Regulation of Hydrogen Peroxide Dosage in a Heterogeneous Photo-Fenton Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Analytical Methods
2.3. Reaction System
2.4. Experimental Procedure
- Open-Loop (OL) (Modeling) experiments: The reaction time began when the peristaltic pump started dosing 0.1 mL/min of a solution of HO with a concentration of 1000 ppm. The initial concentration of HO in the reactor was zero. Samples were taken through time;
- Closed-Loop (CL) (Automatic Control) experiments: The HO dosing was carried out automatically following the control law depicted in the following sections. The online DO measurement, which was used as an indirect control variable instead of true RC measurements, was measured every 5 min to calculate the new HO flow. The reaction started when the peristaltic pump was operated with the first calculated flow. During experiments, samples were taken to analyze the different variables.
2.5. Instrumentation
2.6. Modeling and Control
3. Results and Discussions
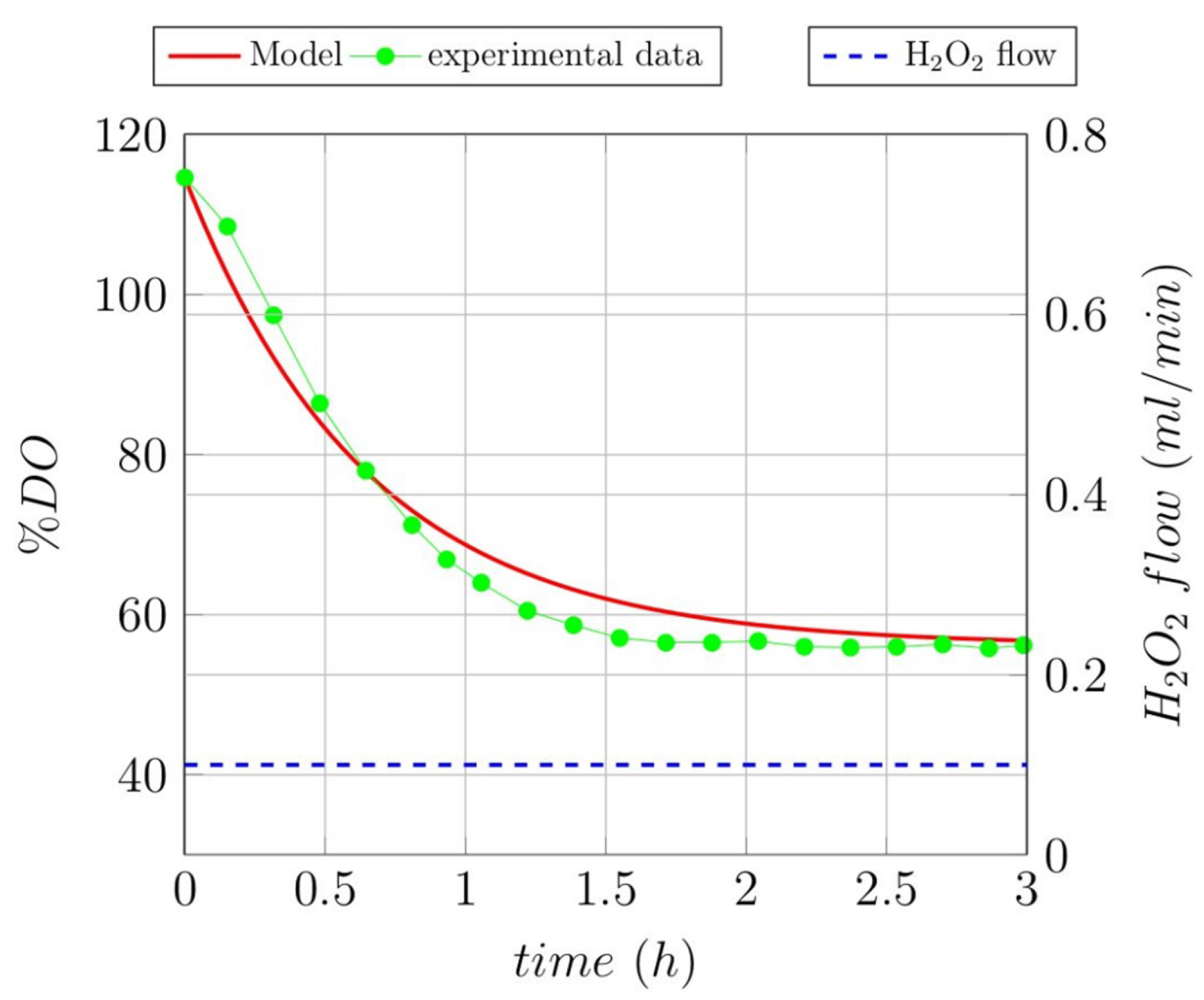
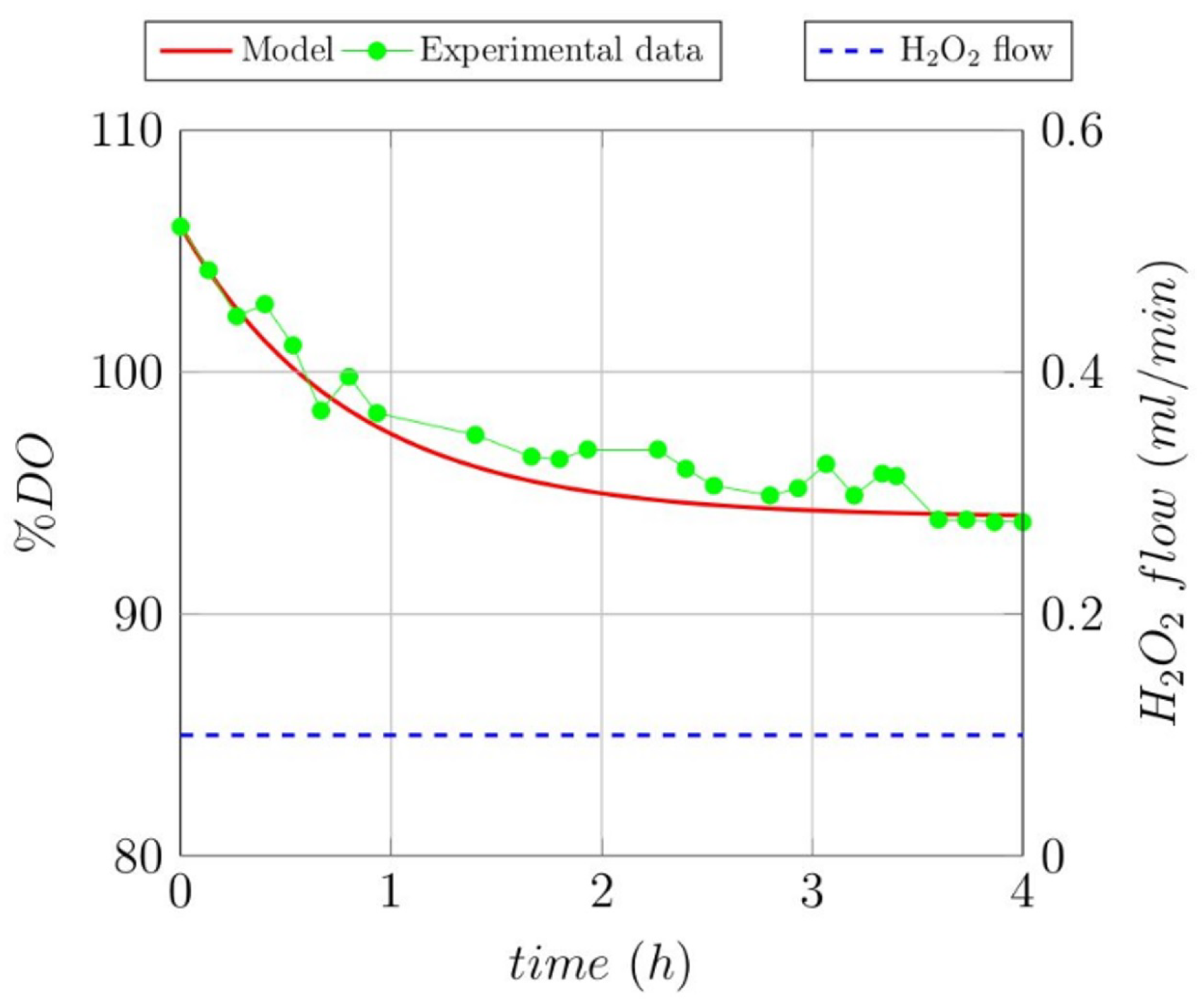
3.1. Open-Loop Experiments
3.2. Modeling Ol Degradation of the Studied Recalcitrant Compounds
3.3. Controllers Design by the Direct Synthesis Method
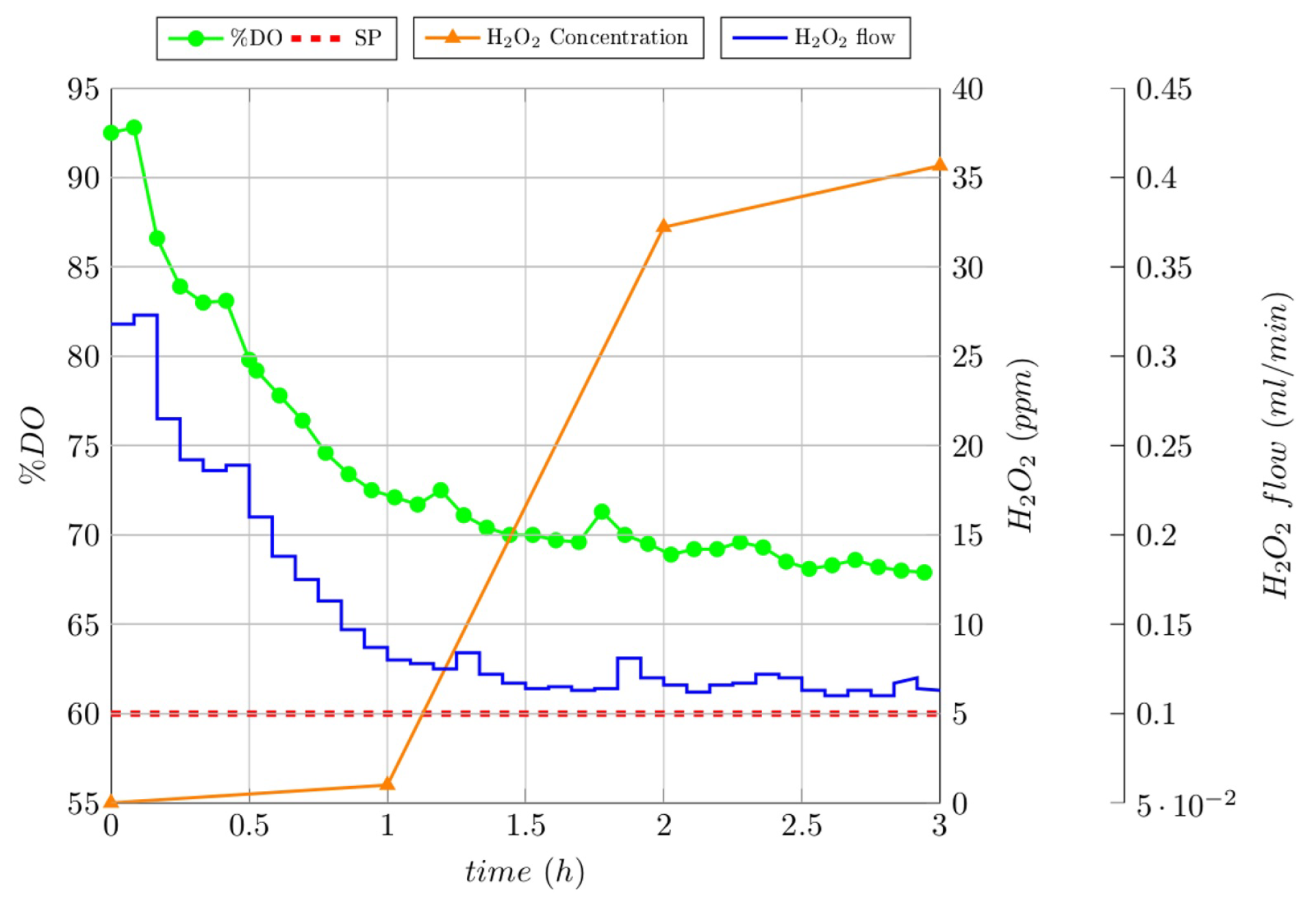
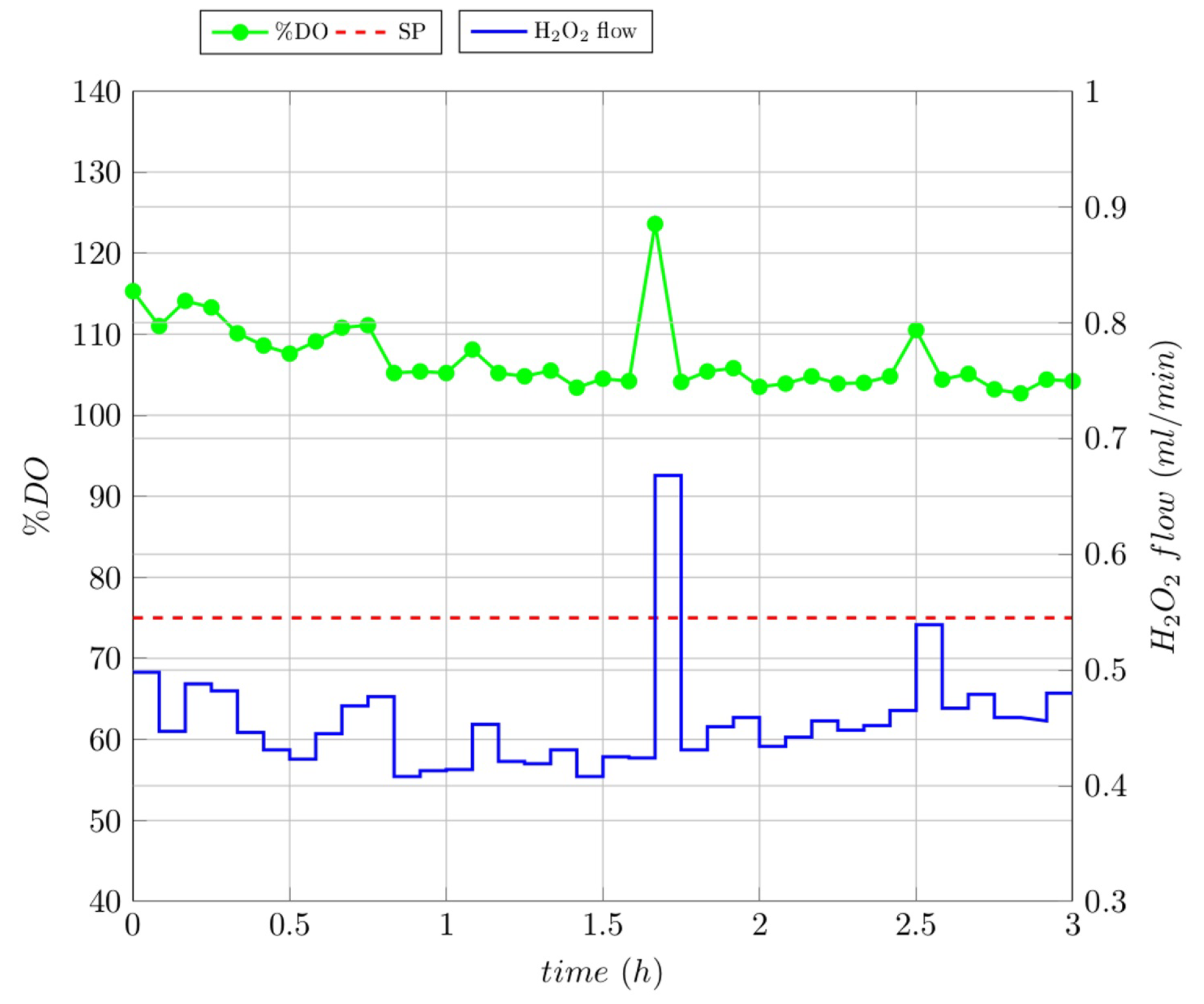
3.4. Closed-Loop Experiments
3.5. Comparison between Ol and Cl Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Anaerobic Digestion |
| AOP | Advanced Oxidation Processes |
| CL | Closed-Loop |
| COD | Chemical Oxygen Demand |
| DO | Dissolved Oxygen |
| HPP | Heterogeneous Photo-Fenton Process |
| OL | Open Loop |
| PI | Proportional Integral |
| RC | Recalcitrant Compounds |
| SP | Set-Point |
| TF | Transfer Function |
| TOC | Total Organic Carbon |
References
- Campos, C.R.; Mesquita, V.A.; Silva, C.F.; Schwan, R.F. Efficiency of physicochemical and biological treatments of vinasse and their influence on indigenous microbiota for disposal into the environment. Waste Manag. 2014, 34, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.F.; Canettieri, E.V.; Souza, S.M.A.; Rodrigues, R.C.L.B.; Acosta Martínez, E. Treatment of Sugarcane Vinasse from Cachaça Production for the Obtainment of Candida utilis CCT 3469 biomass. Biochem. Eng. J. 2019, 148, 131–137. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Cachaça (IBRAC). Serviços Estatísticas Mercado. Available online: https://ibrac.net/servicos/mercado-interno (accessed on 18 October 2021).
- López-López, A.; Davila-Vazquez, G.; León-Becerril, E.; Villegas-García, E.; Gallardo-Valdez, J. Tequila vinasses: Generation and full scale treatment processes. Rev. Environ. Sci. Bio/Technol. 2010, 9, 109–116. [Google Scholar] [CrossRef]
- Consejo Regulador del Tequila (CRT). Estadísticas Consejo Regulator del Tequila. (Statistics Tequila Regulator Council). Available online: http://www.crt.org.mx/ (accessed on 18 October 2021).
- España-Gamboa, E.; Mijangos-Cortes, J.; Barahona-Perez, L.; Dominguez-Maldonado, J.; Hernández-Zarate, G.; Alzate-Gaviria, L. Vinasses: Characterization and treatments. Waste Manag. Res. 2011, 29, 1235–1250. [Google Scholar] [CrossRef] [PubMed]
- García-Sanchez, R.; Ramos-Ibarra, R.; Guatemala-Morales, G.; Arriola-Guevera, E.; Toriz-González, G.; Corona-González, R.I. Photofermentation of Tequila vinasses by Rhodopseudomonas pseudopalustris to produce hydrogen. Int. J. Hydrogen Energy 2018, 43, 15857–15869. [Google Scholar] [CrossRef]
- Iñiguez, G.; Acosta, N.; Martinez, L.; Parra, J.; González, O. Utilización de subproductos de la industria tequilera. Parte 7. Compostaje de bagazo de agave y vinazas tequileras (Use of by-products of the Tequila industry. Part 7. Composting of agave bagasse and Tequila vinasse). Rev. Int. Contam. Ambiental 2005, 21, 37–50. [Google Scholar]
- Pires, J.F.; Ferreira, G.M.R.; Reis, K.C.; Schwan, R.F.; Silva, C.F. Mixed yeasts inocula for simultaneous production of SCP and treatment of vinasse to reduce soil and fresh water pollution. J. Environ. Manag. 2016, 182, 455–463. [Google Scholar] [CrossRef]
- Méndez-Acosta, H.O.; Snell-Castro, R.; Alcaraz-Gonzalez, V.; González-Álvarez, V.; Pelayo-Ortiz, C. Anaerobic treatment of Tequila vinasses in a CSTR-type digester. Biodegradation 2010, 21, 357–363. [Google Scholar] [CrossRef]
- Santos, T.S.; Queiroz Cunha, G.P.; Lacerda Maia, J.; Vital do Carmo, A.L.; Lipra, G.C.; Morais, A.A. Analise dos efluentes da producção de Cachaça e seus impactos sobre os recursos hídricos monitorados em Itabira-MG. Associação Brasileira de Recursos Hídricos. In Proceedings of the XX Simpósio Brasileiro de Recursos Hídricos, Bento Gonçalves, RS, Brasil, 17–22 November 2013. [Google Scholar]
- Robles-González, V.; Galíndez-Mayer, J.; Rinderknecht-Seijas, N.; Poggi-Varaldo, H.M. Treatment of mezcal vinasses: A review. J. Biotechnol. 2012, 157, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbon, F.J.; Nigam, P.; Singh, D.; Marchant, R. Biological treatment of distillery waste for pollution-remediation. J. Basic Microbiol. 1995, 35, 293–301. [Google Scholar] [CrossRef]
- Jiménez, A.M.; Borja, R.; Martın, A. Aerobic-anaerobic biodegradation of beet molasses alcoholic fermentation wastewater. Process Biochem. 2003, 38, 1275–1284. [Google Scholar] [CrossRef]
- Rahim Pouran, S.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J. Ind. Eng. Chem. 2015, 21, 53–69. [Google Scholar] [CrossRef]
- Mills, A.; Davies, R.H.; Worsley, D. Water Purification by Semiconductor Photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.; Brillas, E.; Vilar, V.J. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Poyatos, J.M.; Muñio, M.; Almecija, M.; Torres, J.; Hontoria, E.; Osorio, F. Advanced oxidation processes for wastewater treatment: State of the art. Water Air Soil Pollut. 2010, 205, 187–204. [Google Scholar] [CrossRef]
- Clarizia, L.; Russo, D.; Somma, I.D.; Marotta, R.; Andreozzi, R. Homogeneous photo-Fenton processes at near neutral pH: A review. Appl. Catal. B 2017, 209, 358–371. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef]
- Dowd, K.O.; Pillai, S.C. Photo-Fenton disinfection at near neutral pH: Process, parameter optimization and recent advances. J. Environ. Chem. Eng. 2020, 8, 104063. [Google Scholar]
- Herney-Ramirez, J.; Vicente, M.A.; Madeira, L.M. Heterogeneous photo-Fenton oxidation with pillared clay-based catalysts for wastewater treatment: A review. Appl. Catal. B 2010, 98, 10–26. [Google Scholar] [CrossRef]
- Xiangwei, Y.; Graells, M.; Miralles-Cuevas, S.; Cabrera-Reina, A.; Pérez-Moya, M. An improved hybrid strategy for online dosage of hydrogen peroxide in photo-Fenton processes. J. Environ. Chem. Eng. 2021, 9, 105235. [Google Scholar] [CrossRef]
- Ortega-Gómez, E.; Moreno Úbeda, J.C.; Álvarez Hervás, J.D.; Casas López, J.L.; Santos-Juanes, J.L.; Sánchez Pérez, J.A. Automatic dosage of hydrogen peroxide in solar photo-Fenton plants: Development of a control strategy for efficiency enhancement. J. Hazard. Mater. 2012, 237–238, 223–230. [Google Scholar] [CrossRef]
- Santos-Juanes, L.; García Sánchez, J.L.; Casas López, J.L.; Oller, I.; Malato, S.; Sáchez Pérez, J.A. Dissolved oxygen concentration: A key parameter in monitoring the photo-fenton process. Appl. Catal. B 2011, 104, 316–323. [Google Scholar] [CrossRef]
- Bernardo, E.C.; Egashira, R.; Kawasaki, J. Decolorization of molasses’ wastewater using activated carbon prepared from cane bagasse. Carbon 1997, 35, 1217–1221. [Google Scholar] [CrossRef]
- Souza, B.G. Catalisadores Magnéticos a Base de Ferritas de Co ou Zn de Heteroestruturas TiO2/Ferrita-Avaliação na Degradação Fotocatalitica de Lignina (Magnetic Catalysts Based on Co or Zn of Hetero-Structures TiO2/Ferrite-Evaluation on Photocatalytic Degradation of Lignin). Ph.D. Thesis, Center of Exact Sciences and Technology Research Center on Advanced Materials and Energy, Federal University of São Carlos, São Carlos, Brazil, 2018. [Google Scholar]
- Souza, B.G.; Figueira, G.; Carvalho, M.H.; Alcaraz-Gonzalez, V.; Saldaña-Flores, K.E.; Godinho, M.; Oliveira, A.J.A.; Kiminami, R.H.G.A.; Ruotolo, L.A.M.; Urquieta-Gonzalez, E. A novel synthesis route to obtain magnetic nanocrystalline cobalt ferrite with photo-Fenton activity. Mater. Chem. Phys. 2021, 257, 123741. [Google Scholar] [CrossRef]
- Gómez-Martínez, J.J. Degradación de los Principales Compuestos Recalcitrantes Presentes en Vinazas Tequilera Mediante Foto-Fenton heterogéneo (Degradation of the Main Recalcitrant Compounds Present in Tequila Vinasse by Heterogeneous Photo-Fenton). Master’s Thesis, Chemical Engineering Department, University of Guadalajara, Jalisco, Mexico, 2017. [Google Scholar]
- Souza, B.G. Catalisadores de Fe2O3, NiO e Fe2O3-NiO Suportados in Situ em Ce0,5Zr0,5O2 e Ce0,2Zr0,8O2 - Avaliação na redução de NO com CO (Fe2O3, NiO and Fe2O3-NiO Catalysts Supported in situ in Ce0,5Zr0,5O2 e Ce0,2Zr0,8O2 - Evaluation in the Reduction of NO with CO). Master’s Thesis, Center of Exact Sciences and Technology Research Center on Advanced Materials and Energy, Federal University of São Carlos, São Carlos, Brazil, 2014. [Google Scholar]
- Seborg, E.E.; Edgar, T.F.; Mellichamp, D.A.; Doyle, F.J. Process Dynamics and Control; John Wiley & Sons, Inc.: New York, NY, USA, 2017; pp. 109–110. [Google Scholar]
- Ogunnaike, B.A.; Ray, W.H. Process Dynamics, Modeling and Control; Gubbins, K.E., Ed.; Oxford University Press: New York, NY, USA, 1994; pp. 648–651. [Google Scholar]



| Parameter | Unit | Cachaça [9,11] | Tequila [10] |
|---|---|---|---|
| pH, 25 C | 3.5–4.8 | 3.35 | |
| Temperature | C | 80–100 | 50.4 |
| Biochemical oxygen demand (BOD) | gL | 6–18 | 13–24 |
| Chemical oxygen demand (COD) | gL | 14–39.5 | 28–50 |
| Total suspended solids (TSS) | gL | 0.18 | 12 |
| Volatile suspended solids (VSS) | gL | 20.3 | 9.8 |
| Total volatile fatty acids (acetic acid) | gL | na | 2.5–3.4 |
| Total nitrogen (N) | gL | 0.15–1.19 | 0.243 |
| Total phosphorus (P) | gL | 0.004–0.32 | 0.021 |
| Fats and oils | gL | na | 0.018–0.031 |
| RC | RC Degradation | TOC Removal | COD Removal |
|---|---|---|---|
| Gallic acid | 38% | 19% | 21% |
| Lignin | 28.8% | 21.5% | 20.8% |
| Melanoidin | 72% | 0% | 5% |
| (% DO h/mL) | (h) | |
|---|---|---|
| Gallic acid | −9.97 | 0.65 |
| Lignin | −3.70 | 0.57 |
| Melanoidin | −2.00 | 0.80 |
| (mL/(%DO h)) | (h) | (h) | |
|---|---|---|---|
| Gallic acid | −0.856 | 0.65 | 0.01 |
| Lignin | −1.542 | 0.57 | 0.10 |
| Melanoidin | −0.741 | 0.80 | 0.09 |
| RC | RC Degradation | TOC Removal | COD Removal |
|---|---|---|---|
| Gallic acid | 58% | 29% | 57% |
| Lignin | 37% | 39% | 16% |
| Melanoidin | 68% | 7% | 6.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldaña-Flores, K.E.; Flores-Estrella, R.A.; Alcaraz-Gonzalez, V.; Carissimi, E.; de Souza, B.G.; Ruotolo, L.A.M.; Urquieta-Gonzalez, E. Regulation of Hydrogen Peroxide Dosage in a Heterogeneous Photo-Fenton Process. Processes 2021, 9, 2167. https://doi.org/10.3390/pr9122167
Saldaña-Flores KE, Flores-Estrella RA, Alcaraz-Gonzalez V, Carissimi E, de Souza BG, Ruotolo LAM, Urquieta-Gonzalez E. Regulation of Hydrogen Peroxide Dosage in a Heterogeneous Photo-Fenton Process. Processes. 2021; 9(12):2167. https://doi.org/10.3390/pr9122167
Chicago/Turabian StyleSaldaña-Flores, Karla Estefanía, René Alejandro Flores-Estrella, Victor Alcaraz-Gonzalez, Elvis Carissimi, Bruna Gonçalves de Souza, Luís Augusto Martins Ruotolo, and Ernesto Urquieta-Gonzalez. 2021. "Regulation of Hydrogen Peroxide Dosage in a Heterogeneous Photo-Fenton Process" Processes 9, no. 12: 2167. https://doi.org/10.3390/pr9122167
APA StyleSaldaña-Flores, K. E., Flores-Estrella, R. A., Alcaraz-Gonzalez, V., Carissimi, E., de Souza, B. G., Ruotolo, L. A. M., & Urquieta-Gonzalez, E. (2021). Regulation of Hydrogen Peroxide Dosage in a Heterogeneous Photo-Fenton Process. Processes, 9(12), 2167. https://doi.org/10.3390/pr9122167






