Proprotein Convertase Subtilisin/Kexin Type 9 Gene Variants in Familial Hypercholesterolemia: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy, Inclusion and Exclusion Criteria of Literature
2.2. Data Extraction and Statistical Analysis
3. Results
3.1. The Characteristics of Eligible Studies
3.2. Meta-Analysis: The Proportion of PCSK9 Mutation in FH Patients
3.3. The Frequency of PCSK9 GOF and LOF Variants
3.4. PCSK9 Mutation Proportion In Exon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ApoB | Apolipoprotein B |
| CVD | Cardiovascular disease |
| EGF-A | Epidermal growth factor-like repeat A |
| FH | Familial hypercholesterolemia |
| GOF | Gain-of-function |
| HGVS | Human Genetic Variation Society |
| HRM | High Resolution Melt |
| LDL | Low Density Lipoprotein |
| LDL-C | Low Density Lipoprotein Cholesterol |
| LDLR | Low Density Lipoprotein Receptor |
| LDLRAP1 | Low density lipoprotein receptor adaptor protein 1 |
| LOF | Loss-of-function |
| LOVD | Leiden Open Variation Database |
| N/A | Not available data |
| NARC1 | Neural Apoptosis Regulated Convertase 1 |
| NGS | Next generation sequencing |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| RFLP | Restriction fragment length polymorphism |
| SSCP | Single-strand conformation polymorphism |
| VUS | Variant of uncertain significance |
References
- Goldstein, J.L.; Brown, M.S. Familial hypercholesterolemia. A genetic regulatory defect in cholesterol metabolism. Am. J. Med. 1975, 58, 147–150. [Google Scholar] [CrossRef]
- Veljkovic, N.; Zaric, B.; Djuric, I.; Obradovic, M.; Sudar-Milovanovic, E.; Radak, D.; Isenovic, E.R. Genetic markers for coronary artery disease. Medicina 2018, 54, 36. [Google Scholar] [CrossRef] [PubMed]
- Polychronopoulos, G.; Tziomalos, K. What special considerations must be made for the pharmacotherapeutic management of heterozygous familial hypercholesterolemia? Expert Opin. Pharmacother. 2019, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Żak, J.; Malinowski, B.; Popek, G.; Grześk, G. PCSK9 signaling pathways and their potential importance in clinical practice. EPMA J. 2017, 8, 391–402. [Google Scholar] [CrossRef]
- Burke, A.C.; Dron, J.S.; Hegele, R.A.; Huff, M.W. PCSK9: Regulation and target for drug development for dyslipidemia. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 223–244. [Google Scholar] [CrossRef]
- Abifadel, M.; Rabès, J.P.; Devillers, M.; Munnich, A.; Erlich, D.; Junien, C.; Varret, M.; Boileau, C. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9(PCSK9) gene in cholesterol metabolism and disease. Hum. Mutat. 2009, 30, 520–529. [Google Scholar] [CrossRef]
- Hendricks-Sturrup, R.M.; Lu, C.Y. Understanding implementation challenges to genetic testing for familial hypercholesterolemia in the United States. J. Pers. Med. 2019, 9, 9. [Google Scholar] [CrossRef]
- Hori, M.; Ohta, N.; Takahashi, A.; Masuda, H.; Isoda, R.; Yamamoto, S.; Son, C.; Ogura, M.; Hosoda, K.; Miyamoto, Y.; et al. Impact of LDLR and PCSK9 pathogenic variants in Japanese heterozygous familial hypercholesterolemia patients. Atherosclerosis 2019, 289, 101–108. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Haidich, A.B. Meta-analysis in medical research. Hippokratia 2010, 14, 29–37. [Google Scholar]
- Ryan, R.; Cochrane Consumers and Communication Review Group. Cochrane Consumers and Communication Group: Meta-analysis. Cochrane Consumers and Communication. 2016. Available online: http://cccrg.cochrane.org (accessed on 14 December 2016).
- Lee, C.; Cui, Y.; Song, J.; Li, S.; Zhang, F.; Wu, M.; Li, L.; Hu, D.; Chen, H. Effects of familial hypercholesterolemia-associated genes on the phenotype of premature myocardial infarction. Lipids Health Dis. 2019, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, R.M.; Tugores, A.; Nóvoa, F.J.; Brito-Casillas, Y.; Expósito-Montesdeoca, A.B.; Garay, P.; Bea, A.M.; Riaño, M.; Pocovi, M.; Civeira, F.; et al. The island of Gran Canaria: A genetic isolate for familial hypercholesterolemia. J. Clin. Lipidol. 2019, 13, 618–626. [Google Scholar] [CrossRef]
- Cao, Y.X.; Wu, N.Q.; Sun, D.; Liu, H.H.; Jin, J.L.; Li, S.; Guo, Y.L.; Zhu, C.G.; Gao, Y.; Dong, Q.T.; et al. Application of expanded genetic analysis in the diagnosis of familial hypercholesterolemia in patients with very early-onset coronary artery disease. J. Transl. Med. 2018, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kweon, S.-S.; Shin, M.-H. Detection of Familial Hypercholesterolemia Using Next Generation Sequencing in Two Population-Based Cohorts. Chonnam Med. J. 2018, 54, 31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raal, F.J.; Bahassi, E.M.; Stevens, B.; Turner, T.A.; Stein, E.A. Cascade screening for familial hypercholesterolemia in South Africa the WITS FIND-FH program. J. Am. Coll. Cardiol. 2018, 71, A1768. [Google Scholar] [CrossRef]
- Tada, H.; Kawashiri, M.; Nomura, A.; Teramoto, R.; Hosomichi, K.; Nohara, A.; Inazu, A.; Mabuchi, H.; Tajima, A.; Yamagishi, M. Oligogenic familial hypercholesterolemia, LDL cholesterol, and coronary artery disease. J. Clin. Lipidol. 2018, 12, 1436–1444. [Google Scholar] [CrossRef]
- Tada, H.; Kawashiri, M.A.; Nohara, A.; Inazu, A.; Mabuchi, H.; Yamagishi, M. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur. Heart J. 2017, 38, 1573–1579. [Google Scholar] [CrossRef]
- Xiang, R.; Fan, L.L.; Lin, M.J.; Li, J.J.; Shi, X.Y.; Jin, J.Y.; Liu, Y.X.; Chen, Y.Q.; Xia, K.; Zhao, S.P. The genetic spectrum of familial hypercholesterolemia in the central south region of China. Atherosclerosis 2017, 258, 84–88. [Google Scholar] [CrossRef]
- Zhu, C.G.; Li, S.; Wang, Z.F.; Yin, K.L.; Wu, N.Q.; Guo, Y.L.; Gao, Y.; Li, X.L.; Qing, P.; Liu, G.; et al. Homozygous familiar hypercholesterolemia in China: Case series from the national lipid clinics and literature review. IJC Metab. Endocr. 2017, 14, 75–80. [Google Scholar] [CrossRef]
- Abul-Husn, N.S.; Manickam, K.; Jones, L.K.; Wright, E.A.; Hartzel, D.N.; Gonzaga-Jauregui, C.; O’Dushlaine, C.; Leader, J.B.; Kirchner, H.L.; Lindbuchler, D.M.; et al. Genetic identification of familial hypercholesterolemia within a single U.S. Health care system. Science 2016, 354, 1550–1557. [Google Scholar] [CrossRef]
- Medeiros, A.M.; Alves, A.C.; Bourbon, M. Mutational analysis of a cohort with clinical diagnosis of familial hypercholesterolemia: Considerations for genetic diagnosis improvement. Genet. Med. 2016, 18, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Hori, M.; Takahashi, A.; Ogura, M.; Makino, H.; Tamanaha, T.; Fujiyama, H.; Miyamoto, Y.; Harada-Shiba, M. Proprotein convertase subtilisin/kexin 9 V4I variant with LDLR mutations modifies the phenotype of familial hypercholesterolemia. J. Clin. Lipidol. 2016, 10, 547.e5–555.e5. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kawashiri, M.A.; Yoshida, T.; Teramoto, R.; Nohara, A.; Konno, T.; Inazu, A.; Mabuchi, H.; Yamagishi, M.; Hayashi, K. Lipoprotein(A) in familial hypercholesterolemia with proprotein convertase subtilisin/kexin type 9 (PCSK9) gain-of-function mutations. Circ. J. 2016, 80, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dron, J.S.; Ban, M.R.; Robinson, J.F.; McIntyre, A.D.; Alazzam, M.; Zhao, P.J.; Dilliott, A.A.; Cao, H.; Huff, M.W.; et al. Polygenic Versus Monogenic Causes of Hypercholesterolemia Ascertained Clinically. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2439–2445. [Google Scholar] [CrossRef]
- Mabuchi, H.; Nohara, A.; Noguchi, T.; Kobayashi, J.; Kawashiri, M.; Inoue, T.; Mori, M.; Tada, H.; Nakanishi, C.; Yagi, K.; et al. Genotypic and phenotypic features in homozygous familial hypercholesterolemia caused by proprotein convertase subtilisin/kexin type 9 (PCSK9) gain-of-function mutation. Atherosclerosis 2014, 236, 54–61. [Google Scholar] [CrossRef]
- Maglio, C.; Mancina, R.M.; Motta, B.M.; Stef, M.; Pirazzi, C.; Palacios, L.; Askaryar, N.; Borén, J.; Wiklund, O.; Romeo, S. Genetic diagnosis of familial hypercholesterolaemia by targeted next-generation sequencing. J. Intern. Med. 2014, 276, 396–403. [Google Scholar] [CrossRef]
- Saavedra, Y.G.L.; Dufour, R.; Davignon, J.; Baass, A. PCSK9 R46L, lower LDL, and cardiovascular disease risk in familial hypercholesterolemia a cross-sectional cohort study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2700–2705. [Google Scholar] [CrossRef]
- Ahmed, W.; Whittall, R.; Riaz, M.; Ajmal, M.; Sadeque, A.; Ayub, H.; Qamar, R.; Humphries, S.E. The genetic spectrum of familial hypercholesterolemia in Pakistan. Clin. Chim. Acta 2013, 421, 219–225. [Google Scholar] [CrossRef]
- Vandrovcova, J.; Thomas, E.R.A.; Atanur, S.S.; Norsworthy, P.J.; Neuwirth, C.; Tan, Y.; Kasperaviciute, D.; Biggs, J.; Game, L.; Mueller, M.; et al. The use of next-generation sequencing in clinical diagnosis of familial hypercholesterolemia. Genet. Med. 2013, 15, 948–957. [Google Scholar] [CrossRef]
- Abifadel, M.; Guerin, M.; Benjannet, S.; Rabès, J.P.; Le Goff, W.; Julia, Z.; Hamelin, J.; Carreau, V.; Varret, M.; Bruckert, E.; et al. Identification and characterization of new gain-of-function mutations in the PCSK9 gene responsible for autosomal dominant hypercholesterolemia. Atherosclerosis 2012, 223, 394–400. [Google Scholar] [CrossRef]
- Palacios, L.; Grandoso, L.; Cuevas, N.; Olano-Martín, E.; Martinez, A.; Tejedor, D.; Stef, M. Molecular characterization of familial hypercholesterolemia in Spain. Atherosclerosis 2012, 221, 137–142. [Google Scholar] [CrossRef]
- Noguchi, T.; Katsuda, S.; Kawashiri, M.; Tada, H.; Nohara, A.; Inazu, A.; Yamagishi, M.; Kobayashi, J.; Mabuchi, H. The E32K variant of PCSK9 exacerbates the phenotype of familial hypercholesterolaemia by increasing PCSK9 function and concentration in the circulation. Atherosclerosis 2010, 210, 166–172. [Google Scholar] [CrossRef]
- Strøm, T.B.; Holla, Ø.L.; Cameron, J.; Berge, K.E.; Leren, T.P. Loss-of-function mutation R46L in the PCSK9 gene has little impact on the levels of total serum cholesterol in familial hypercholesterolemia heterozygotes. Clin. Chim. Acta 2010, 411, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Abifadel, M.; Rabès, J.P.; Jambart, S.; Halaby, G.; Gannagé-Yared, M.H.; Sarkis, A.; Beaino, G.; Varret, M.; Salem, N.; Corbani, S.; et al. The molecular basis of familial hypercholesterolemia in Lebanon: Spectrum of LDLR mutations and role of PCSK9 as a modifier gene. Hum. Mutat. 2009, 30, E682–E691. [Google Scholar] [CrossRef]
- Homer, V.M.; Marais, A.D.; Charlton, F.; Laurie, A.D.; Hurndell, N.; Scott, R.; Mangili, F.; Sullivan, D.R.; Barter, P.J.; Rye, K.A.; et al. Identification and characterization of two non-secreted PCSK9 mutants associated with familial hypercholesterolemia in cohorts from New Zealand and South Africa. Atherosclerosis 2008, 196, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Tosi, I.; Toledo-Leiva, P.; Neuwirth, C.; Naoumova, R.P.; Soutar, A.K. Genetic defects causing familial hypercholesterolaemia: Identification of deletions and duplications in the LDL-receptor gene and summary of all mutations found in patients attending the Hammersmith Hospital Lipid Clinic. Atherosclerosis 2007, 194, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Berge, K.E.; Ose, L.; Leren, T.P. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.; Beil, F.U. The E670G SNP in the PCSK9 gene is associated with polygenic hypercholesterolemia in men but not in women. BMC Med. Genet. 2006, 7, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Allard, D.; Amsellem, S.; Abifadel, M.; Trillard, M.; Devillers, M.; Luc, G.; Krempf, M.; Reznik, Y.; Girardet, J.P.; Fredenrich, A.; et al. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum. Mutat. 2005, 26, 497. [Google Scholar] [CrossRef]
- Sun, X.M.; Eden, E.R.; Tosi, I.; Neuwirth, C.K.; Wile, D.; Naoumova, R.P.; Soutar, A.K. Evidence for effect of mutant PCSK9 on apolipoprotein B secretion as the cause of unusually severe dominant hypercholesterolaemia. Hum. Mol. Genet. 2005, 14, 1161–1169. [Google Scholar] [CrossRef]
- Leren, T.P. Mutations in the PCSK9 gene in Norwegian subjects with autosomal dominant hypercholesterolemia. Clin. Genet. 2004, 65, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.R.; Lightstone, F.C.; Cheng, F. In Silico Insights into Protein–Protein Interaction Disruptive Mutations in the PCSK9-LDLR Complex. Int. J. Mol. Sci. 2020, 21, 1550. [Google Scholar] [CrossRef] [PubMed]
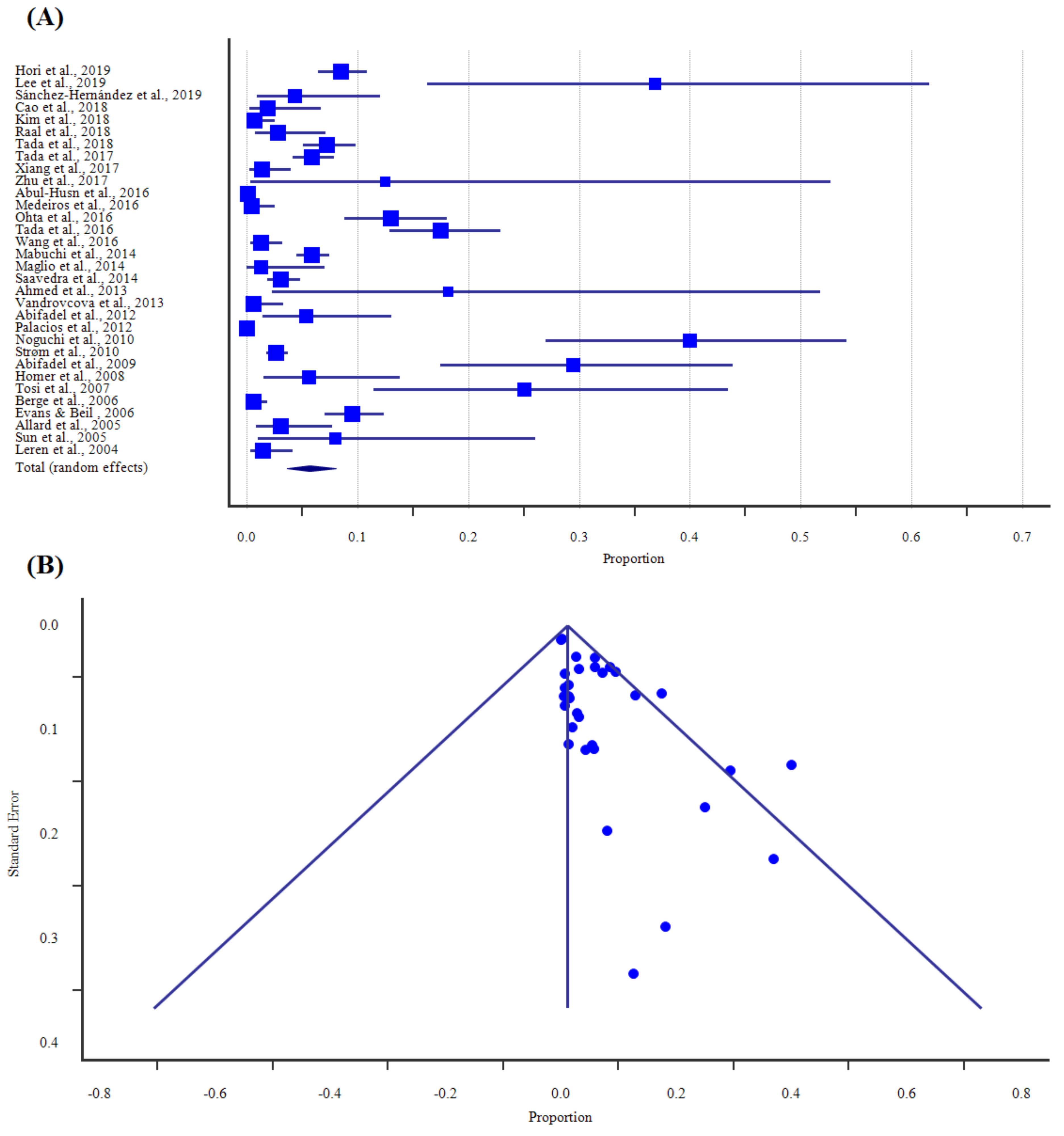
| Study, Year | Region | Method | Sample Size | Proportion (%) | 95% CI | Weight (%) |
|---|---|---|---|---|---|---|
| Hori et al., 2019 [8] | Asia | Sequencing | 650 | 8.46 | 6.44–10.87 | 3.55 |
| Lee et al., 2019 [12] | Asia | Sequencing | 19 | 36.84 | 16.30–61.64 | 2.05 |
| Sánchez-Hernández et al., 2019 [13] | Europe | NGS | 70 | 4.29 | 0.89–12.02 | 2.98 |
| Cao et al., 2018 [14] | Asia | NGS | 105 | 1.91 | 0.23–6.71 | 3.17 |
| Kim et al., 2018 [15] | Asia | NGS | 283 | 0.71 | 0.09–2.53 | 3.45 |
| Raal et al., 2018 [16] | Africa | NGS | 141 | 2.84 | 0.78–7.10 | 3.28 |
| Tada et al., 2018 [17] | Asia | NGS | 500 | 7.20 | 5.09–9.83 | 3.53 |
| Tada et al., 2017 [18] | Asia | Sequencing | 636 | 5.82 | 4.13–7.93 | 3.55 |
| Xiang et al., 2017 [19] | Asia | Sequencing | 219 | 1.37 | 0.28–3.95 | 3.40 |
| Zhu et al., 2017 [20] | Asia | NGS | 8 | 12.50 | 0.32–52.65 | 1.33 |
| Abul-Husn et al., 2016 [21] | North America | NGS | 6015 | 0.12 | 0.05–0.24 | 3.63 |
| Medeiros et al., 2016 [22] | Europe | Sequencing | 220 | 0.46 | 0.01–2.51 | 3.40 |
| Ohta et al., 2016 [23] | Asia | Sequencing | 224 | 12.95 | 8.85–18.06 | 3.40 |
| Tada et al., 2016 [24] | Asia | Sequencing | 240 | 17.50 | 12.91–22.91 | 3.42 |
| Wang et al., 2016 [25] | North America | NGS | 313 | 1.28 | 0.35–3.24 | 3.47 |
| Mabuchi et al., 2014 [26] | Asia | Invader assay | 1055 | 5.88 | 4.54–7.47 | 3.59 |
| Maglio et al., 2014 [27] | Europe | NGS | 77 | 1.30 | 0.03–7.03 | 3.03 |
| Saavedra et al., 2014 [28] | North America | RFLP | 582 | 3.09 | 1.84–4.84 | 3.54 |
| Ahmed et al., 2013 [29] | Asia | HRM, RFLP | 11 | 18.18 | 2.28–51.78 | 1.58 |
| Vandrovcova et al., 2013 [30] | Europe | NGS | 168 | 0.60 | 0.02–3.27 | 3.33 |
| Abifadel et al., 2012 [31] | Europe | Sequencing | 75 | 5.33 | 1.47–13.10 | 3.02 |
| Palacios et al., 2012 [32] | Europe | Microarray | 5430 | 0.02 | 0.0005–0.10 | 3.63 |
| Noguchi et al., 2010 [33] | Asia | SSCP, RFLP | 55 | 40.00 | 27.02–54.09 | 2.85 |
| Strøm et al., 2010 [34] | Europe | Sequencing | 1130 | 2.66 | 1.80–3.80 | 3.59 |
| Abifadel et al., 2009 [35] | Asia | Sequencing | 51 | 29.41 | 17.49–43.83 | 2.80 |
| Homer et al., 2008 [36] | (*) | Sequencing | 71 | 5.63 | 1.56–13.80 | 2.99 |
| Tosi et al., 2007 [37] | Europe | Sequencing | 32 | 25.00 | 11.46–43.41 | 2.47 |
| Berge et al., 2006 [38] | Europe | Sequencing | 475 | 0.63 | 0.13–1.84 | 3.52 |
| Evans & Beil, 2006 [39] | Europe | RFLP | 506 | 9.49 | 7.08–12.38 | 3.53 |
| Allard et al., 2005 [40] | Europe | Sequencing | 130 | 3.08 | 0.85–7.69 | 3.25 |
| Sun et al., 2005 [41] | Europe | Sequencing | 25 | 8.00 | 0.98–26.03 | 2.28 |
| Leren et al., 2004 [42] | Europe | Sequencing | 209 | 1.44 | 0.30–4.14 | 3.39 |
| Total (random effects) | 19,725 | 5.67 | 3.68–8.05 | 100 | ||
| Heterogeneity: Chi2 = 1070.12; df = 31 (p < 0.0001); I2 = 97.10% | ||||||
| Region | GOF (%) | 95%CI | p Value | LOF (%) | 95%CI | p Value |
|---|---|---|---|---|---|---|
| Asia | 6.26 | 4.45–8.36 | <0.0001 | 22.14 | 18.04–26.70 | 0.14 |
| Africa | 7.14 | N/A | N/A | 2.24 | 0.54–5.95 | 0.17 |
| Europe | 1.84 | 0.29–4.70 | <0.0001 | 2.55 | 0.97–4.85 | <0.0001 |
| North America | 0.30 | 0.002–1.11 | 0.06 | 1.56 | 0.03–5.37 | 0.0015 |
| Oceania | 1.75 | N/A | N/A | N/A | N/A | N/A |
| Location | Chromosome Position (GRCh38) | Amino Acid Change | Nucleotide Change | Annotation | LOVD | Clinvar | Article Remark | References |
|---|---|---|---|---|---|---|---|---|
| Exon 1 | Chr1:55039847 | p.(Val4Ile) | c.10G > A | Missense | Pathogenic | Conflicting | GOF | [8,14,23] |
| Chr1:55039880-55039902 | p.(Leu21dup/tri) | c.61_63dup/triCTG | In-frame insertion | N/A | N/A | LOF | [8,13,20,23,27,31,33,35,37] | |
| Chr1:55039931 | p.(Glu32Lys) | c.94G > A | Missense | Pathogenic | Conflicting | GOF | [8,17,18,23,24,26,33] | |
| Chr1:55039940 | p.(Asp35Tyr) | c.103G > T | Missense | N/A | VUS | GOF | [31] | |
| Chr1:55039974 | p.(Arg46Leu) | c.137G > T | Missense | Likely benign | Likely benign | LOF | [16,28,34,38] | |
| Chr1:55039995 | p.(Ala53Val) | c.158C > T | Missense | Benign | Benign | LOF | [5,8,23,33] | |
| Chr1:55040022 | p.(Ala62Asp) | c.185C > A | Missense | N/A | GOF | GOF | [22] | |
| Exon 2 | Chr1:55043888 | p.(Glu85Lys) | c.253G > A | Missense | N/A | VUS | VUS | [8,15] |
| Chr1:55043902 | p.(Ser89=) | c.267G > A | Synonymous substitution | N/A | Likely benign | Likely benign | [16] | |
| Chr1:55043912 | p.(Arg93Cys) | c.277C > T | Missense | Pathogenic | Conflicting | LOF | [5,8,12,23] | |
| Chr1:55043922 | p.(Arg96Leu) | c.287G > T | Missense | N/A | N/A | GOF | [19] | |
| Chr1:55043948 | p.(Arg105Trp) | c.313C > T | Missense | N/A | VUS | GOF | [19] | |
| Chr1:55043949 | p.(Arg105Gln) | c.314G > A | Missense | VUS | VUS | LOF | [5,29] | |
| Chr1:55043951 | p.(Gly106Arg) | c.316G > A | Missense | N/A | N/A | LOF | [5,38] | |
| Chr1:55043958 | p.(Leu108Arg) | c.323T > G | Missense | N/A | GOF | GOF | [31] | |
| Chr1:55044016 | p.(Ser127Arg) | c.381T > A | Missense | N/A | GOF | GOF | [5,31,36] | |
| Chr1:55044020 | p.(Asp129Asn) | c.385G > A | Missense | Pathogenic | Conflicting | GOF | [5,8,30] | |
| Chr1:55044021 | p.(Asp129Gly) | c.386A > G | Missense | N/A | Conflicting | GOF | [5,36] | |
| Chr1:55044031 | p.(Glu132Asp) | c.396G > C | Missense | N/A | N/A | VUS | [8] | |
| Exon 3 | Chr1:55046587 | p.(Pro155Leu) | c.464C > T | Missense | N/A | VUS | VUS | [29] |
| Chr1:55046594 | p.(Asn157Lys) | c.471C > A | Missense | N/A | VUS | LOF | [5,38,42] | |
| Chr1:55046626 | p.(Ala168Glu) | c.503C > A | Missense | N/A | VUS | VUS | [36] | |
| Chr1:55046626 | p.(Ala168Val) | c.503C > T | Missense | N/A | N/A | VUS | [8] | |
| Chr1:55046640 | p.(Pro173Ser) | c.517C > T | Missense | N/A | VUS | Benign | [12] | |
| Exon 4 | Chr1:55052381 | p.(Pro209Leu) | c.626C > T | Missense | N/A | N/A | VUS | [14] |
| Chr1:55052398 | p.(Arg215His) | c.644G > A | Missense | Pathogenic | Conflicting | GOF | [8,14,21] | |
| Chr1:55052408 | p.(Arg218Ser) | c.654A > T | Missense | N/A | VUS | GOF | [5,40] | |
| Exon 5 | Chr1:55052701 | p.(Arg237Trp) | c.709C > T | Missense | VUS | Conflicting | LOF | [22,25,36,38] |
| Chr1:1:55052779 | p.(Gly263Ser) | c.787G > A | Missense | N/A | Conflicting | LOF | [8,23,33] | |
| Chr1:55052783 | p.(Thr264Ile) | c.791C > T | Missense | N/A | Conflicting | VUS | [8,23] | |
| Exon 7 | Chr1:55057404 | p.(Arg357His) | c.1070G > A | Missense | VUS | VUS | GOF | [5,40] |
| Chr1:55057454 | p.(Asp374Asn) | c.1120G > A | Missense | N/A | VUS | VUS | [21] | |
| Chr1:55057454 | p.(Asp374His) | c.1120G > C | Missense | N/A | GOF | GOF | [5,22] | |
| Chr1:55057454 | p.(Asp374Tyr) | c.1120G > T | Missense | Pathogenic | GOF | GOF | [5,32,41,42] | |
| Exon 8 | Chr1:55058106 | p.(His417Gln) | c.1251C > A | Missense | N/A | Conflicting | GOF | [5,25] |
| Chr1:55058125 | p.(Ile424Val) | c.1270A > G | Missense | N/A | Likely benign | VUS | [8,23] | |
| Chr1:55058182 | p.(Ala443Thr) | c.1327G > A | Missense | Benign | Conflicting | LOF | [5,40] | |
| Exon 9 | Chr1:55058524 | p.(Val460=) | c.1380A > G | Synonymous substitution | Benign | Benign | VUS | [37] |
| Chr1:55058543 | p.(Pro467Ala) | c.1399C > G | Missense | Pathogenic | Conflicting | GOF | [22] | |
| Chr1:55058549 | p.(Arg469Trp) | c.1405C > T | Missense | Pathogenic | Conflicting | GOF | [21,25,40] | |
| Chr1:55058564 | p.(Val474Ile) | c.1420G > A | Missense | Benign | Likely benign | LOF | [5,33,37] | |
| Chr1:55058576 | p.(Ala478Thr) | c.1432G > A | Missense | N/A | Conflicting | VUS | [8] | |
| Chr1:55058630 | p.(Arg496Trp) | c.1486C > T | Missense | Pathogenic | Conflicting | GOF | [8,21,23] | |
| Chr1:55058639 | p.(Arg499Cys) | c.1495C > T | Missense | N/A | VUS | VUS | [15] | |
| Chr1:55058640 | p.(Arg499His) | c.1496G > A | Missense | N/A | VUS | VUS | [13] | |
| Exon 10 | Chr1:1:55059492 | p.(Gly504Trp) | c.1510G > T | Missense | N/A | VUS | VUS | [8,23] |
| Chr1:55059519 | p.(Asn513Asp) | c.1537A > G | Missense | N/A | VUS | VUS | [25] | |
| Exon 11 | Chr1:55061485 | p.(Ala598Thr) | c.1792G > A | Missense | VUS | VUS | LOF | [12] |
| Exon 12 | Chr1:55063391 | p.(Gly629Asp) | c.1886G > A | Missense | N/A | N/A | VUS | [8] |
| Chr1:55063435 | p.(Val644Ile) | c.1930G > A | Missense | N/A | N/A | VUS | [8] | |
| Chr1: 55063450 | p.(Ala649Thr) | c.1945G > A | Missense | N/A | N/A | VUS | [8] | |
| Chr1:55063459 | p.(Asn652Asp) | c.1954A > G | Missense | N/A | VUS | Benign | [12] | |
| Chr1:55063509 | p.(Ser668Arg) | c.2004C > A | Missense | N/A | Conflicting | LOF | [8,23,33] | |
| Chr1:55063514 | p.(Gly670Lys) | c.2009G > A | Missense | Benign | Likely benign | VUS | [33,39] | |
| Chr1: 55063550 | p.(Arg682Gln) | c.2045G > A | Missense | N/A | N/A | VUS | [8] |
| ASIAN | GAIN-OF-FUNCTION | |||||
| Location (GRCh38) | Nucleotide Change | Amino Acid Change | Proportion (%) | 95%CI | p Value | |
| Chr1:55039847 | c.10G > A | p.(Val4Ile) | 3.63 | 0.31–10.35 | 0.02 | |
| Chr1:55039931 | c.94G > A | p.(Glu32Lys) | 6.58 | 5.77–7.44 | 0.62 | |
| Chr1:55043922 | c.287G > T | p.(Arg96Leu) | 0.46 | N/A | N/A | |
| Chr1:55043948 | c.313C > T | p.(Arg105Trp) | 0.46 | N/A | N/A | |
| Chr1:55044020 | c.385G > A | p.(Asp129Asn) | 0.15 | N/A | N/A | |
| Chr1:55052398 | c.644G > A | p.(Arg215His) | 0.33 | 0.05–1.05 | 0.18 | |
| Chr1:55058630 | c.1486C > T | p.(Arg496Trp) | 0.68 | 0.25–1.48 | 0.95 | |
| LOSS-OF-FUNCTION | ||||||
| Chr1:55039902-55039903 | c.63_64insCTG | p.(Leu21dup/tri) | 16.20 | 6.91–28.44 | 0.0022 | |
| Chr1:55039995 | c.158C > T | p.(Ala53Val) | 5.63 | 1.34–12.61 | 0.09 | |
| Chr1:55043912 | c.277C > T | p.(Arg93Cys) | 10.66 | 3.28–56.19 | 0.0001 | |
| Chr1:55043948 | c.314G > A | p.(Arg105Gln) | 9.09 | N/A | N/A | |
| Chr1:55052698 | c.787G > A | p.(Gly263Ser) | 2.01 | 0.71–4.41 | 0.24 | |
| Chr1:55058564 | c.1420G > A | p.(Val474Ile) | 7.27 | N/A | N/A | |
| Chr1:55061485 | c.1792G > A | p.(Ala598Thr) | 5.26 | N/A | N/A | |
| Chr1:55063509 | c.2004C > A | p.(Ser668Arg) | 0.93 | 0.17–2.89 | 0.28 | |
| EUROPEAN | GAIN-OF-FUNCTION | |||||
| Chr1:55039940 | c.103G > T | p.(Asp35Tyr) | 1.33 | N/A | N/A | |
| Chr1:55043958 | c.323T > G | p.(Leu108Arg) | 1.33 | N/A | N/A | |
| Chr1:55044016 | c.381T > A | p.(Ser127Arg) | 1.33 | N/A | N/A | |
| Chr1:55044020 | c.385G > A | p.(Asp129Asn) | 0.60 | N/A | N/A | |
| Chr1:55052408 | c.654A > T | p.(Arg218Ser) | 0.77 | N/A | N/A | |
| Chr1:55057404 | c.1070G > A | p.(Arg357His) | 0.77 | N/A | N/A | |
| Chr1:55057454 | c.1120G > T | p.(Asp374Tyr) | 1.44 | 0.001–5.84 | 0.0001 | |
| Chr1:55058549 | c.1405C > T | p.(Arg469Trp) | 0.77 | N/A | N/A | |
| LOSS-OF-FUNCTION | ||||||
| Chr1:55039902-55039903 | c.63_64insCTG | p.(Leu21dup/tri) | 4.17 | 0.10–9.40 | 0.03 | |
| Chr1:55039974 | c.137G > T | p.(Arg46Leu) | 2.11 | 0.36–5.25 | 0.0009 | |
| Chr1:55046594 | c.471C > A | p.(Asn157Lys) | 0.41 | 0.07–1.02 | 0.50 | |
| Chr1:55052701 | c.709C > T | p.(Arg237Trp) | 0.45 | N/A | N/A | |
| Chr1:55058182 | c.1327G > A | p.(Ala443Thr) | 0.77 | N/A | N/A | |
| Chr1:55058564 | c.1420G > A | p.(Val474Ile) | 18.75 | N/A | N/A | |
| AMERICAN | GAIN-OF-FUNCTION | |||||
| Chr1:55052398 | c.644G > A | p.(Arg215His) | 0.02 | N/A | N/A | |
| Chr1:55058106 | c.1251C > A | p.(His417Gln) | 0.32 | N/A | N/A | |
| Chr1:55058549 | c.1405C > T | p.(Arg469Trp) | 0.32 | N/A | N/A | |
| Chr1:55058630 | c.1486C > T | p.(Arg496Trp) | 0.1 | N/A | N/A | |
| Exon | GOF (%) | 95%CI | p Value | LOF (%) | 95%CI | p Value |
|---|---|---|---|---|---|---|
| 1 | 6.46 | 4.89–8.23 | 0.0007 | 6.54 | 3.36–10.67 | <0.0001 |
| 2 | 1.13 | 0.29–2.48 | 0.04 | 10.91 | 0.05–41.07 | <0.0001 |
| 3 | N/A | N/A | N/A | 0.41 | 0.08–1.23 | 0.50 |
| 4 | 0.28 | 0.01–0.91 | 0.02 | N/A | N/A | N/A |
| 5 | N/A | N/A | N/A | 1.02 | 0.45–1.96 | 0.10 |
| 6 | N/A | N/A | N/A | N/A | N/A | N/A |
| 7 | 1.78 | 0.15–5.16 | <0.0001 | N/A | N/A | N/A |
| 8 | 0.32 | N/A | N/A | 0.77 | N/A | N/A |
| 9 | 0.42 | 0.11–0.94 | 0.03 | 12.69 | 3.80–25.77 | 0.12 |
| 10 | N/A | N/A | N/A | N/A | N/A | N/A |
| 11 | N/A | N/A | N/A | 5.26 | N/A | N/A |
| 12 | N/A | N/A | N/A | 0.93 | 0.17–2.89 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, N.H.; Truong, P.K.; Lao, T.D.; Le, T.A.H. Proprotein Convertase Subtilisin/Kexin Type 9 Gene Variants in Familial Hypercholesterolemia: A Systematic Review and Meta-Analysis. Processes 2021, 9, 283. https://doi.org/10.3390/pr9020283
Pham NH, Truong PK, Lao TD, Le TAH. Proprotein Convertase Subtilisin/Kexin Type 9 Gene Variants in Familial Hypercholesterolemia: A Systematic Review and Meta-Analysis. Processes. 2021; 9(2):283. https://doi.org/10.3390/pr9020283
Chicago/Turabian StylePham, Nang Hoang, Phuong Kim Truong, Thuan Duc Lao, and Thuy Ai Huyen Le. 2021. "Proprotein Convertase Subtilisin/Kexin Type 9 Gene Variants in Familial Hypercholesterolemia: A Systematic Review and Meta-Analysis" Processes 9, no. 2: 283. https://doi.org/10.3390/pr9020283
APA StylePham, N. H., Truong, P. K., Lao, T. D., & Le, T. A. H. (2021). Proprotein Convertase Subtilisin/Kexin Type 9 Gene Variants in Familial Hypercholesterolemia: A Systematic Review and Meta-Analysis. Processes, 9(2), 283. https://doi.org/10.3390/pr9020283






