Rhizopus oligosporus-Assisted Valorization of Coconut Endosperm Waste by Black Soldier Fly Larvae for Simultaneous Protein and Lipid to Biodiesel Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Rhizopus Oligosporus Spore Suspension for Inoculation
2.2. Procurement of BSFL
2.3. Experimental Setups to Grow BSFL in the Presence of Various R. oligosporus Inoculum Sizes
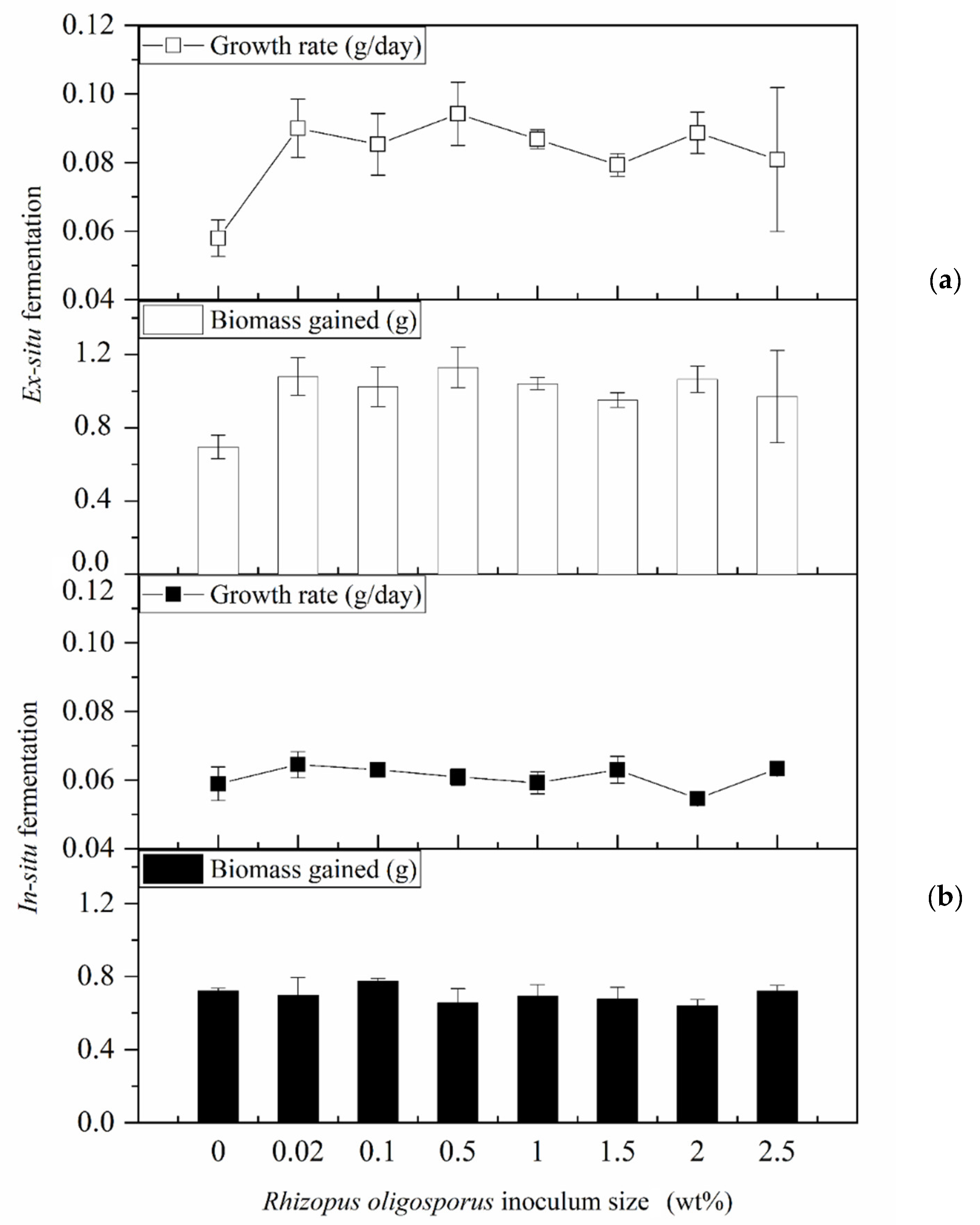
2.4. Larval Growth Analyses
2.5. Larval Biochemical Analyses
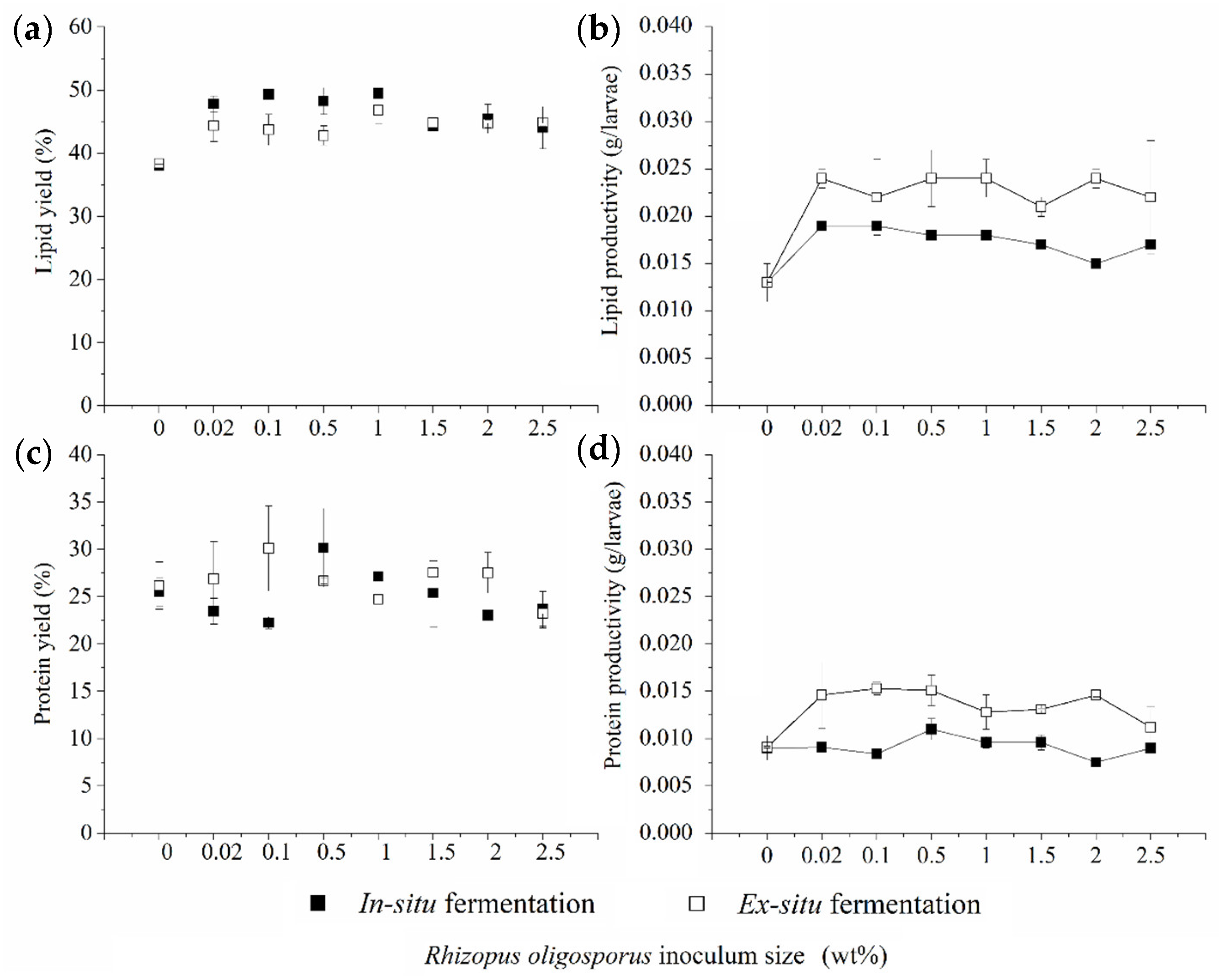
2.5.1. Lipid
2.5.2. Protein
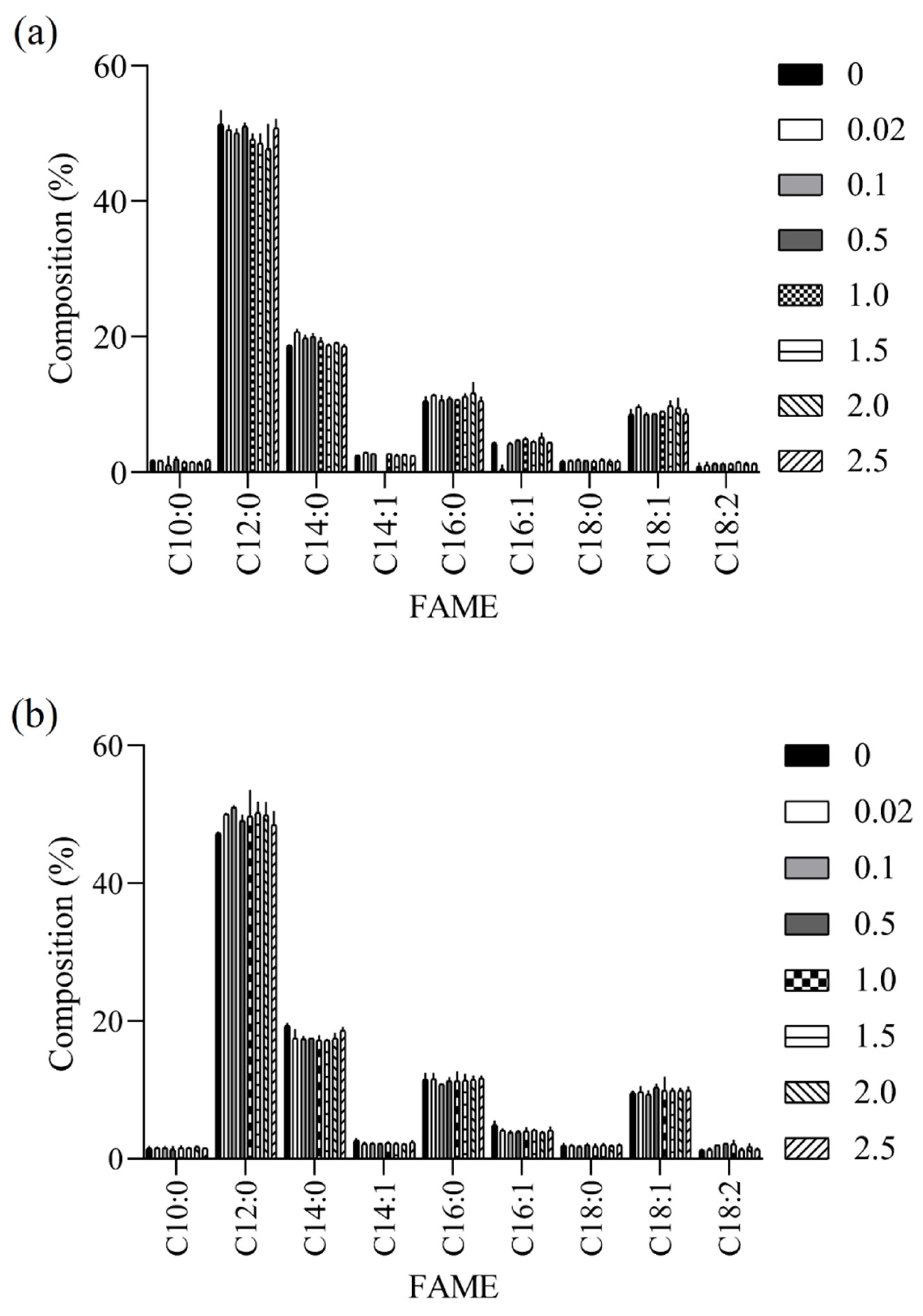
2.5.3. Fatty Acid Methyl Esters
2.6. Statistical Verification
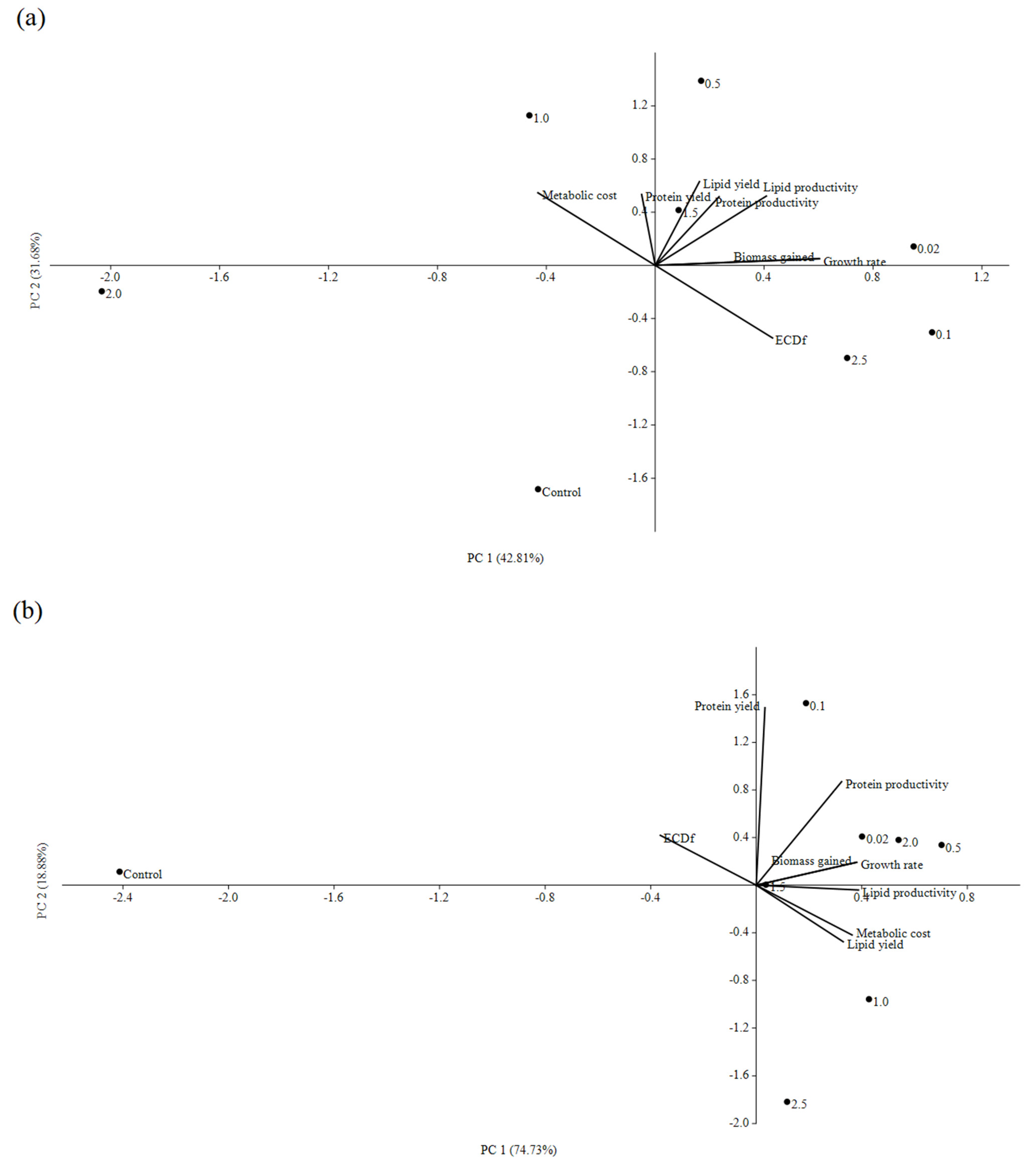
3. Results and Discussion
3.1. Impacts of In Situ and Ex Situ Fermentations on BSFL Growth Performances
3.2. Lipid and Protein Derived from BSFL Feeding on In Situ and Ex Situ Fermented CEW
3.3. Quality of BSFL-Based Biodiesels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Liu, S.; Ji, H.; Yu, H. Effect of replacing dietary fish meal with black soldier fly larvae meal on growth and fatty acid composition of jian carp (cyprinus carpio var. Jian). Aquac. Nutr. 2018, 24, 424–433. [Google Scholar] [CrossRef]
- Schiavone, A.; Dabbou, S.; Petracci, M.; Zampiga, M.; Sirri, F.; Biasato, I.; Gai, F.; Gasco, L. Black soldier fly defatted meal as a dietary protein source for broiler chickens: Effects on carcass traits, breast meat quality and safety. Animal 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; Dabbou, S.; De Marco, M.; Cullere, M.; Biasato, I.; Biasibetti, E.; Capucchio, M.; Bergagna, S.; Dezzutto, D.; Meneguz, M. Black soldier fly larva fat inclusion in finisher broiler chicken diet as an alternative fat source. Animal 2018, 12, 2032–2039. [Google Scholar] [CrossRef]
- Fisher, H.J.; Collins, S.A.; Hanson, C.; Mason, B.; Colombo, S.M.; Anderson, D.M. Black soldier fly larvae meal as a protein source in low fish meal diets for atlantic salmon (salmo salar). Aquaculture 2020, 521, 734978. [Google Scholar] [CrossRef]
- Gimbun, J.; Ali, S.; Kanwal, C.; Shah, L.A.; Ghazali, N.H.M.; Cheng, C.K.; Nurdin, S. Biodiesel production from rubber seed oil using a limestone based catalyst. Adv. Mater. Phys. Chem. 2012, 2, 138–141. [Google Scholar] [CrossRef]
- Gimbun, J.; Ali, S.; Kanwal, C.; Shah, L.A.; Ghazali, N.H.M.; Cheng, C.K.; Nurdin, S. Biodiesel production from rubber seed oil using activated cement clinker as catalyst. Procedia Eng. 2013, 53, 13–19. [Google Scholar] [CrossRef]
- Muhamad, S.; Gimbun, J.; Cheng, C.; Nurdin, S. Enhancement of biodiesel yield from high ffa malaysian rubber seed oil with sodium methoxide treated limestone. J. Eng. Technol. Jet 2013, 4, 123–132. [Google Scholar]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Q.; Zhang, J.; Yu, Z. Double the biodiesel yield: Rearing black soldier fly larvae, hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 2012, 41, 75–79. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Zheng, L.; Liu, Y.; Zhang, Y.; Yu, Z.; Ma, Z.; Li, Q. Simultaneous utilization of glucose and xylose for lipid accumulation in black soldier fly. Biotechnol. Biofuels 2015, 8, 117. [Google Scholar] [CrossRef]
- Xenopoulos, E.; Giannikakis, I.; Chatzifragkou, A.; Koutinas, A.; Papanikolaou, S. Lipid production by yeasts growing on commercial xylose in submerged cultures with process water being partially replaced by olive mill wastewaters. Processes 2020, 8, 819. [Google Scholar] [CrossRef]
- Elfeky, N.; Elmahmoudy, M.; Bao, Y. Manipulation of culture conditions: Tool for correlating/improving lipid and carotenoid production by rhodotorula glutinis. Processes 2020, 8, 140. [Google Scholar] [CrossRef]
- Pinzi, S.; Leiva, D.; López-García, I.; Redel-Macías, M.D.; Dorado, M.P. Latest trends in feedstocks for biodiesel production. Biofuels Bioprod. Biorefining 2014, 8, 126–143. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Liang, S.-H.; Li, S.-Y.; Su, C.-H.; Chien, C.-C.; Chen, Y.-J.; Huong, D.T.M. Direct transesterification of black soldier fly larvae (hermetia illucens) for biodiesel production. J. Taiwan Inst. Chem. Eng. 2018, 85, 165–169. [Google Scholar] [CrossRef]
- Win, S.S.; Ebner, J.H.; Brownell, S.A.; Pagano, S.S.; Cruz-Diloné, P.; Trabold, T.A. Anaerobic digestion of black solider fly larvae (bsfl) biomass as part of an integrated biorefinery. Renew. Energ. 2018, 127, 705–712. [Google Scholar] [CrossRef]
- Rehman, K.; Liu, X.; Wang, H.; Zheng, L.; Rehman, R.; Cheng, X.; Li, Q.; Li, W.; Cai, M.; Zhang, J.; et al. Effects of black soldier fly biodiesel blended with diesel fuel on combustion, performance and emission characteristics of diesel engine. Energy Convers. Manag. 2018, 173, 489–498. [Google Scholar] [CrossRef]
- Hasnol, S.; Kiatkittipong, K.; Kiatkittipong, W.; Wong, C.Y.; Khe, C.S.; Lam, M.K.; Show, P.L.; Oh, W.D.; Chew, T.L.; Lim, J.W. A review on insights for green production of unconventional protein and energy sources derived from the larval biomass of black soldier fly. Processes 2020, 8, 523. [Google Scholar] [CrossRef]
- Wang, C.; Qian, L.; Wang, W.; Wang, T.; Deng, Z.; Yang, F.; Xiong, J.; Feng, W. Exploring the potential of lipids from black soldier fly: New paradigm for biodiesel production (i). Renew. Energy 2017, 111, 749–756. [Google Scholar] [CrossRef]
- Pamintuan, K.R.S.; Cajayon, J.A.B.; Dableo, G.B. Growth characteristics and lipid content of black soldier fly (hermetia illucens) larva reared in milkfish offal and mixed vegetable wastes. In Proceedings of the 2019 6th International Conference on Biomedical and Bioinformatics Engineering, Shanghai, China, 3–15 November 2019; pp. 163–168. [Google Scholar]
- Zheng, L.; Hou, Y.; Wu, L.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, W.; Lu, X.; Zhu, F.; Liu, W.; Wang, X.; Lei, C. Bioconversion performance and life table of black soldier fly (hermetia illucens) on fermented maize straw. J. Clean. Prod. 2019, 230, 974–980. [Google Scholar] [CrossRef]
- Howdeshell, T. Bioconversion of Lignocellulosic Biomass by the Black Soldier Fly in Combination with Solid State Fermentation for Biofuel and Larval Biomass Production; University of Saskatchewan: Saskatoon, SK, Canada, 2016. [Google Scholar]
- Wong, C.Y.; Lim, J.W.; Chong, F.K.; Lam, M.K.; Uemura, Y.; Tan, W.N.; Bashir, M.J.K.; Lam, S.M.; Sin, J.C.; Lam, S.S. Valorization of exo-microbial fermented coconut endosperm waste by black soldier fly larvae for simultaneous biodiesel and protein productions. Environ. Res. 2020, 185, 109458. [Google Scholar] [CrossRef]
- Wong, C.-Y.; Rosli, S.-S.; Uemura, Y.; Ho, Y.C.; Leejeerajumnean, A.; Kiatkittipong, W.; Cheng, C.-K.; Lam, M.-K.; Lim, J.-W. Potential protein and biodiesel sources from black soldier fly larvae: Insights of larval harvesting instar and fermented feeding medium. Energies 2019, 12, 1570. [Google Scholar] [CrossRef]
- Mohd-Noor, S.-N.; Wong, C.-Y.; Lim, J.-W.; Uemura, Y.; Lam, M.-K.; Ramli, A.; Bashir, M.J.; Tham, L. Optimization of self-fermented period of waste coconut endosperm destined to feed black soldier fly larvae in enhancing the lipid and protein yields. Renew. Energy 2017, 111, 646–654. [Google Scholar] [CrossRef]
- Polanowska, K.; Grygier, A.; Kuligowski, M.; Rudzińska, M.; Nowak, J. Effect of tempe fermentation by three different strains of rhizopus oligosporus on nutritional characteristics of faba beans. Lebensm. Wiss. Technol. 2020, 122, 109024. [Google Scholar] [CrossRef]
- Handoyo, T.; Morita, N. Structural and functional properties of fermented soybean (tempeh) by using rhizopus oligosporus. Int. J. Food Prop. 2006, 9, 347–355. [Google Scholar] [CrossRef]
- Nelofer, R. Nutritional enhancement of barley in solid state fermentation by rhizopus oligosporus mL-10. Nutr. Food Sci. Int. J. 2018, 6. [Google Scholar] [CrossRef]
- Kim, W.; Bae, S.; Park, H.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Koh, Y.-H. The larval age and mouth morphology of the black soldier fly, hermetia illucens (diptera: Stratiomyidae). Int. J. Indust. Entomol. 2010, 21, 185–187. [Google Scholar]
- Diclaro, J.W.; Kaufman, P.E. Black soldier fly hermetia illucens linnaeus (insecta: Diptera: Stratiomyidae). Eeny 2009, 461, 1–3. [Google Scholar]
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of daiy manure by black soldier fly (diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag. 2011, 31, 1316–1320. [Google Scholar] [CrossRef]
- Nielsen, S.S. Protein nitrogen determination. In Food Analysis Laboratory Manual; Springer: Berlin/Heidelberg, Germany, 2010; pp. 39–46. [Google Scholar]
- Lim, J.-W.; Mohd-Noor, S.-N.; Wong, C.-Y.; Lam, M.-K.; Goh, P.-S.; Beniers, J.; Oh, W.-D.; Jumbri, K.; Ghani, N.A. Palatability of black soldier fly larvae in valorizing mixed waste coconut endosperm and soybean curd residue into larval lipid and protein sources. J. Environ. Manag. 2019, 231, 129–136. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Feng, X.M.; Eriksson, A.R.B.; Schnürer, J. Growth of lactic acid bacteria and rhizopus oligosporus during barley tempeh fermentation. Int. J. Food Microbiol. 2005, 104, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Vong, W.C.; Hua, X.Y.; Liu, S.-Q. Solid-state fermentation with rhizopus oligosporus and yarrowia lipolytica improved nutritional and flavour properties of okara. LWT 2018, 90, 316–322. [Google Scholar] [CrossRef]
- Mei Feng, X.; Ostenfeld Larsen, T.; Schnürer, J. Production of volatile compounds by rhizopus oligosporus during soybean and barley tempeh fermentation. Int. J. Food Microbiol. 2007, 113, 133–141. [Google Scholar] [CrossRef]
- Wong, C.Y.; Mohd Aris, M.N.; Daud, H.; Lam, M.K.; Yong, C.S.; Abu Hasan, H.; Chong, S.; Show, P.L.; Hajoeningtijas, O.D.; Ho, Y.C. In-situ yeast fermentation to enhance bioconversion of coconut endosperm waste into larval biomass of hermetia illucens: Statistical augmentation of larval lipid content. Sustainability 2020, 12, 1558. [Google Scholar] [CrossRef]
- Wong, C.Y.; Ho, Y.C.; Lim, J.W.; Show, P.L.; Chong, S.; Chan, Y.J.; Ho, C.D.; Mohamad, M.; Wu, T.Y.; Lam, M.K. In-situ yeast fermentation medium in fortifying protein and lipid accumulations in the harvested larval biomass of black soldier fly. Processes 2020, 8, 337. [Google Scholar] [CrossRef]
- Appaiah, P.; Sunil, L.; Kumar, P.P.; Krishna, A.G. Composition of coconut testa, coconut kernel and its oil. J. Am. Oil Chem. Soc. 2014, 91, 917–924. [Google Scholar] [CrossRef]
- Karatay, S.E.; Dönmez, G. Improving the lipid accumulation properties of the yeast cells for biodiesel production using molasses. Bioresour. Technol. 2010, 101, 7988–7990. [Google Scholar] [CrossRef]

| Feedstock | BSFL | BSFL [20] | Wet White Coconut Kernel [40] | Yeast [41] | |
|---|---|---|---|---|---|
| FAME | |||||
| C8:0 | - | - | 8.1% | - | |
| C10:0 | 1.6% | 3.8% | 7.8% | - | |
| C12:0 | 49.5% | 27.8% | 50.5% | - | |
| C14:0 | 17.8% | 8.1% | 16.1% | - | |
| C14:1 | 2.3% | - | - | - | |
| C16:0 | 11.4% | 14.2% | 6.8% | 26.2% | |
| C16:1 | 4.1% | 4.5% | - | - | |
| C18:0 | 1.9% | 7.6% | 2.3% | 37.3% | |
| C18:1 | 9.8% | 22.5% | 5.6% | 22.3% | |
| C18:2 | 1.6% | 1.8% | 1.8% | 6.5% | |
| C18:3 | - | 2.1% | - | - | |
| Others | - | 7.6% | 1% | 2.8% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, C.Y.; Kiatkittipong, K.; Kiatkittipong, W.; Lim, J.W.; Lam, M.K.; Wu, T.Y.; Show, P.L.; Daud, H.; Goh, P.S.; Sakuragi, M.; et al. Rhizopus oligosporus-Assisted Valorization of Coconut Endosperm Waste by Black Soldier Fly Larvae for Simultaneous Protein and Lipid to Biodiesel Production. Processes 2021, 9, 299. https://doi.org/10.3390/pr9020299
Wong CY, Kiatkittipong K, Kiatkittipong W, Lim JW, Lam MK, Wu TY, Show PL, Daud H, Goh PS, Sakuragi M, et al. Rhizopus oligosporus-Assisted Valorization of Coconut Endosperm Waste by Black Soldier Fly Larvae for Simultaneous Protein and Lipid to Biodiesel Production. Processes. 2021; 9(2):299. https://doi.org/10.3390/pr9020299
Chicago/Turabian StyleWong, Chung Yiin, Kunlanan Kiatkittipong, Worapon Kiatkittipong, Jun Wei Lim, Man Kee Lam, Ta Yeong Wu, Pau Loke Show, Hanita Daud, Pei Sean Goh, Mina Sakuragi, and et al. 2021. "Rhizopus oligosporus-Assisted Valorization of Coconut Endosperm Waste by Black Soldier Fly Larvae for Simultaneous Protein and Lipid to Biodiesel Production" Processes 9, no. 2: 299. https://doi.org/10.3390/pr9020299
APA StyleWong, C. Y., Kiatkittipong, K., Kiatkittipong, W., Lim, J. W., Lam, M. K., Wu, T. Y., Show, P. L., Daud, H., Goh, P. S., Sakuragi, M., & Elfis. (2021). Rhizopus oligosporus-Assisted Valorization of Coconut Endosperm Waste by Black Soldier Fly Larvae for Simultaneous Protein and Lipid to Biodiesel Production. Processes, 9(2), 299. https://doi.org/10.3390/pr9020299










