-metals (Cu2+, Na+). Here are references to articles that studied the solubility of fullerenol C60(OH)24 or mixed fullerenols, based on it (for example, fullerenol-d) [,,,,,,,].
Solubility diagrams in systems with rare earth elements were quite similar: They did not form new compounds, the diagrams consisted of two branches: As a rule, long branches of crystallization of fullerenol crystal-hydrates and short branches of crystallization of rare earth salt crystal-hydrates. At the same time, on the branches of crystallization of fullerenol crystal-hydrates, as a rule, the effect of a strong decrease in the solubility of the latter was observed as the concentrations of rare earth salts increased, i.e., a strong salting-out of fullerenol from saturated solutions.
In addition to the purely scientific interest in the study of phase diagrams in systems with nanoclusters, there is also a practical interest. We point out two possible areas of application of the obtained data:
1. Rare earth salts can serve as a very effective salting-out agent for fullerenols from aqueous solutions. Taking into account the very low concentration of fullerenols in saturated solutions near nonvariant eutonic points (sometimes tenths and even hundredths of a mass % of fullerenol) such salting-out of fullerenol takes place almost without loss of the latter. Recovery of rare earths in this case is also trivial and is reduced to calcining eutonic sediments in air to burn fullerenol residues. Currently, the salting of fullerenols (and other water-soluble derivatives) is carried out, using methanol or ethanol or methyl acetate (which are much less effective). With repeated recrystallization of the sediment from aqueous solutions, a significant part of a very expensive product—fullerenol—is lost, and the salting agent itself must be regenerated.
2. On the other hand, fullerenol can be considered as a reagent for the separation of rare earth elements by the method of multi-stage recrystallization. Perhaps they will be effective in separating such pairs of rare earths as, for example, Nd-Pr. The classical separation of this pair of lanthanides by liquid extraction cascades is labor-intensive, multi-stage, and relatively inefficient.
Questions of theoretical or model consideration of solubility of nonelectrolytes in solutions of electrolytes in, as a rule, polar solvents, have been considered in the scientific literature for a long time—since the end of the 19th—beginning of the 20th centuriy (see, for example, the classical works of Sechenov [,,]). First, the solubility of atmospheric gases (O2, N2, CO2, Ar, etc.) in sea and ocean water (aqueous solutions of the mutual system Na+, K+, Ca2+, Mg2+//Cl−, SO42−, HCO3−—H2O) was considered, later the solubility of solid nonelectrolytes and weak electrolytes (for example, phenol—C6H5OH, urea—CO(NH2)2, glucose—C6H12O6, ascorbic acid, sugars, fats, proteins). At the same time, solubility in non-aqueous solvents, such as methanol-CH3OH, ethanol-C2H5OH, acetonitrile-CH3CN, etc., were also considered in water in the presence of salts acids and bases—strong electrolytes. Recently, the range of water-soluble nonelectrolytes and weak electrolytes has expanded to systems with water-soluble nanoclusters, such as fullerenols, adducts of fullerenes with amino acids, carboxylic acids, proteins, etc. Studying the compatibility (solubility) of the latter with physiological fluids, such as: Saline solutions, blood, lymph, liquor, gastric juice, etc., which from a physicochemical point of view, are solutions of strong electrolytes, is an important task in the food industry, pharmacology, physiology, and medicine.
A simple semi-empirical equation describing the solubility of nonelectrolytes and weak electrolytes the concentration of electrolytes in polar solvents (linear dependence of the logarithm of solubility of non-electrolyte on the concentration of the electrolyte) was proposed by Sechenov, and is still sometimes used in describing simple systems of this type. In this paper, we present a model justification of the Sechenov equation and obtain a three-term modification of the latter, which will allow us to quantitatively describe solubility in such systems.
2. Experimental Part and Discussion of the Results
The solubility in ternary water-salt systems containing the chlorides NdCl3, PrCl3, YCl3, TbCl3, and water-soluble fullerenol C60(OH)24 at 25 °C was studied by isothermal saturation in ampoules: PrCl3—C60(OH)24-H2O, NdCl3-C60(OH)24-H2O, YCl3-C60(OH)24-H2O, and TbCl3-C60(OH)24-H2O. Saturation was carried out for 6 h. Under the conditions of a shaker thermostat (temperature control accuracy ΔT ≤ 0.05 K, pressure—745 mm Hg, shaking frequency v = 1.5 Hz). After that, the saturated solutions were settled for 30 min, after which samples were taken from the ampoules for analysis.
The analysis for the content of rare earth elements was carried out by atomic absorption spectroscopy (Perkin Elmer PinAAcle 500 atomic absorption spectrometer), the relative error in the determination of rare earth elements is δREM3+ ≈ 1 ÷ 3 rel. mass % at high concentrations of rare earth metals CREM3+ ≈ 10 ÷ 700 g/dm3 and δREM3+ ≈ 3 ÷ 5 rel. mass % at low concentrations of rare earth metals CREM3+ ≈ 1 ÷ 10 g/dm3 (here and after: REM = Pr, Nd, Y, Tb).
The analysis on the content of fullerenol were carried out by electron spectrophotometry, according to the Bouguer-Lambert-Ber law (spectro-photometer UV 1280) by the optical density at the wavelength λ = 400 nm − D400 (see Figure 1 and Figure 2):
C(g/dm3) = 0.937*D400 (width of the cuvette l = 1 cm)
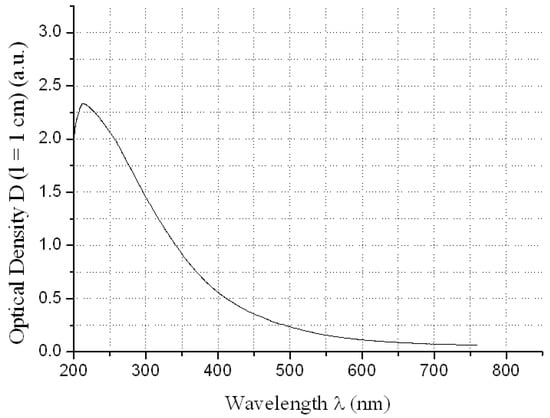
Figure 1.
Electronic spectrum of water solution of C60(OH)24 at concentration CC60(OH)24 ≈ 0.625 g/dm3 relatively distilled H2O.
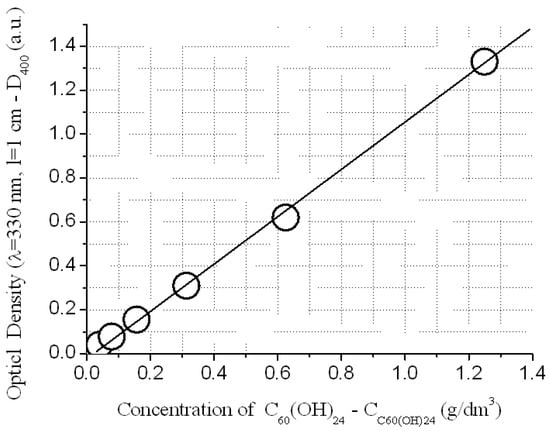
Figure 2.
Implementation of the Bouguer-Lambert-Ber law of light absorption of the water solution of C60(OH)24 at λ = 400 nm.
The absorption of REMCl3 in the near ultraviolet region at λ = 400 nm can be neglected (see Figure 3, Figure 4, Figure 5 and Figure 6)—REM solutions are practically absolutely transparent in the classical violet region of the visible part of the spectrum. The relative error in the determination of fullerenol was δC60(OH)24 ≈ 3–5 rel. mass % at high C60(OH)24 concentrations: CC60(OH)24 ≈ 0.5 ÷ 5.6 g/dm3 and δC60(OH)24 ≈ 5–10 rel. mass % at low C60(OH)24 concentrations: CC60(OH)24 ≈ 0.1 ÷ 0.5 g/dm3.
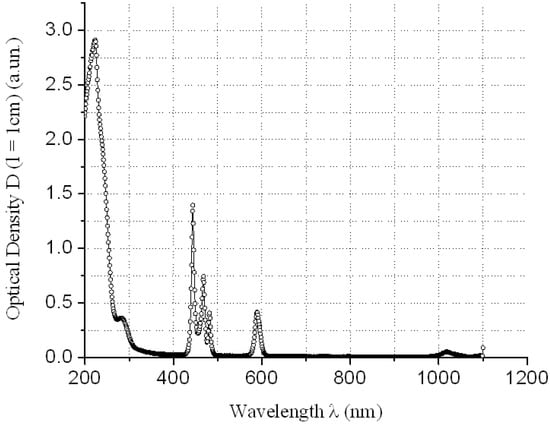
Figure 3.
Electronic spectrum of water solution of PrCl3 at concentration CPrCl3 ≈ 50.7 g/dm3 relatively distilled H2O.
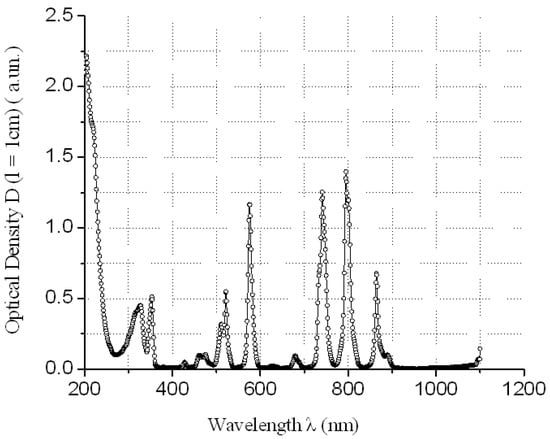
Figure 4.
Electronic spectrum of water solution of NdCl3 at concentration CNdCl3 ≈ 51.6 g/dm3 relatively distilled H2O.
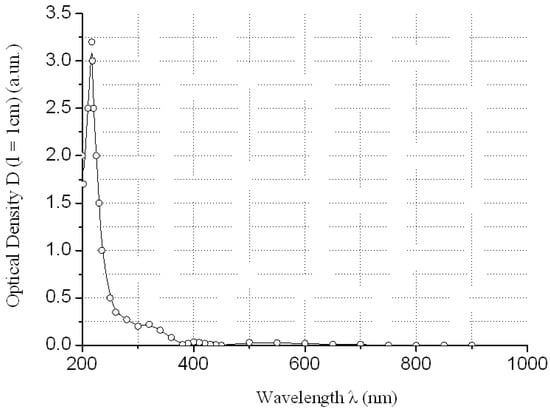
Figure 5.
Electronic spectrum of water solution of TbCl3 at concentration CTbCl3 ≈ 21.3 g/dm3 relatively distilled H2O.
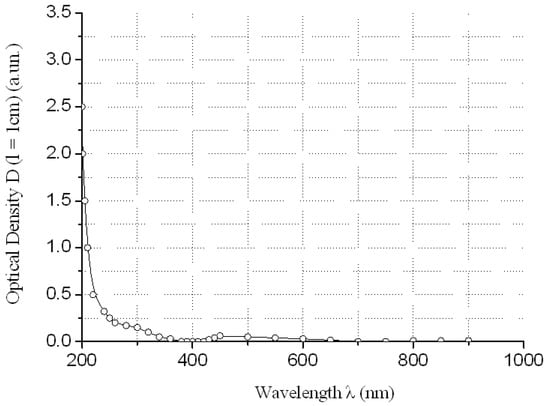
Figure 6.
Electronic spectrum of water solution of YCl3 at concentration CYCl3 ≈ 24.9 g/dm3 relatively distilled H2O.
To determine the molality concentrations of components, it was also necessary to be able to translate volume concentrations of C (g/dm3) into weight concentrations of C (mass. %) or molality m (mole/kg H2O). To do this, it was necessary to determine the densities of saturated ternary solutions. The determination was carried out using quartz pycnometers with a working volume of about V 5 cm3, the standard liquid was distilled water. The error in determining the density was .% with temperature control accuracy T ~ 0.05 K.
Data on solubility in triple systems PrCl3-C60(OH)24-H2O, NdCl3-C60(OH)24-H2O, YCl3-C60(OH)24-H2O, and TbCl3-C60(OH)24-H2O at 25 °C are presented in Figure 7, Figure 8, Figure 9 and Figure 10 and in Table 1 in molalities of rare earth salts and fullerenol.
where: mi, Ci, Mi is the molality, concentration in mass. %, and the molecular weight of the i-th component of the solution.
mc60(OH)24 = C60(OH)24/CH2O*(1000/MC60(OH)24); mREM = CREMCl3/CH2O*(1000/MREMCl3)
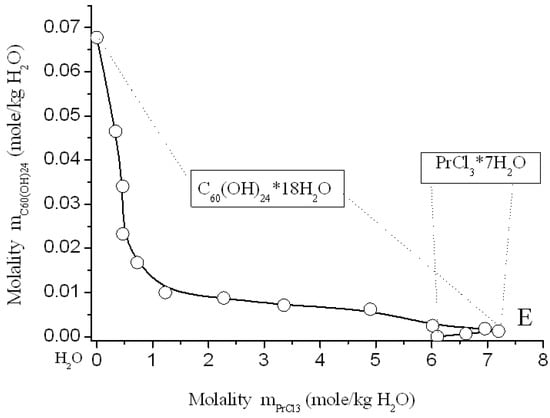
Figure 7.
Solubility diagram in ternary system C60(OH)24—PrCl3—H2O at 25 °C.
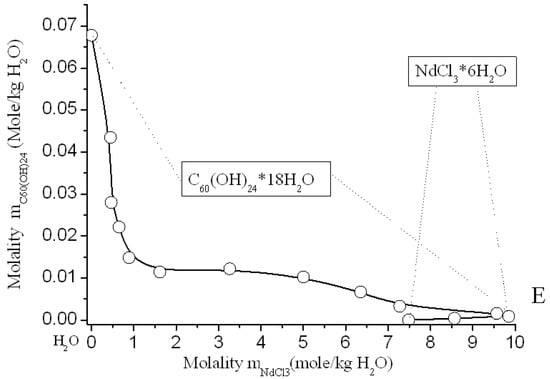
Figure 8.
Solubility diagram in ternary system C60(OH)24—NdCl3—H2O at 25 °C.
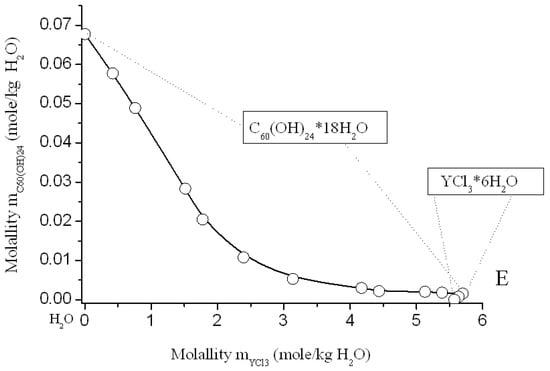
Figure 9.
Solubility diagram in ternary system C60(OH)24—YCl3—H2O at 25 °C.
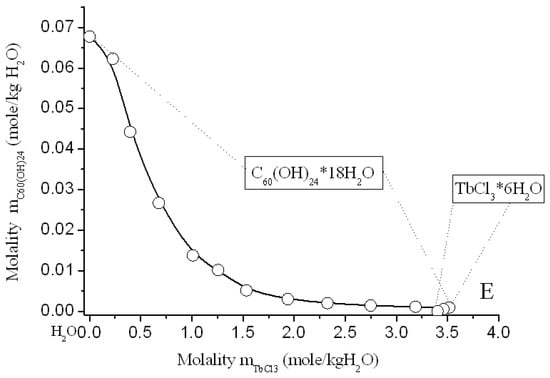
Figure 10.
Solubility diagram in ternary system C60(OH)24—TbCl3—H2O at 25 °C.

Table 1.
Solubility in ternary systems PrCl3-C60(OH)24-H2O, NdCl3-C60(OH)24-H2O, YCl3-C60(OH)24-H2O, TbCl3-C60(OH)24-H2O at 25 °C and pressure 745 mm Hg. (Purity C60(OH)24—98.5 mass. %—HPLC, REMCl3 (REM = Pr, Nd, Tb, Y) = 99 mass.%).
Solubility diagrams in four ternary systems are simple eutonic, both consist of two branches, corresponding to the crystallization of fullerenol crystal-hydrate and rare-earth chloride crystal-hydrates, and contain one eutonic nonvariant point corresponding to saturation with both solid phases [,,]. On the long branches of C60(OH)24*18H2O crystallization, a pronounced salting-out effect is observed—the solubility of C60(OH)24*18H2O decreases by almost 2 orders of magnitude compared to the solubility of fullerenol in pure water. On very short branches of crystallization of NdCl3*6H2O, PrCl3*7H2O, YCl3*6H2O, and TbCl3*6H2O, the effect of salting-in is clearly observed, the solubility of crystal-hydrates of four chlorides increases markedly. The long crystallization curves themselves C60(OH)24*18H2O in both cases have a rare concave-convex σ-id character with an inflection point:
dmC60(OH)24/dmREMCl3 = 0 and d2mC60(OH)24/dmREMCl32 = 0
3. Modeling of C60(OH)24*18H2O Crystallization Branches in Ternary Systems REMCl3-C60(OH)24—H2O at 25 °C
3.1. Sechenov Equation of the Solubility of Nonelectrolyte in Electrolyte Solutions
For the primary modeling of the branches of crystallization of C60(OH)24*18H2O in ternary systems PrCl3—C60(OH)24—H2O, NdCl3—C60(OH)24—H2O, YCl3—C60(OH)24—H2O, TbCl3—C60(OH)24—H2O at 25 °C, we used the equation of Sechenov (one parameter Sechenov Equation-SE-1):
where: C0C60(OH)24 and CC60(OH)24 solubility of C60(OH)24 in a binary system C60(OH)24—water and solubility of C60(OH)24 in a ternary system—an aqueous solution of REMCl3 (REM = Pr, Nd, Y, Tb), CREMCl3 is the concentration of REMCl3 in a saturated solution, and AS is the constant of the Sechenov equation. The scale of concentrations—C is generally uncertain (it can be CV—g/dm3, molarity M-mole/dm3, molality m-mole/kg of solvent, and mole fraction X—a.u.). As is known, this equation often quite adequately describes the solubility of a nonelectrolyte or a weak electrolyte (usually gaseous) in solutions of a strong electrolyte in polar solvents (H2O, CH3OH, CH3CN…) [,,]. The results of the approximation are shown below in Figure 11, Figure 12, Figure 13 and Figure 14 and in Table 2. The result of the approximation is generally not satisfactory enough, because, first, it is not accurate enough (see Figure the value of the standard deviations of the calculated values ln(m0C60(OH)24/mC60(OH)24) from the experimentally obtained ones), and, secondly, the calculated curves do not at all convey the σ-id character of the experimental curves with inflection points (see Figure 11, Figure 12, Figure 13 and Figure 14). The reasons for this discrepancy are as follows:
ln(C0C60(OH)24/CC60(OH)24) = ASCREMCl3
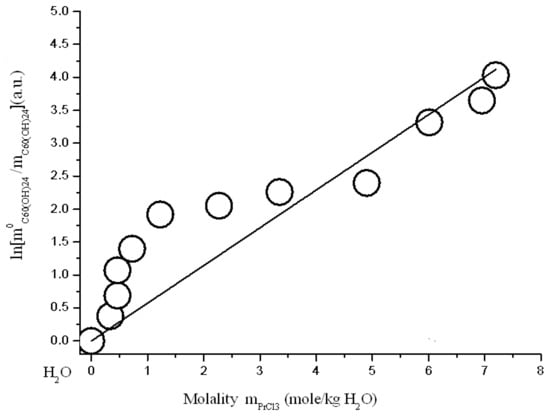
Figure 11.
Branch of crystallization C60(OH)24*18H2O in the diagram of solubility in ternary system C60(OH)24—PrCl3—H2O at 25 °C (strict line -s calculation according to Sechenov equation SE-1 (4)).
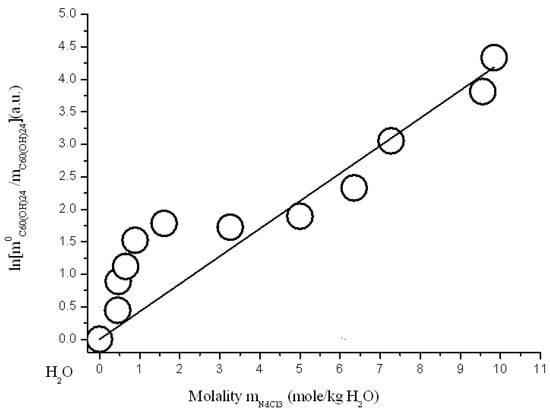
Figure 12.
Branch of crystallization C60(OH)24*18H2O in the diagram of solubility in ternary system C60(OH)24—NdCl3—H2O at 25 °C (strict line -s calculation according to Sechenov equation SE-1 (4)).
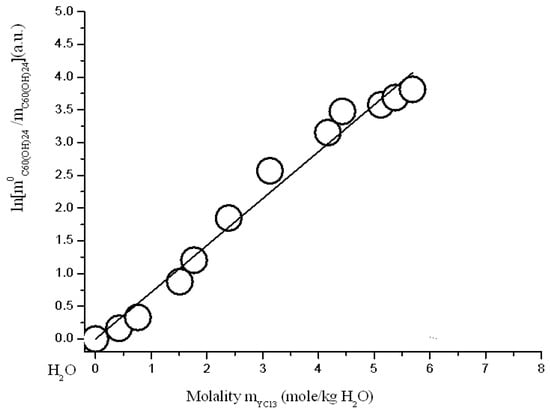
Figure 13.
Branch of crystallization C60(OH)24*18H2O in the diagram of solubility in ternary system C60(OH)24—YCl3—H2O at 25 °C (strict line -s calculation according to Sechenov equation SE-1 (4)).
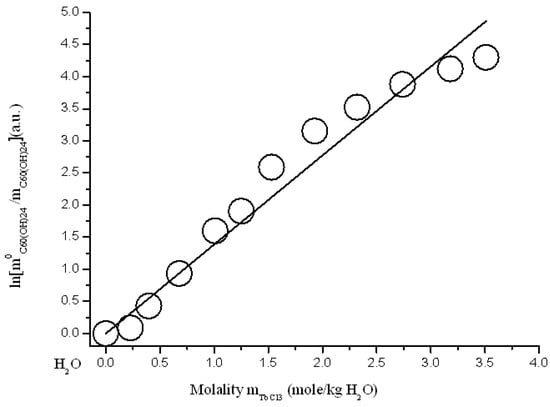
Figure 14.
Branch of crystallization C60(OH)24*18H2O in the diagram of solubility in ternary system C60(OH)24—TbCl3—H2O at 25 °C (strict line -s calculation according to Sechenov equation SE-1 (4)).

Table 2.
Model parameters for the description of C60(OH)24*18H2O crystallization branches in triple systems PrCl3—C60(OH)24—H2O, NdCl3—C60(OH)24—H2O, YCl3—C60(OH)24—H2O, TbCl3—C60(OH)24—H2O at 25 °C. The approximation quality parameters (χ2/DoF and R2) are also presented there. The Pearson criterion of approximation quality χ2/DoF is given by the relation: χ2/DoF = (1/v)∑[F(obs)i–F(appr)i]2/F(obs)i, where: Summation is carried out for all experimental points from i = 1 to i =N—the number of experimental points (in our case, 14, Table).
The empirical Sechenov equation is written for the solubility branch of the unsolvated solid phase, and in our case the crystallization branch of the crystal-hydrate is described, i.e., the thermodynamic potential (solubility product-SP) of C60(OH)24*18H2O, which retains its value on the crystallization branch of this compound, has the form:
where: aH2O is the activity of H2O in saturated solution, γC60(OH)24 is the activity coefficient C60(OH)24 in the molality scale with asymmetric normalization of redundant functions.
lnSP(C60(OH)24*18H2O) = ln (mC60(OH)24) + ln(γC60(OH)24) + 18lnaH2O
The C60(OH)24 nanoclusters themselves form a complex hierarchical sequence of associates: First-order associates are formed from monomers, then second-order associates are formed from first-order associates, and so on. The aqueous solutions of C60(OH)24 themselves are characterized by huge positive deviations from ideality, which often lead to the loss of diffusion stability by the solution [,].
It is unlikely that in saturated aqueous solutions PrCl3—C60(OH)24—H2O, NdCl3—C60(OH)24—H2O, YCl3—C60(OH)24—H2O, TbCl3—C60(OH)24—H2O electrolytes PrCl3, NdCl3, YCl3, and TbCl3 can be considered as very strong, it is likely the formation of ion pairs and larger ion associates in solutions.
3.2. Model Description of One-Parameter Sechenov Solubility Equation (SE-1)
Due to the fact that the Sechenov equation is historically considered to be empirical, we will give a derivation of this equation. Naturally, such a conclusion will be based on a semi-empirical model, in our case, the Pitzer model of strong electrolytes [,], and it is naturally impossible to call it strictly thermodynamic. The same conclusion is easily obtained using other semi-empirical models, such as the Bromley model [] or the regular solutions model []. So, consider the branch of crystallization of unsolvated nonelectrolyte (NE) in a solution containing a polar solvent and a strong electrolyte (E = MνcXνa), νc and νa—number of cations and anions in molecule E:
where: mNE, γNE—molality and activity coefficient of NE in a ternary solution of NE-E-H2O, m0NE, γ0NE—also in a binary system of NE-H2O, SP(NE)—thermodynamic solubility product of NE. So:
lnSP(NE) = ln(mNE)+ ln(γNE) = ln(m0NE)+ ln(γ0NE) = const
ln(m0NE/mNE) = const + ln(γNE)
According to the physical sense, const = 0, because if: mNE → m0NE, ln(γNE) → 0 due to the conditions of asymmetric normalization for both E and NE.
ln(m0NE/mNE) = ln(γNE)
Take this to describe the concentration dependence of the ln(γNE) simplified model of Pitzer [] taking into account only the second virial coefficients (λij) in the expansion of the excess Gibbs energy of solution (Gex) on the numbers of moles of the cation and anion of the electrolyte-E (nc, na) and number of moles of NE (nNE). We shall not take into account NE-NE interactions, as well as NE concentration in solution, are usually much smaller than the electrolyte, in our case, by 2 orders of magnitude. The physical sense of the second virial coefficients (λij) is the following: It is reduced to RT energy of specific non-electrostatic interactions of 1 mole of i particles and 1 mole of j particles.
Then:
where: nS is the moles number of the solvent in 1 kg, and I is the molar ionic strength of the solution:
Gex/RT = nSf(I) + 1/nS [(λc-cnc2) + (λa-ana2) + (λc-ancna) + (λc-NEncnNE) + (λa-NEnanNE)]
I = ½ [(nc/nS)zc2 + na/nS)za2]
Function:
characterizes the energy of non-specific electrostatic interactions, according to the Debye-Hückel theory, Aφ is the Debye-Hückel constant, which depends on the temperature and permittivity of the solvent, and b = 1.2 [] s, the non-variable Pitzer parameter. We assume, additionally, as in the Pitzer model, that λij(I) (if i,j are cation and anion) can exclusively be functions of I and do not depend on nNE. In addition, assume that λc-NE and λa-NE do not depend on I, because one of interacting particles (NE) has no charge. Then, given the fact that:
where:
f(I) = (−4AφIb)ln(1 + bI1/2)
So, the Sechenov equations in model SE-1 (8) and (4) are proved.
3.3. Model Description of Modified Three-Parameter Sechenov Solubility Equation (SEM-3)
To increase the accuracy of the approximation, we used a modified Sechenov solubility equation (SEM-3—three parameter Sechenov equation model):
where: As, Bs, Ds are the empirical (fitting) constants of the model SEM-3. As authors know, such an approximation is original and at the same time forced due to the nontrivial convex-concave course of the solubility branches of the nonelectrolyte in the studied systems. The appearance of a quadratic and cubic term in equation (15) is quite justified, since, for example, the expression for the activity coefficient of electrolyte, in the most popular now semi-empirical model of K. Pitzer [,], includes variable parameters with weights m2 and m3-these are the parameters and, respectively in binary systems and in ternary systems.
ln(m0C60(OH)24/mC60(OH)24) = AsmREMCl3 + BsmREMCl32 + CsmREMCl33
Let us consider the ternary system E(electrolyte)-NE(non-electrolyte)-S(solvent) and take into account virial coefficients of high order:
here: and are the third and fourth virial coefficients pf the decomposition of function on the molar numbers of ions and NE. Pitzer [] proposed to consider independent on I, some of them (corresponds to the three ions with the same charge) are equal to zero . Let us assume this, and additionally, that are also independent on I, and some of them are also equal to zero: .
So, one can easily find that:
And after simplifying:
So, the modified three-parameter Sechenov Equation (15) is proven and:
The simulation results are shown in Figure 15, Figure 16, Figure 17 and Figure 18 and in Table 2. As can be seen from the figures and the table: Firstly, the accuracy of the approximation has increased dramatically (the Parkinson’s approximation accuracy criterion χ2/DoF is 3–8 times higher) and, secondly, the model begins to perfectly describe the nontrivial concave-convex σ-id course of the crystallization curve. The last three-parameter SEM-3 allows us to expand the scope of the model for describing the branches of solubility of non-electrolytes in electrolyte solutions to high concentrations of the latter (to a molalities range from 4 to 7 mol per kg of solvent).
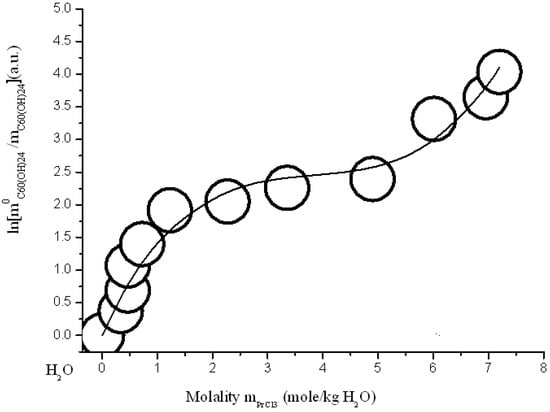
Figure 15.
Branch of crystallization C60(OH)24*18H2O in the diagram of solubility in ternary system C60(OH)24—PrCl3—H2O at 25 °C (convex-conxave line—calculation according to modified three-parameter Sechenov equation SEM-3 (15)).
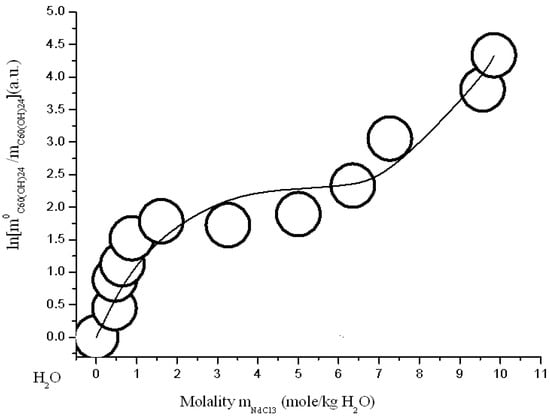
Figure 16.
Branch of crystallization C60(OH)24*18H2O in the diagram of solubility in ternary system C60(OH)24—NdCl3—H2O at 25 °C (convex-conxave line—calculation according to modified three-parameter Sechenov equation SEM-3 (15)).
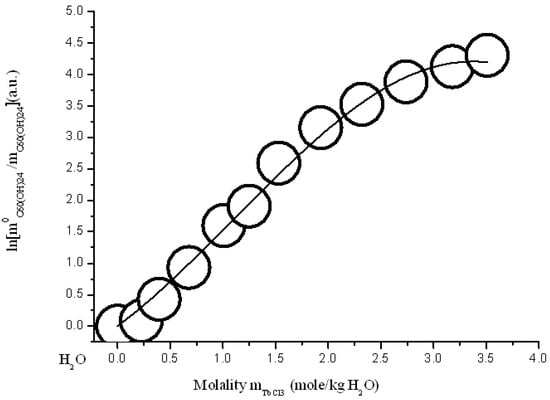
Figure 17.
Branch of crystallization C60(OH)24*18H2O in the diagram of solubility in ternary system C60(OH)24—TbCl3—H2O at 25 °C (convex-conxave line—calculation according to modified three-parameter Sechenov equation SEM-3 (15)).
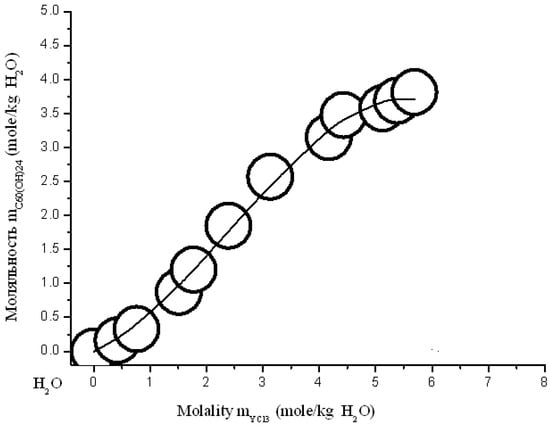
Figure 18.
Branch of crystallization C60(OH)24*18H2O in the diagram of solubility in ternary system C60(OH)24—YCl3—H2O at 25 °C (convex-conxave line—calculation according to modified three-parameter Sechenov equation—SEM-3 (15)).
Curiously, no two-parameter model is capable of such a description (see Table 2).
3.4. Model Description of Modified Three-Parameter Sechenov Solubility Equation for the Crystallization of Crystal Solvates of Non-Electrolyte (SEM(CS)-3)
Earlier, we noted that all solubility equations should be used for the calculation of the solubility of pure non-solvated non-electrolyte-NE without solvent molecules in the solid phases. This applies to both models—SE-1 and SEM-3. Let us again consider the ternary system nonelectrolyte (NE)–electrolyte (E)–solvent (S), and describe the branch of crystallization of crystal solvate (NE*NSS, NS—number of solvent molecules in solid crystal solvate), in our case it is crystal hydrate C60(OH)24*18H2O. For direct use of the equation of the SEM-3 model (Equation (15)), one should perform one simple mathematical operation—go from a natural set of composition variables (molalities of electrolyte and non-electrolyte—mE, mNE) to modified the set of composition variables (molalities of electrolyte and crystal solvate of non-electrolyte—):
In these variables, we will proceed to a different modification of SEM-3 equation, namely to SEM(CS)-3 equation:
where: As-cs, Bs-cs, and Ds-cs are the empirical (fitting) parameters of the model equation SEM(CS)-3 (25). In our case, it has following form:
where: REM = rare earth metals—Pr, Nd, Y, Tb.
The parameters of the SEM(CS)-3 model for both four systems are also represented in Table 2 (in the bottom). One can see that the SEM(SC)-3 equation can describe the crystallization curve of solubility of C60(OH)24*18H2O with nearly absolutely the same accuracy as that of model SEM-3 in the direct component molalities (parameters of accuracy of approximation χ2/DoF and R2 are almost the same). We should probably accept this conclusion, which matches the results of simulation model expected and common, at least until solubility of nonelectrolyte (NE) in the electrolyte (E) is relatively low, and the number of solvent molecules in solid crystal solvate (NS), on the contrary, is not too large. In other words, as a rule, the Sechenov equations (SE-1 and SE-3) can be used to describe both systems with crystallization of unsolvated non-electrolyte and systems with crystallization of crystal-solvates. In Figure 19, as an example, and for the comparison, the results of calculation according to the SEM(CS)-3 model are represented for ternary system C60(OH)24—PrCl3—H2O at 25 °C.
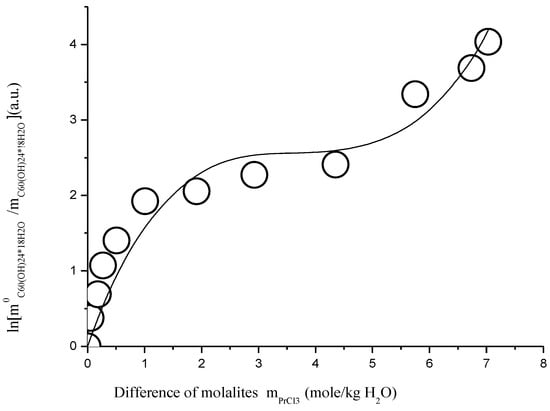
Figure 19.
Branch of crystallization C60(OH)24*18H2O in the diagram of solubility in ternary system C60(OH)24—TbCl3—H2O at 25 °C (convex-conxave line—calculation according to modified three-parameter Sechenov equation SEM(CS)-3—Equation (26)).
4. Conclusions
Solubility in triple systems C60(OH)24—PrCl3—H2O, C60(OH)24—NdCl3—H2O, C60(OH)24—TbCl3—H2O, and C60(OH)24—YCl3—H2O at 25 °C was studied. Solubility diagrams in four ternary systems are simple eutonic, consist of two branches, corresponding to the crystallization of fullerenol crystal-hydrate and rare earth chloride crystal-hydrates, and contain one nonvariant point corresponding to saturation with both solid phases. On the long branches of C60(OH)24*18H2O crystallization, a pronounced salting-out effect is observed—the solubility of C60(OH)24 decreases by more than 2 orders of magnitude compared to the solubility of fullerenol in pure water. On very short branches of crystallization of PrCl3*7H2O, NdCl3*6H2O, YCl3*7H2O, and TbCl3*6H2O, the effect of salting-in is clearly observed, the solubility of all chlorides increases markedly. All diagrams are fairly accurately approximated by classical one-parameter Sechenov equation and very accurately approximated by the three-parameter modified Sechenov equation in the whole concentration range up to 4-7 molalities of electrolytes.
Author Contributions
Conceptualization, N.A.C., K.N.S.; methodology, A.K., V.A.K., N.A.K., investigation, N.A.K., A.K., N.A.C., K.A.T. and M.V.C.; writing—original draft preparation, K.N.S., B.K.S., L.V.G., and Z.K.S.; writing—review and editing, N.A.C., B.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Russian Foundation for Basic Research (RFBR) (Project No. 22-08-00031 A) and the Ministry of Science and Higher Education of the Russian Federation (institution 785.00.X6019, mnemonic code of the application topic 0785-2021-0002). Research was performed using the equipment of the Resource Centers “GeoModel”, Center for Chemical Analysis and Materials Research of Research park of St. Petersburg State University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keskinov, V.A.; Semenov, K.N.; Gol’cov, T.C.; Charykov, N.A.; Podol’skii, N.E.; Kurilenko, A.V.; Shaimardanov, Z.K.; Shaimardanova, B.K.; Kulenova, N.A. Phase diagrams of fullerenol-d–LaCl3–H2O and fullerenol-d–GdCl3–H2O Systems at 25 °C. Russ. J. Phys. Chem. A 2019, 93, 2555–2558. [Google Scholar] [CrossRef]
- Petrov, A.A.; Keskinov, V.A.; Semenov, K.N.; Charykov, N.A.; Letenko, D.G.; Nikitin, V.A. Formation of a new adduct based on fullerene tris-malonate samarium salt C60-[C60(=C(COO)2)3]Sm2. Russ. J. Phys. Chem. 2017, 91, 549–554. [Google Scholar] [CrossRef]
- Yuryev, G.O.; Keskinov, V.A.; Semenov, K.N.; Charykov, N.A. Phase equilibria in a ternary fullerenol-d(c60(OH)22–24)–Smcl3–H2O system at 25°C. Russ. J. Phys. Chem. A 2017, 91, 797–799. [Google Scholar] [CrossRef]
- Semenov, K.N.; A Charykov, N.; Postnov, V.N.; Sharoyko, V.V.; Murin, I.V. Phase equilibria in fullerene-containing systems as a basis for development of manufacture and application processes for nanocarbon materials. Russ. Chem. Rev. 2016, 85, 38–59. [Google Scholar] [CrossRef]
- Semenov, K.N.; Kanterman, I.G.; Charykov, N.A.; Murin, I.V.; Kritchenkov, A.S. Solid-liquid phase equilibria in the fullerenol-d-CuCl2-H2O system at 25 °C. Russ. J. Phys. Chem. A 2014, 88, 1073–1075. [Google Scholar] [CrossRef]
- Semenov, K.N.; Kanterman, I.G.; Charykov, N.A.; Keskinov, V.A.; Kulenova, N.A. Solubility in the ternary system fullerenol-d-uranyl sulfate-water at 25 °C. Radiochemistry 2014, 56, 493–495. [Google Scholar] [CrossRef]
- Zolotarev, A.A.; Lushin, A.I.; Charykov, N.A.; Semenov, K.N.; Namazbaev, V.I.; Keskinov, V.A.; Kritchenkov, A.S. Impact Resistance of Cement and Gypsum Plaster Nanomodified by Water-Soluble Fullerenols. Ind. Eng. Chem. Res. 2013, 52, 14583–14591. [Google Scholar] [CrossRef]
- Semenov, K.N.; Charykov, N.A. Solubility Diagram of a Fullerenol-d-NaCl-H2O System at 25 °C. Russ. J. Phys. Chem. 2012, 86, 1636–1638. [Google Scholar] [CrossRef]
- Setschenov, I.M. Ueber die Absorbtion der Kohlensauren durch Salzlosungen, 6th ed.; Académie impériale des sciences: Petersb., Russia, 1886. [Google Scholar]
- Hermann, C.; Dewes, I.; Schumpe, A. The estimation of gas solubilities in salt solutions. Chem. Eng. Sci. 1995, 50, 1673–1675. [Google Scholar] [CrossRef]
- Sechenov, Ivan Mikhailovich (1829–1905); Autobiographical notes of Ivan Mikhailovich Sechenov; Scientific Word: Moscow, Russia, 1907; Volume XVI, 195p, Available online: https://dlib.rsl.ru/01003744204 (accessed on 1 July 2015). (In Russian)
- Charykova, M.V.; Charykov, N.A. Thermodynamic Modeling of the Process of Evaporite Sedimentation; Science: S-Petersburg, Russia, 2003. [Google Scholar]
- Charykov, N.A. St Petersburg State Institute of Technology (Technical University) different types of non-variant points and non-variant phase processes in the phase diagrams of binary, ternary and multicomponent systems. Bull. St. Petersburg State Inst. Technol. (Techn. Univ.) 2019, 3–20. [Google Scholar] [CrossRef]
- Charykov, N.A.; Charykova, M.V.; Semenov, K.N.; Keskinov, V.A.; Kurilenko, A.V.; Shaimardanov, Z.K.; Shaimardanova, B.K. Multiphase Open Phase Processes Differential Equations. Processes 2019, 7, 148. [Google Scholar] [CrossRef]
- Charykov, N.; Semenov, K.; Keskinov, V.; Kulenova, N.; Shaimardanov, Z.; Gerasimova, L.; Kanbar, A.; Letenko, D. Cryometry and excess thermodynamic functions in water soluble of the fullerenol C60(OH)24. Nanosyst. Phys. Chem. Math. 2020, 11, 205–213. [Google Scholar] [CrossRef]
- Sharoyko, V.V.; Ageev, S.V.; Meshcheriakov, A.A.; Akentiev, A.V.; Noskov, B.A.; Rakipov, I.T.; Charykov, N.A.; Kulenova, N.A.; Shaimardanova, B.K.; Podolsky, N.E.; et al. Physicochemical study of water-soluble C60(OH)24 fullerenol. J. Mol. Liq. 2020, 311, 113360. [Google Scholar] [CrossRef]
- Pitzer, K.S. Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys. Chem. 1973, 77, 268–277. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Kim, J.J. Thermodynamics of electrolytes. IV. Activity and osmotic coefficients for mixed electrolytes. J. Am. Chem. Soc. 1974, 96, 5701–5707. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).