Solubility and Activation of Hydrogen in the Non-Catalytic Upgrading of Venezuela Orinoco, China Liaohe, and China Fengcheng Atmospheric Residues
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Upgrading Processing
2.3. Determination of Hydrogen Transfer Ability
2.4. Preparation of Metalloporphyrin-Anthracene Mixtures
3. Results and Discussion
3.1. Hydrogen Solubility in Reaction
3.2. Hydrogen Transfer of the Residues
3.3. Activation of the Hydrogen
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.; Que, G. Determination of Hydrogen Solubility in Petroleum Fractions. Pet. Refin. Eng. 1999, 29, 33–36. [Google Scholar]
- Cai, H.Y.; Shaw, J.M.; Chung, K.H. Hydrogen Solubility Measurements in Heavy Oil and Bitumen Cuts. Fuel 2001, 80, 1055–1063. [Google Scholar] [CrossRef]
- Florusse, L.J.; Peters, C.J.; Pàmies, J.C.; Vega, L.F.; Meijer, H. Solubility of Hydrogen in Heavy n-Alkanes: Experiments and Saft Modeling. AIChE J. 2003, 49, 3260–3269. [Google Scholar] [CrossRef]
- Ji, S.; Wang, Z.; Guo, A.; Zhou, Y.; Chen, K. Determination of Hydrogen Solubility in Heavy Fractions of Crude Oils by a Modified Direct Method. J. Chem. Eng. Data 2013, 58, 3453–3457. [Google Scholar] [CrossRef]
- Nasery, S.; Barati-Harooni, A.; Tatar, A.; Najafi-Marghmaleki, A.; Mohammadi, A.H. Accurate Prediction of Solubility of Hydrogen in Heavy Oil Fractions. J. Mol. Liq. 2016, 222, 933–943. [Google Scholar] [CrossRef]
- Bai, X.; Guo, R.; Zeng, Z.; Chen, Z.; Zhang, L.; Xu, Z.; Xu, C. Effect of Asphaltene Contents on Hydrogen Solubility in Heavy Oils. CIESC J. 2019, 70, 4012–4020. [Google Scholar]
- Liu, Y.; Liu, C. Effects of hydrogen sulphide on residue hydrodemetallization. ACTA Pet. Sin. 2010, 26, 389–393. [Google Scholar]
- Guo, A.; Wang, Z.; Zhang, H.; Zhang, X.; Wang, Z. Hydrogen transfer and coking propensity of petroleum residues under thermal processing. Energy Fuels 2010, 24, 3093–3100. [Google Scholar] [CrossRef]
- Rankel, L.A.; Rollmann, L.D. Catalytic activity of metals in petroleum and their removal. Fuel 1983, 62, 44–46. [Google Scholar] [CrossRef]
- Gao, Y.; Shen, B.; Liu, J. Distribution of nickel and vanadium in venezuela crude oil. Pet. Sci. Technol. 2013, 31, 509–515. [Google Scholar] [CrossRef]
- Ji, S.; Zhou, Y.; Ge, D.; Chen, K.; Wang, Z. Synergism of Hydrogen and Oil Composition in Noncatalytic Upgrading of Petroleum Residues. Pet. Sci. Technol. 2014, 32, 2903–2910. [Google Scholar] [CrossRef]
- Park, J.; Robinson, R.L., Jr.; Gasem, K.A.M. Solubilities of Hydrogen in Heavy Normal Paraffins at Temperatures from 323.2 to 423.2 K and Pressures to 17.4 MPa. J. Chem. Eng. Data 1995, 40, 241–244. [Google Scholar] [CrossRef]
- Park, J.; Robinson, R.L., Jr.; Gasem, K.A.M. Solubilities of Hydrogen in Aromatic Hydrocarbons from 323 to 433 K and Pressures to 21.7 MPa. J. Chem. Eng. Data 1996, 41, 70–73. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Cleanliness and Compatibility of Residual Fuels by Spot Test. ASTM D4740-04. Available online: https://www.antpedia.com/standard/6926892.html (accessed on 15 March 2021).
- Ali, M.F.; Abbas, S. A review of methods for the demetallization of residual fuel oils. Fuel Process. Technol. 2006, 87, 573–584. [Google Scholar] [CrossRef]

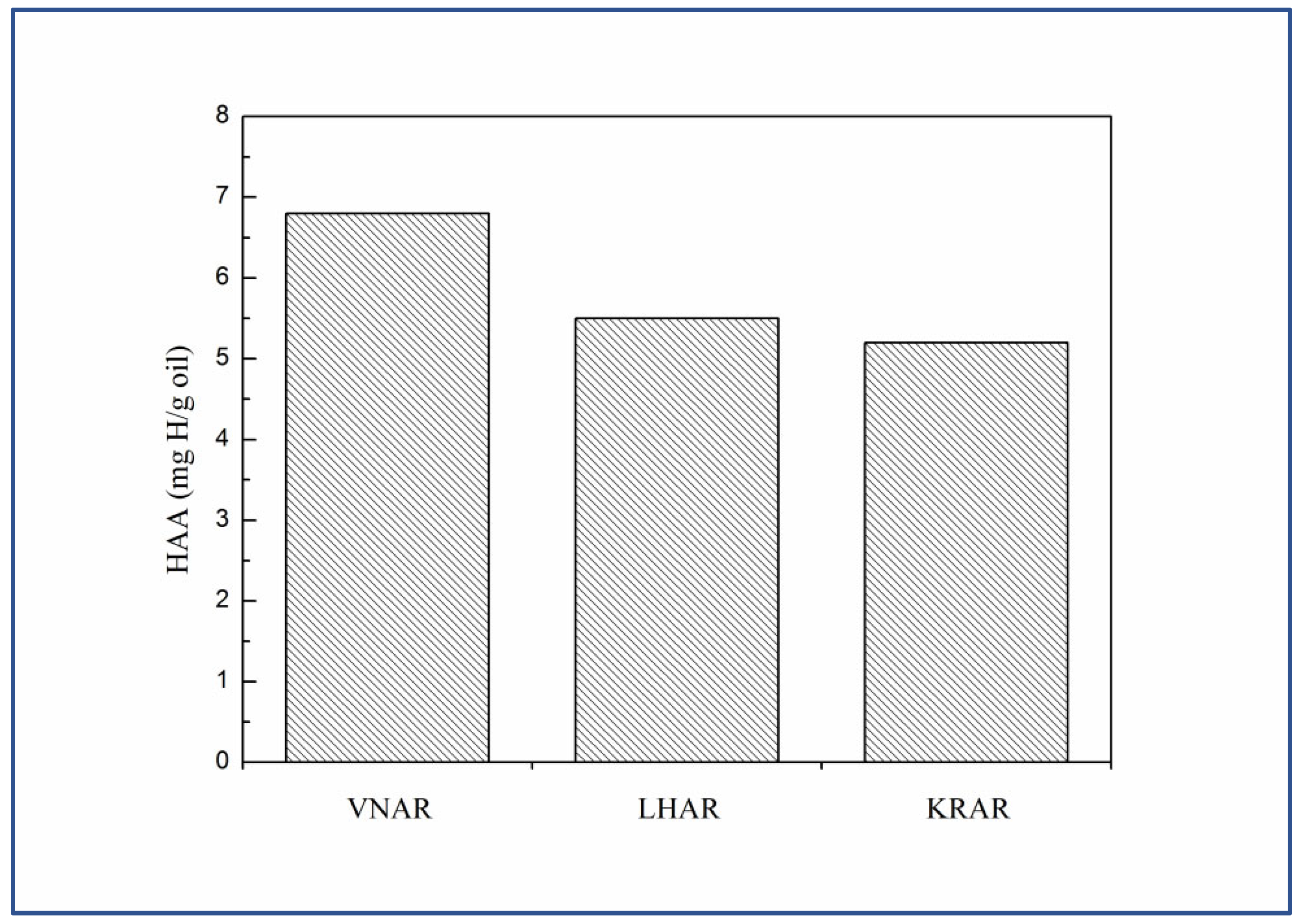

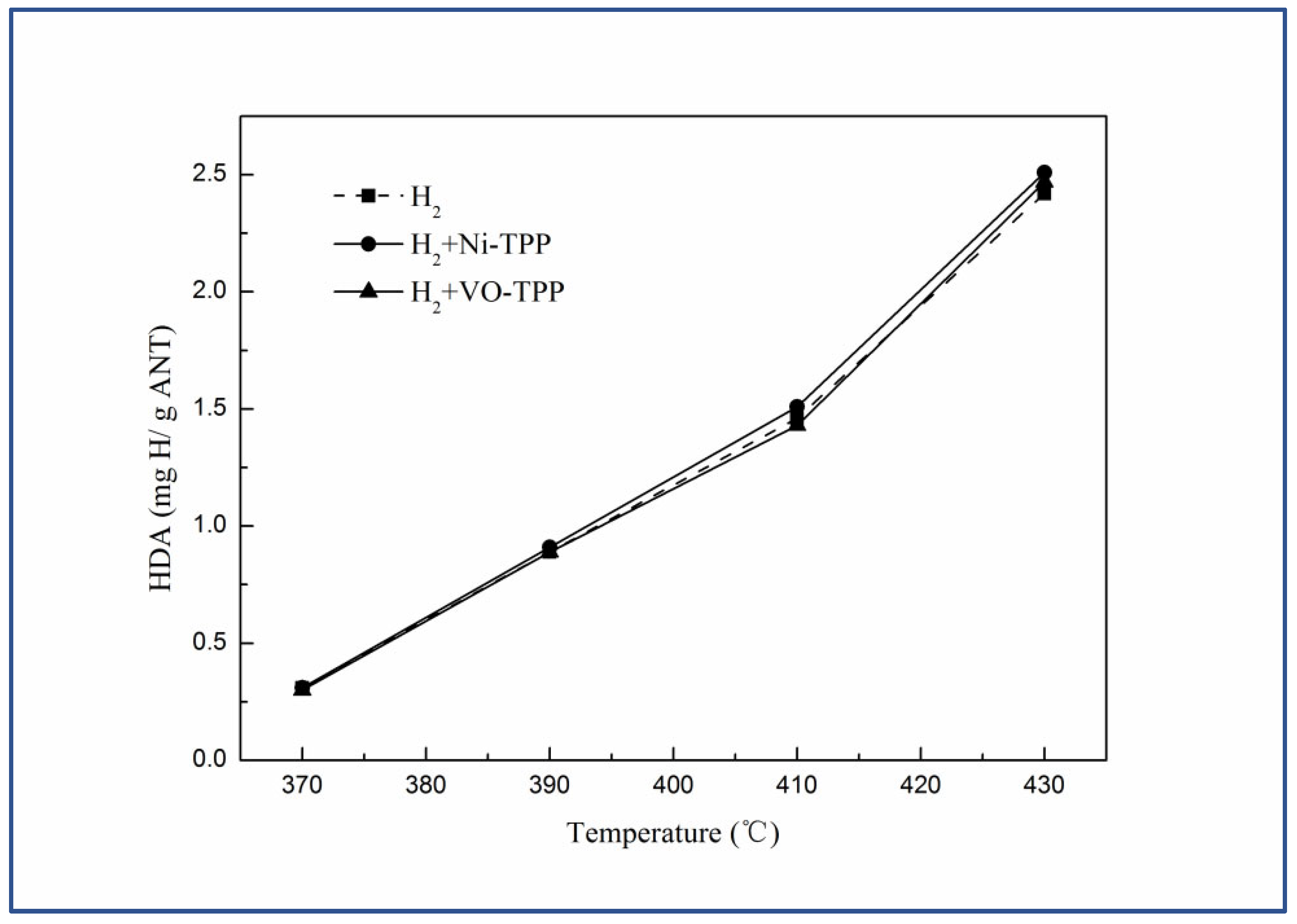

| Sample | VNAR | LHAR | KRAR | P-VNAR | P-LHAR | P-KRAR |
|---|---|---|---|---|---|---|
| Density, g/cm3 (20 °C) | 1.0297 | 0.9821 | 0.9650 | |||
| dynamic viscosity, mPa·s (100 °C) | 2995.0 | 476.6 | 765.3 | 249.3 | 155.5 | 126.6 |
| molecular weight | 781.7 | 686.3 | 736.8 | 586.6 | 620.7 | 702.9 |
| elemental analysis | ||||||
| C, wt % | 83.82 | 86.83 | 85.89 | 84.28 | 86.86 | 86.55 |
| H, wt % | 9.96 | 11.42 | 11.53 | 10.18 | 11.49 | 12.03 |
| S, wt % | 4.31 | 0.36 | 0.36 | 3.76 | 0.38 | 0.23 |
| N, wt % | 0.71 | 0.86 | 0.92 | 0.81 | 0.97 | 0.83 |
| H/C atomic ratio | 1.416 | 1.567 | 1.600 | 1.439 | 1.576 | 1.656 |
| Nickel, ppmw | 113 | 68.7 | 43.5 | |||
| Vanadium, ppmw | 417 | 1.81 | 0.77 | |||
| average structural parameters a | ||||||
| Aromaticity, % | 0.333 | 0.247 | 0.216 | 0.327 | 0.243 | 0.190 |
| number of paraffinic rings | 3.509 | 2.726 | 3.194 | 2.714 | 2.551 | 2.642 |
| number of aromatic rings | 4.039 | 2.568 | 2.341 | 2.864 | 2.227 | 1.911 |
| boiling range, °C | ||||||
| IBP~350 | 1.5 | 1.8 | 1.5 | 10.7 | 5.0 | 6.0 |
| 350~420 | 3.5 | 5.2 | 10.1 | 18.3 | 9.5 | 11.7 |
| 420~500 | 19.0 | 24.0 | 20.9 | 26.0 | 27.0 | 22.8 |
| 500~FBP | 76.0 | 69.0 | 67.5 | 45.0 | 58.5 | 59.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, S.; Zeng, A. Solubility and Activation of Hydrogen in the Non-Catalytic Upgrading of Venezuela Orinoco, China Liaohe, and China Fengcheng Atmospheric Residues. Processes 2021, 9, 2274. https://doi.org/10.3390/pr9122274
Ji S, Zeng A. Solubility and Activation of Hydrogen in the Non-Catalytic Upgrading of Venezuela Orinoco, China Liaohe, and China Fengcheng Atmospheric Residues. Processes. 2021; 9(12):2274. https://doi.org/10.3390/pr9122274
Chicago/Turabian StyleJi, Shunfeng, and Anran Zeng. 2021. "Solubility and Activation of Hydrogen in the Non-Catalytic Upgrading of Venezuela Orinoco, China Liaohe, and China Fengcheng Atmospheric Residues" Processes 9, no. 12: 2274. https://doi.org/10.3390/pr9122274
APA StyleJi, S., & Zeng, A. (2021). Solubility and Activation of Hydrogen in the Non-Catalytic Upgrading of Venezuela Orinoco, China Liaohe, and China Fengcheng Atmospheric Residues. Processes, 9(12), 2274. https://doi.org/10.3390/pr9122274






