Optical Chemical Sensor Based on 2,2-Furildioxime in Sol-Gel Matrix for Determination of Ni2+ in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sol-Gel Matrix
2.3. Analytical Procedure
3. Results and Discussion
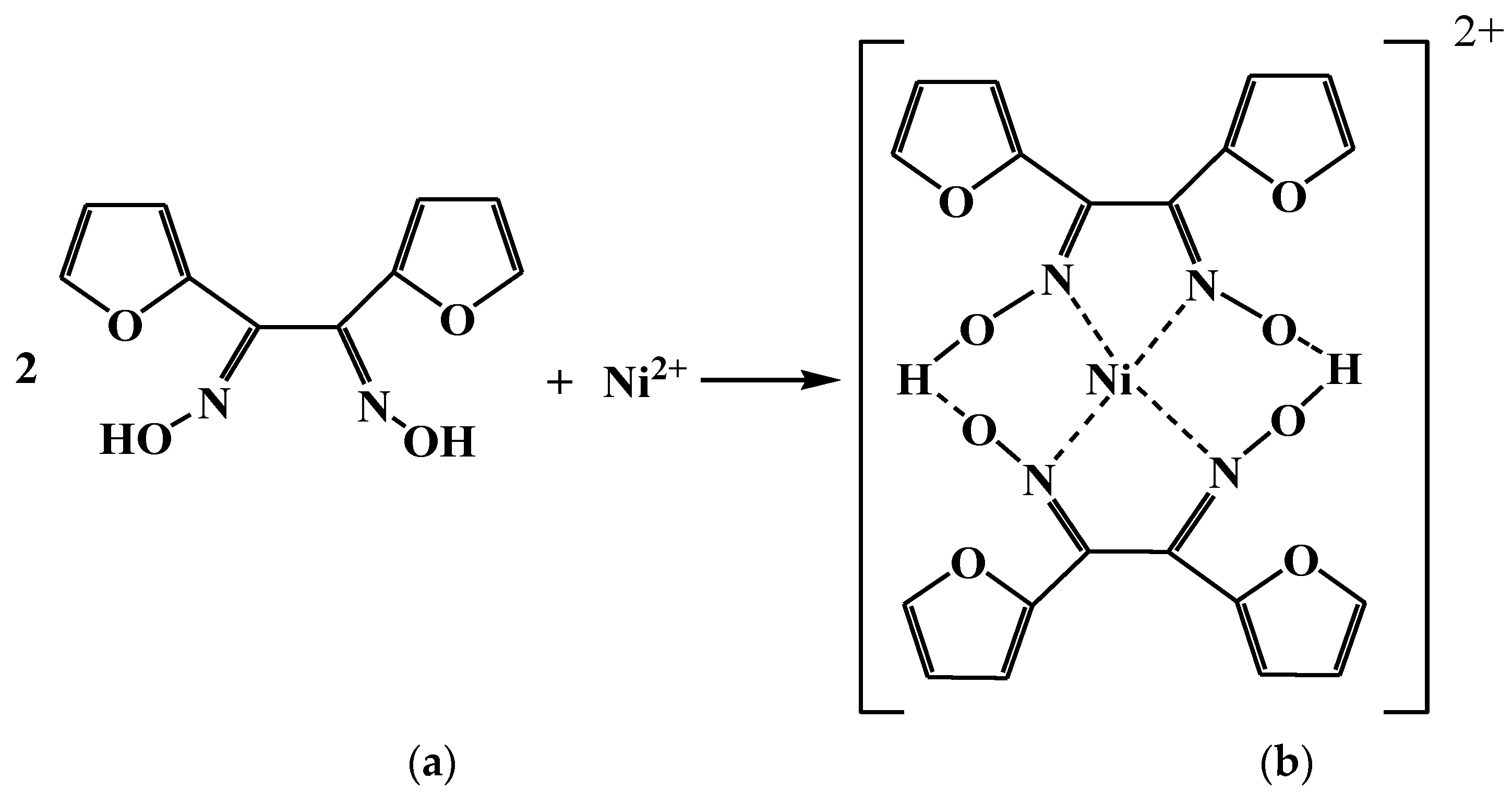
3.1. Characterization of Sensor
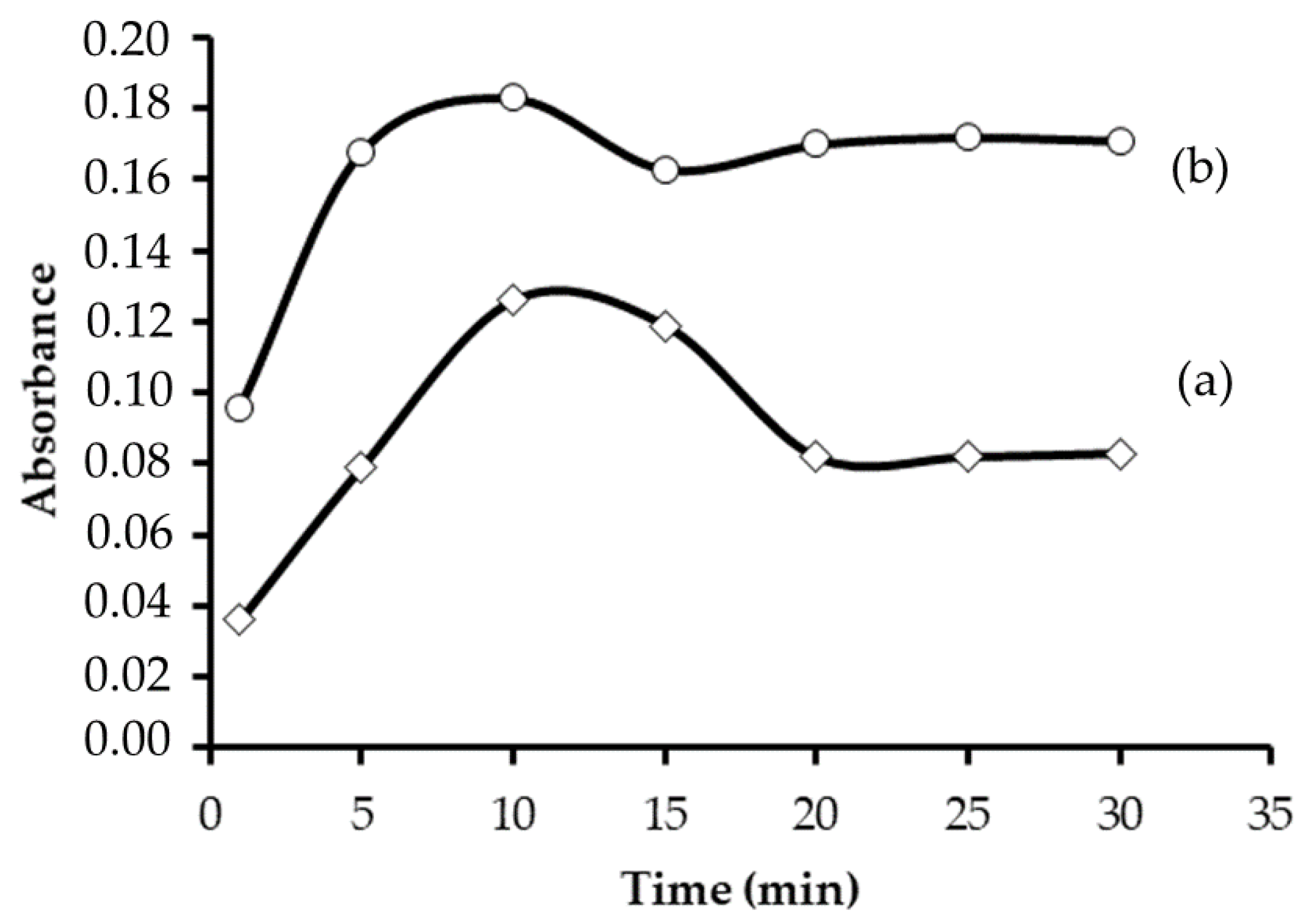
3.2. Effect of pH and Contact Time
3.3. Calibration Curve of the Sensor
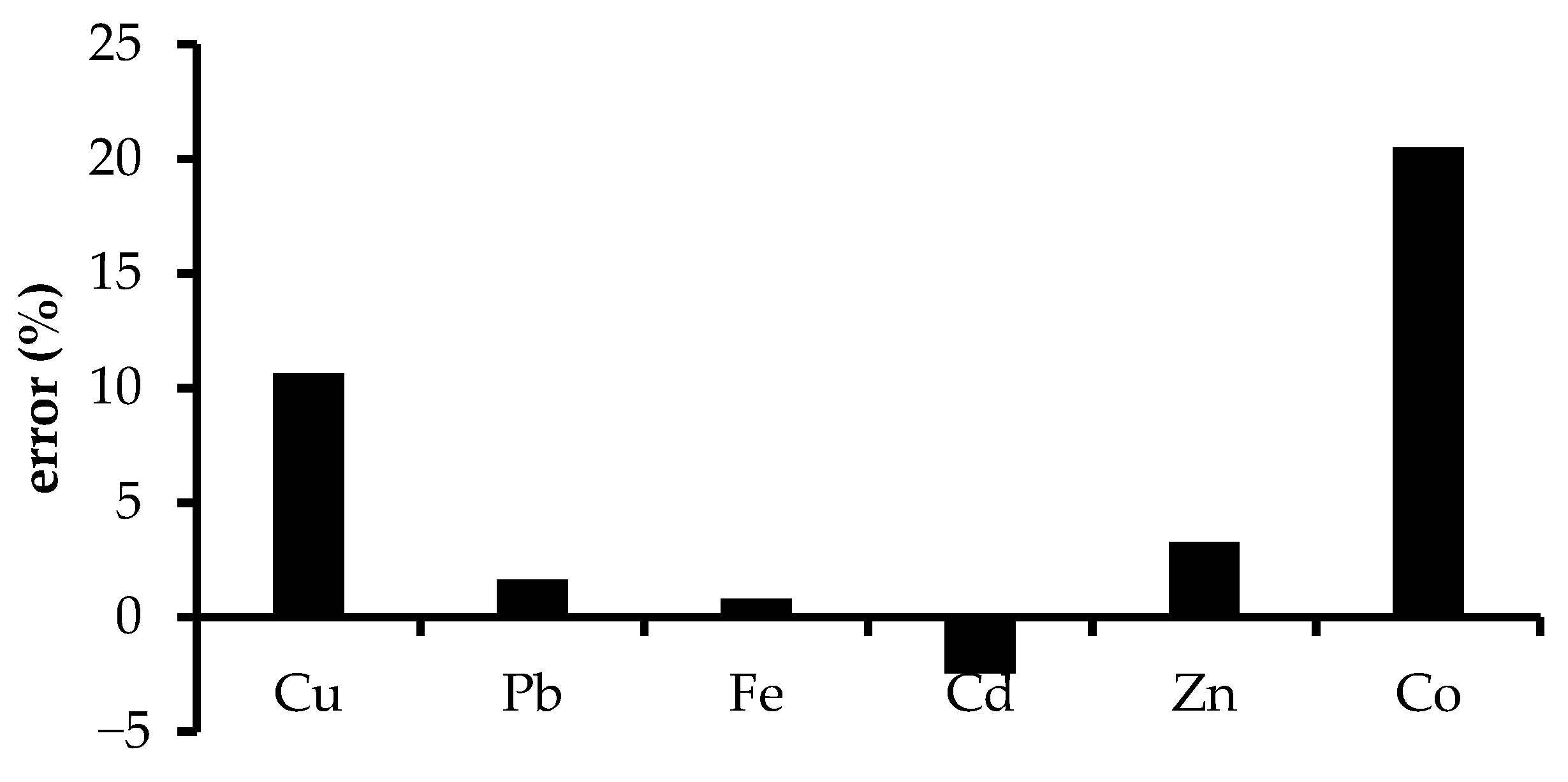
3.4. Selectivity
3.5. Precision and Accuracy
3.6. Application of Sensor in Seawater Sample
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Parsaee, Z. Electrospin nanofibers decorated with bio-sonochemically synthesized gold nanoparticles as an ultrasensitive probe in amalgam-based mercury (II) detection system. Ultrason. Sonochem. 2018, 44, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Parsaee, Z.; Haratipour, P.; Lariche, M.J.; Vojood, A. A novel highly performance nano chemosensor for copper (II) ion based on an ultrasound-assisted synthesized diphenylamine-based Schiff base: Design, fabrication and density functional theory calculations. Ultrason. Sonochem. 2018, 41, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Shahamiri, A.; Hajati, S.; Mirtamizdoust, B. A novel PVC-membrane optical sensor for highly sensitive and selective determination of Cu2+ ion based on synthesized (E)-N′-(pyridin-2-ylmethylene) isonicotin-ohydrazide. J. Mol. Liq. 2014, 199, 483–488. [Google Scholar] [CrossRef]
- Hashemi, P.; Zarjani, R.A. A wide range pH optical sensor with mixture of neutral red and thionin immobilized on an agarose film coated glass slide. Sens. Actuators B Chem. 2008, 135, 112–115. [Google Scholar] [CrossRef]
- Kim, M.D.; Dergunov, S.A.; Lindner, E.; Pinkhassik, E. Dye-loaded porous nano-capsules immobilized in a permeable polyvinyl alcohol matrix: A versatile optical sensor platform. Anal. Chem. 2012, 84, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Chen, T. Phenol red immobilized PVA membrane for an optical pH sensor with two determination ranges and long-term stability. Sens. Actuators B Chem. 2005, 107, 311–316. [Google Scholar] [CrossRef]
- Dashtian, K.; Zare-Dorabei, R. Preparation and characterization of a novel optical chemical sensor for determination of trace amounts of Praseodymium ion by UV/Vis spectrophotometry. Sens. Actuators B Chem. 2017, 242, 586–594. [Google Scholar] [CrossRef]
- Azeman, N.H.; Arsad, N.; Bakar, A.A. Polysaccharides as the sensing material for metal ion detection-based optical sensor applications. Sensors 2020, 20, 3924. [Google Scholar] [CrossRef]
- Shahamirifard, S.A.; Ghaedi, M.; Montazerozohori, M. Design a sensitive optical thin film sensor based on incorporation of isonicatinohydrazide derivative in sol-gel matrix for determination of trace amounts of copper (II) in fruit juice: Effect of sonication time on immobilization approach. Ultrason. Sonochem. 2018, 42, 723–730. [Google Scholar] [CrossRef]
- Maybodi, A.S.; Rezaei, V. A new sol-gel optical sensor with nonporous structure for determination of trace zinc. Sens. Actuators B Chem. 2014, 199, 418–423. [Google Scholar] [CrossRef]
- Parsaee, Z.; Karachi, N.; Razavi, R. Ultrasound assisted fabrication of a novel optode base on a triazine based schiff base immobilized on TEOS for copper detection. Ultrason. Sonochem. 2018, 47, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Shahamirifard, S.A.; Ghaedi, M.; Hajati, S. A new silver (I) ions optical sensor based on nanoporous thin film of sol-gel by Rose Bengal dye. Sens. Actuators B Chem. 2018, 259, 20–29. [Google Scholar] [CrossRef]
- Lev, O.; Tsionsky, M.; Rabinovich, L.; Glezer, V.; Sampath, S.; Pankratov, I.; Gun, J. Organically modified sol-gel sensors. Anal. Chem. 1995, 67, 22A–30A. [Google Scholar] [CrossRef]
- Darmawan, A.; Smart, S.; Julbe, A.; da Costa, J.C.D. Iron oxide silica derived from sol-gel synthesis. Materials 2011, 4, 448–456. [Google Scholar] [CrossRef]
- Anonymous. Ministry of Environment Republic of Indonesia No.9 Year 2006 Concerning Wastewater Quality Standards for Nickel ore Mining in Business and Activities; 2006.
- Fay, M.; Wilbur, S.; Abadin, H.; Ingerman, L.; Swarts, S.G. Toxicological Profile for Nickel; Agency for Toxic Substances and Disease Registry (ATSDR): Atlanta, Georgia, 2005. [Google Scholar]
- Sugiyono, A. Overview of nickel industry in Indonesia. MAMBERAMO NOW 1998, 2, 1–8. [Google Scholar]
- Silva, E.L.; Roldan, P.D.S.; Gine, M.F. Simultaneous preconcentration of copper, cadmium and nickel in Water Samples by Cloud Point Extraction using 4-(2-pyridylazo)-resorcinol and their determination by inductively coupled plasma optic emission spectrometry. Hazard. Mater. 2009, 171, 1133–1138. [Google Scholar] [CrossRef]
- Mizuguchi, H.; Zhang, Y.-F.; Onodera, H.; Nishizawa, S.; Shida, J. On-site determination of trace nickel in liquid sample for semiconductor manufacturing by highly sensitive solid-phase colorimetry with α-furil dioxime. Chem. Lett. 2008, 37, 792–793. [Google Scholar] [CrossRef]
- Bobrowski, A. Catalytic adsorptive stripping voltammetric determination of cobalt and nickel as their Furil dioxime complexes. Electroanalysis 2004, 16, 1536–1541. [Google Scholar] [CrossRef]
- Murthy, Y.L.N.; Govindh, B.; Diwakar, B.S.; Nagalakshmi, K. A simple inexpensive detection method of Nickel in Water using Optical Sensor. Int. J. Chem. Tech. Res. 2011, 3, 1285–1291. [Google Scholar]
- Hakim, M.S. Colorimetric Detection of Ni(II) Using Thin Film Sensor with Ligand Alfa-Furildioxime in Silica Gel Matrix. Master’s Thesis, Universitas Gadjah Mada, Yogyakarta, Indonesia, 30 August 2019. [Google Scholar]
- De Sousa, C.S.; Korn, M. Effects of ultrasonic irradiation on the spectrophotometric determination of nickel with dimethylglyoxime. Anal. Chim. Acta 2001, 444, 309–315. [Google Scholar] [CrossRef]
- Memon, N.; Memon, S.; Solangi, A.R.; Soomro, R.; Soomro, R. Single-channel flow injection spectrophotometric determination of nickel using furildioxime in micellar solution. Sci. World. J. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed]




| Element | Percentage (%) | |
|---|---|---|
| Before | After Ni2+ Detection | |
| C | 17.85 | 16.82 |
| O | 62.37 | 60.68 |
| Ni | n.d. | 0.10 |
| Si | 19.78 | 22.40 |
| Water Sample | Sensor Method | Recovery (%) | |
|---|---|---|---|
| Ni2+ Added (mg L−1) | Ni2+ Founded (mg L−1) | ||
| A | 0 | 0.25 | - |
| 1 | 1.07 | 83.19 | |
| 3 | 3.03 | 92.95 | |
| B | 0 | 0.35 | - |
| 1 | 1.19 | 83.87 | |
| 3 | 3.02 | 89.22 | |
| Sample B | Optical Sensor | AAS | tcount |
|---|---|---|---|
| Ni2+ (mg L−1) | Ni2+ (mg L−1) | ||
| 1 | 0.331 | 0.364 | 0.218 |
| 2 | 0.352 | 0.458 | |
| 3 | 0.373 | 0.271 | |
| Average | 0.352 ± 0.020 | 0.364 ± 0.093 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suherman, S.; Hakim, M.S.; Kuncaka, A. Optical Chemical Sensor Based on 2,2-Furildioxime in Sol-Gel Matrix for Determination of Ni2+ in Water. Processes 2021, 9, 280. https://doi.org/10.3390/pr9020280
Suherman S, Hakim MS, Kuncaka A. Optical Chemical Sensor Based on 2,2-Furildioxime in Sol-Gel Matrix for Determination of Ni2+ in Water. Processes. 2021; 9(2):280. https://doi.org/10.3390/pr9020280
Chicago/Turabian StyleSuherman, Suherman, Muh. Supwatul Hakim, and Agus Kuncaka. 2021. "Optical Chemical Sensor Based on 2,2-Furildioxime in Sol-Gel Matrix for Determination of Ni2+ in Water" Processes 9, no. 2: 280. https://doi.org/10.3390/pr9020280
APA StyleSuherman, S., Hakim, M. S., & Kuncaka, A. (2021). Optical Chemical Sensor Based on 2,2-Furildioxime in Sol-Gel Matrix for Determination of Ni2+ in Water. Processes, 9(2), 280. https://doi.org/10.3390/pr9020280






