Investigation of the Ambient Temperature Influence on the PEMFC Characteristics: Modeling from a Single Cell to a Stack
Abstract
:1. Introduction
2. Materials and Methods
2.1. MEA Simulation Model
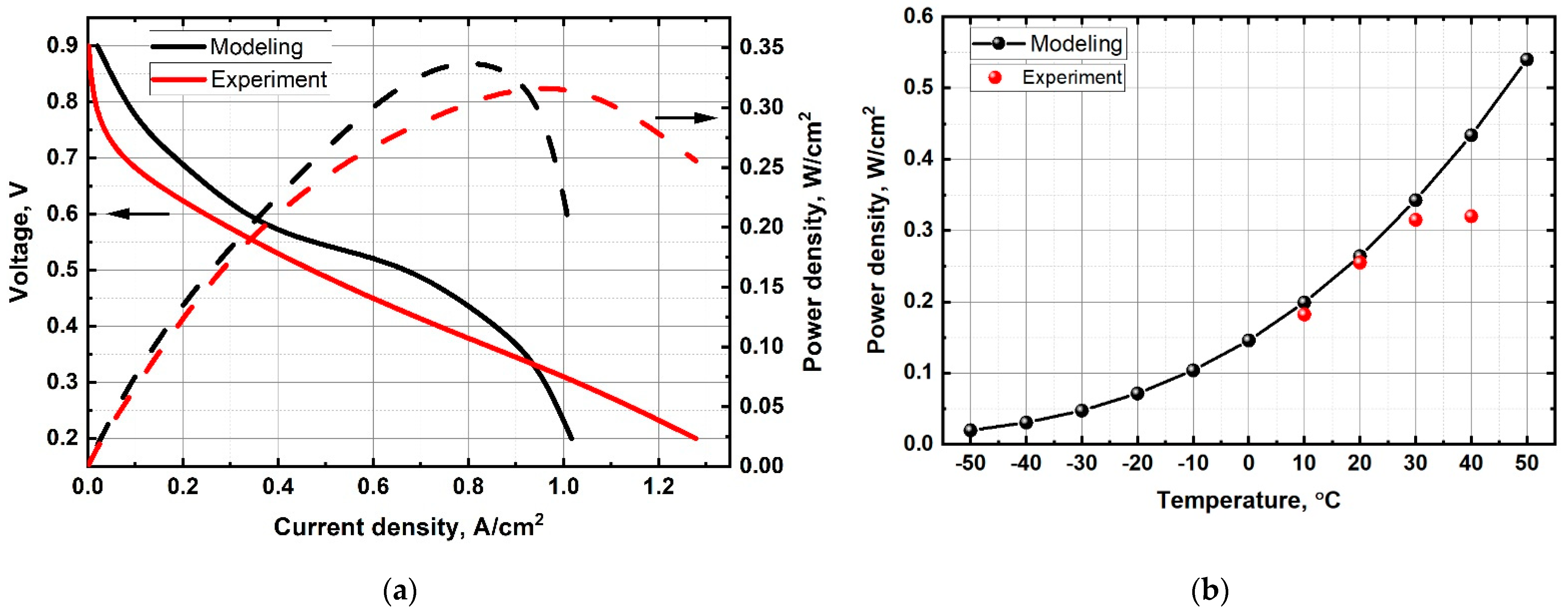
2.2. PEM Fuel Cell Hardware Experimental Data
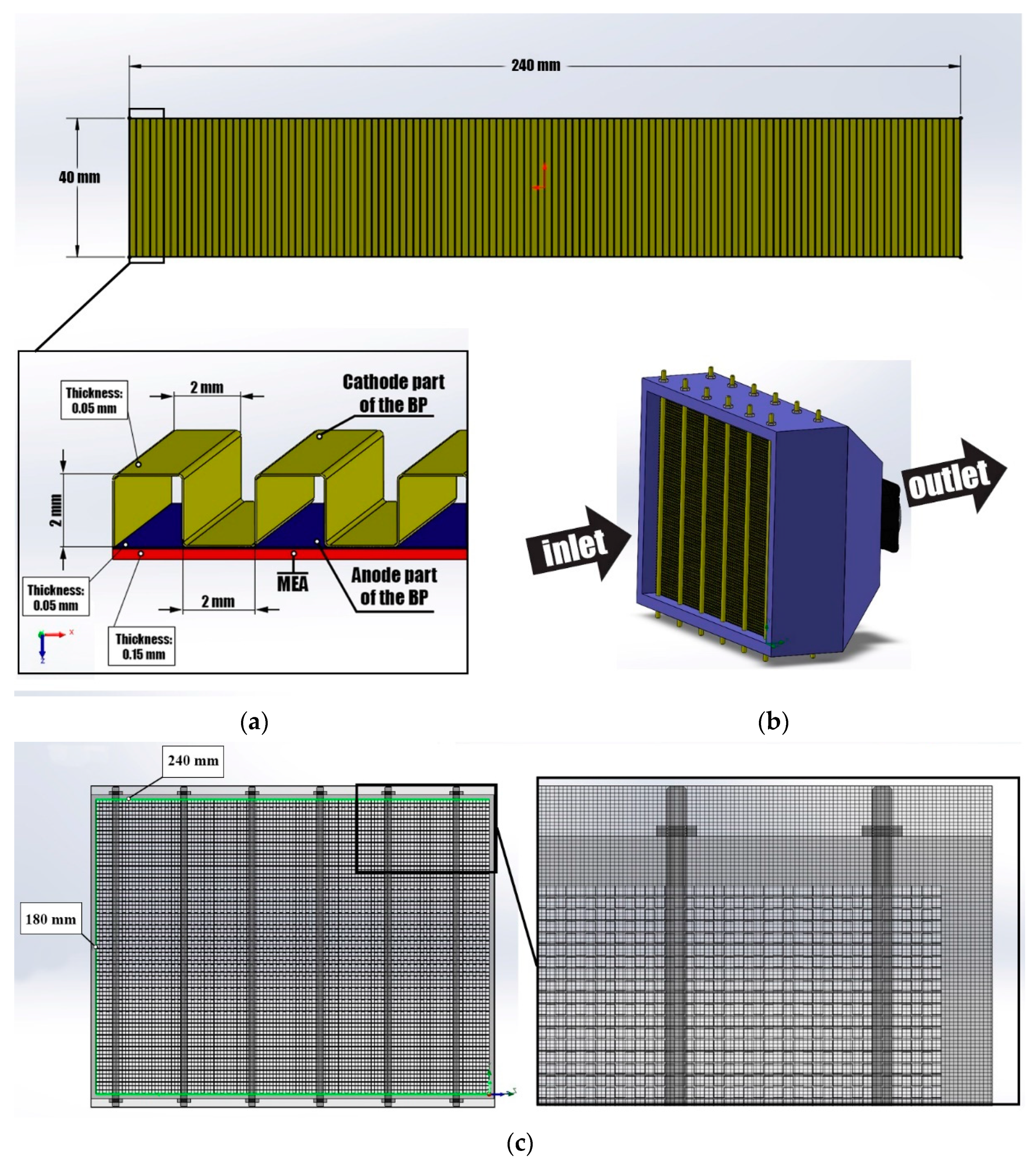
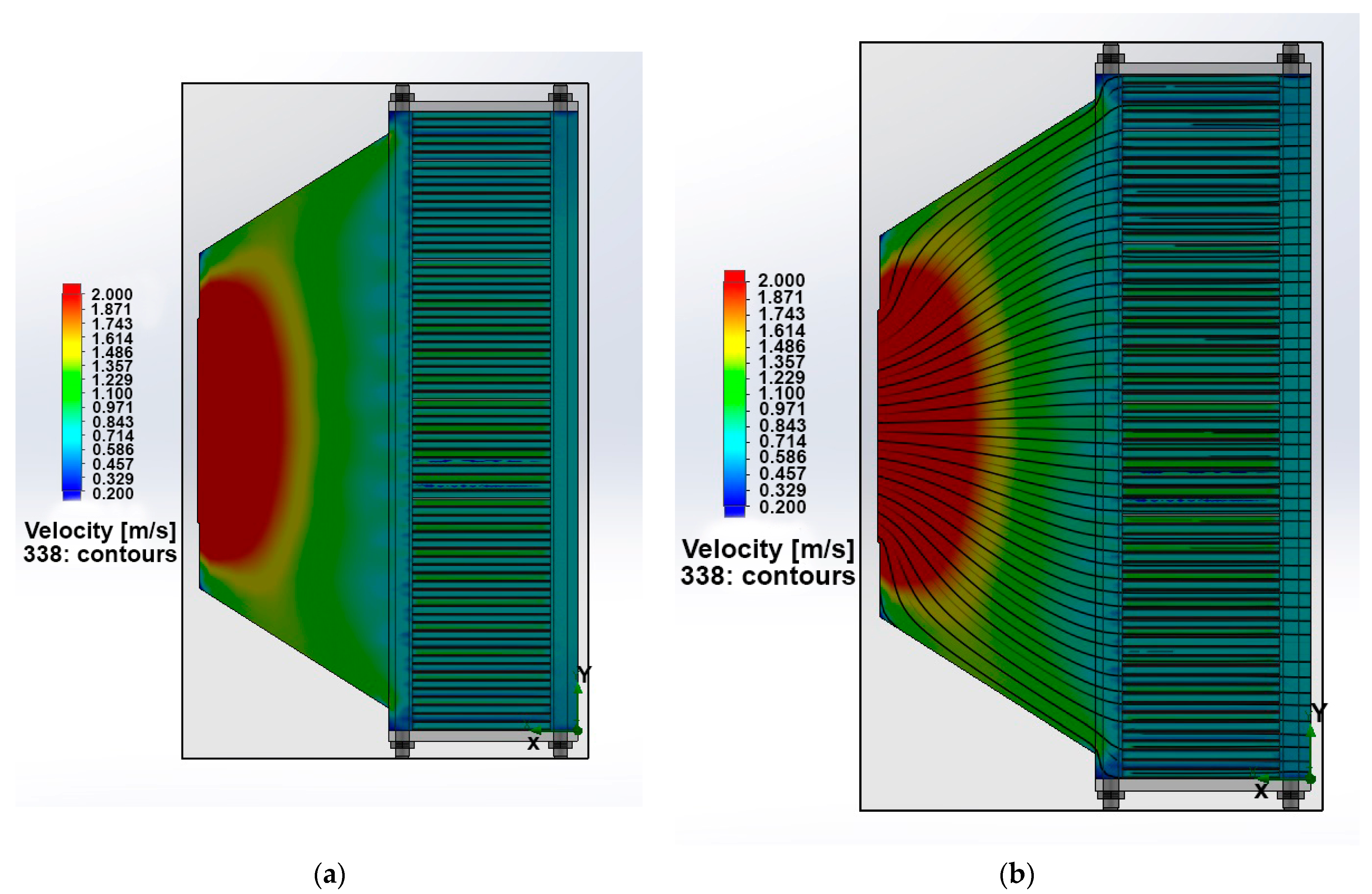
2.3. PEMFC Stack Simulation Model
- −
- Air-cooled PEMFC stack;
- −
- Number of PEMFC in stack—75 units;
- −
- Fuel cell consists of a MEA and BP;
- −
- Material of BP is titanium;
- −
- BP size—40 × 240 mm;
- −
- BP consists of 2 parts (cathode and anode);
- −
- Anode part of the BP has smooth configuration;
- −
- Cathode part of the BP has a channels configuration—pitch—2 mm; height—2 mm; width—2 mm;
- −
- 2 end-plates provided at the longitudinal, opposing ends of the PEMFC stack;
- −
- Plurality of tie rods, passing through a peripheral region of each end plate for positioning the PEMFC stack between the 2 end-plates;
- −
- 2 fans with confuser for supplying an oxidizing agent and cooling a PEMFC stack;
- −
- Fittings for supplying fuel (H2), electrical leads.
- −
- Fluid cells—3,352,729 units;
- −
- Solid cells—3,465,737 units;
- −
- Fluid cells contacting solids—2,500,284 units;
- −
- Cooling and oxidizer agent –air;
- −
- Flow rate of cooling and oxidizer agent—0.008 m3/s;
- −
- Heat emission as a result of H2 oxidation—50% of the nominal stack power;
- −
- Heat transfer coefficient from PEMFC stack to outside –0*;
- −
- Atmospheric pressure—1 atm, 101,325 Pa (in the inlet of the PEMFC stack);
- −
- Ambient temperature—30 °C.
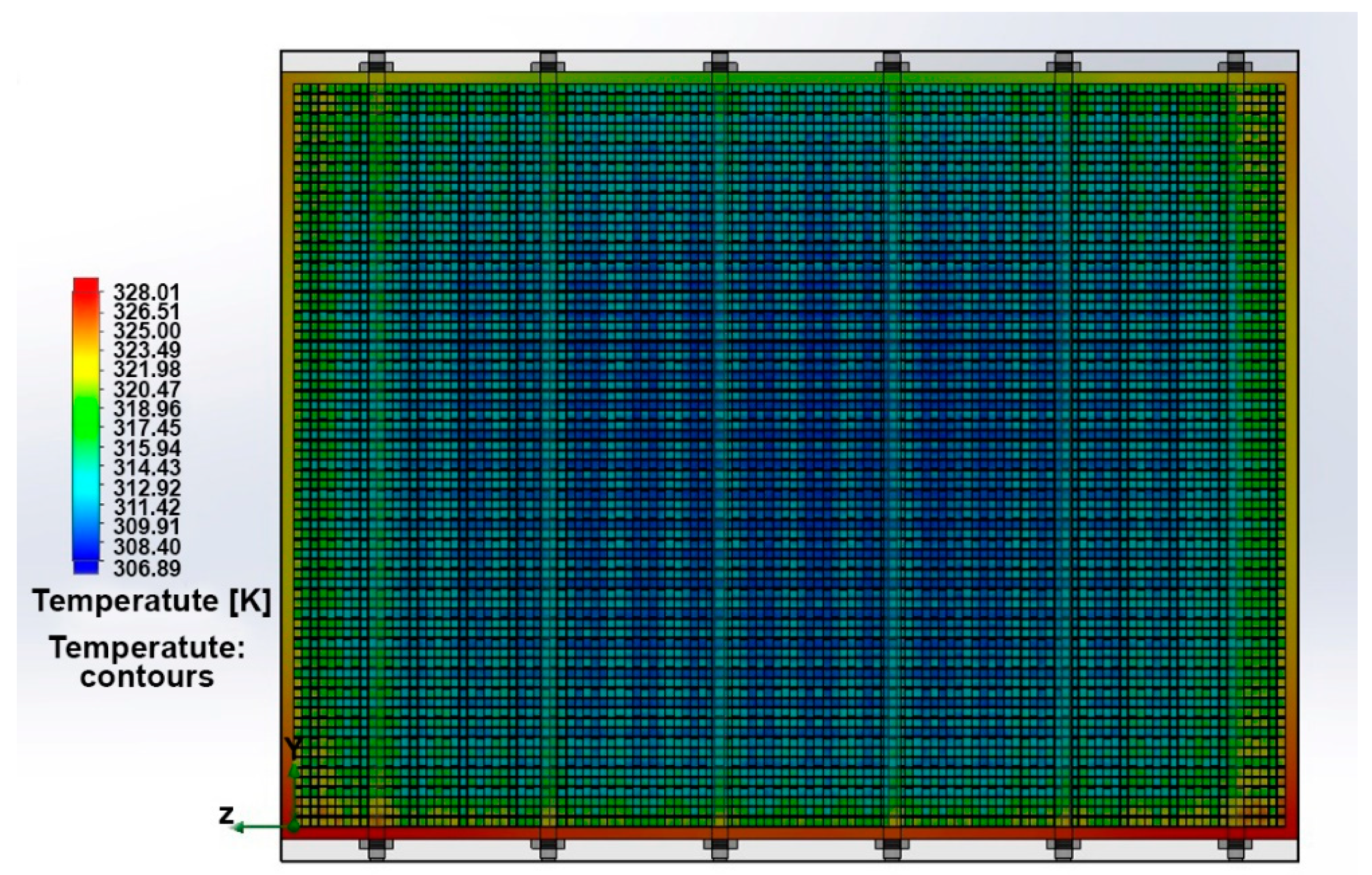
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| a | Active surface area density [1/m] |
| aa | Prefactor in ka [m/s] |
| ad | Prefactor in kd [m/s] |
| alg | Liquid-gas interfacial area density prefactor [1/m] |
| C | Total interstitial gas concentration [mol/m3] |
| DX | Fickean diffusion coefficient of gas X [m2/s] |
| DX,ref | Diffusivity of X at reference conditions [m2/s] |
| Dλ | Diffusion coefficient of dissolved water [m2/s] |
| F | Faraday constant (96,485.333 C/mol) |
| f | Water volume fraction in ionomer [–] |
| ∆G | Gibbs free energy difference [J/mol] |
| ∆H | Enthalpy of formation of liquid water [J/mol] |
| Had | Water ab-/desorption enthalpy [J/mol] |
| Hec | Evaporation/condensation enthalpy [J/mol] |
| I | Cell current density [A/m2] |
| i | Electrochemical reaction rate [A/m3] |
| i0 | Exchange current density [A/m2] |
| je | Electronic flux [A/m2] |
| jp | Protonic flux [A/m2] |
| jT | Heat flux [W/m2] |
| jλ | Flux of dissolved water [mol/m2s] |
| jX | Flux of gas X [mol/m2s] |
| js | Liquid water flux [mol/m2s] |
| k | Thermal conductivity [W/mK] |
| ka | Water absorption transfer coefficient [m/s] |
| kc | Water condensation transfer coefficient [m/s] |
| kd | Water desorption transfer coefficient [m/s] |
| ke | Water evaporation transfer coefficient [m/s] |
| L | Layer thickness [m] |
| Mw | Molar mass of water [kg/mol] |
| n | Interfacial unit normal vector [–] |
| P | Absolute gas pressure [Pa] |
| PA | Gas pressure in anode gas channel [Pa] |
| PC | Gas pressure in cathode gas channel [Pa] |
| Pref | Reference pressure (1 atm, 101,325 Pa) |
| Psat | Saturation water vapor pressure [Pa] |
| pc | Capillary pressure [Pa] |
| pX | Partial pressure of gas X [Pa] |
| R | Gas constant (8.31446 J/mol K) |
| RPEM | Membrane resistance [Ωm2] |
| r | Equivalent capillary radius [m] |
| RH | Relative gas humidity [–] |
| RHA | Relative humidity in anode gas channel [–] |
| RHC | Relative humidity in cathode gas channel [–] |
| s | Liquid water saturation [–] |
| sC | Saturation at cathode GDL/GC interface [–] |
| sim | Immobile liquid water saturation [–] |
| sred | Reduced liquid water saturation [–] |
| SF | Substantial reaction rate [mol/m3s] |
| Se | Electron reaction rate [A/m3] |
| Sp | Proton reaction rate [A/m3] |
| ST | Heat source [W/m3] |
| ST,e | Joule heat source of electrons [W/m3] |
| ST,p | Joule heat source of protons [W/m3] |
| ST,r | Reaction heat source [W/m3] |
| ST,ad | Water ab-/desorption heat source [W/m3] |
| ST,ec | Evaporation/condensation heat source [W/m3] |
| Sλ | Dissolved water reaction rate [mol/m3s] |
| SX | Reaction rate of gas X [mol/m3s] |
| Ss | Liquid water reaction rate [mol/m3s] |
| Sad | Water ab-/desorption source [mol/m3s] |
| Sec | Evaporation/condensation source [mol/m3s] |
| ∆S | Reaction entropy [J/mol K] |
| ∆SHOR | Hydrogen oxidation reaction entropy [J/mol K] |
| ∆SORR | Oxygen reduction reaction entropy [J/mol K] |
| T | Absolute temperature [K] |
| TA | Temperature of anode plate and GC [K] |
| TC | Temperature of cathode plate and GC [K] |
| Tref | Reference temperature (80 °C, 353.15 K) |
| T | Mean MEA temperature [K] |
| U | Cell voltage [V] |
| Vm | Acid equivalent volume of membrane [m3/mol] |
| Vw | Molar volume of liquid water [m3/mol] |
| x | Through-plane coordinate [m] |
| xX | Mole fraction of gas X [–] |
| Water vapor mole fraction in anode GC [–] | |
| Water vapor mole fraction in cathode GC [–] | |
| Hydrogen mole fraction in anode GC [–] | |
| Oxygen mole fraction in cathode GC [–] | |
| Saturation water vapor mole fraction [–] | |
| Mole fraction of hydrogen in dry fuel gas [–] | |
| Mole fraction of oxygen in dry oxidant gas [–] | |
| β | Half-reaction symmetry factor [–] |
| γ | Surface tension of water [N/m] |
| γc | Water condensation rate [1/s] |
| γe | Water evaporation rate [1/s] |
| εi | Ionomer volume fraction [–] |
| εp | Pore space volume fraction (porosity) [–] |
| η | Activation overpotential [V] |
| θ | Effective contact angle [deg] |
| k | Hydraulic permeability [m2] |
| kabs | Absolute (intrinsic) permeability [m2] |
| λ | Ionomer water content [–] |
| λeq | Equilibrium ionomer water content [–] |
| λ | Mean ionomer water content [–] |
| μ | Dynamic viscosity of liquid water [Pa s] |
| ξ | Electro-osmotic drag coefficient [–] |
| 𝜎e | Electric conductivity [S/m] |
| 𝜎p | Protonic conductivity [S/m] |
| τ | Pore tortuosity [–] |
| 𝜙e | Electrode phase potential [V] |
| 𝜙p | Electrolyte phase potential [V] |
| ∆𝜙 | Galvani potential difference [V] |
| ∆𝜙0 | Reversible potential difference [V] |
References
- Zhao, C.; Xing, S.; Chen, M.; Liu, W.; Wang, H. Optimal design of cathode flow channel for air-cooled PEMFC with open cathode. Int. J. Hydrogen Energy 2020, 45, 17771–17781. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells: Theory and Practice; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- O’hayre, R.; Cha, S.W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Jeong, S.U.; Cho, E.A.; Kim, H.-J.; Lim, T.-H.; Oh, I.-H.; Kim, S.H. A study on cathode structure and water transport in air-breathing PEM fuel cells. J. Power Sources 2006, 159, 1089–1094. [Google Scholar] [CrossRef]
- Wu, J.; Galli, S.; Lagana, I.; Pozio, A.; Monteleone, G.; Yuan, X.Z.; Martin, J.; Wang, H. An air-cooled proton exchange membrane fuel cell with combined oxidant and coolant flow. J. Power Sources 2009, 188, 199–204. [Google Scholar] [CrossRef]
- Sasmito, A.P.; Birgersson, E.; Mujumdar, A.S. A novel flow reversal concept for improved thermal management in polymer electrolyte fuel cell stacks. Int. J. Therm. Sci. 2012, 54, 242–252. [Google Scholar] [CrossRef]
- Sasmito, A.; Lum, K.; Birgersson, E.; Mujumdar, A. Computational study of forced air-convection in open-cathode polymer electrolyte fuel cell stacks. J. Power Sources 2010, 195, 5550–5563. [Google Scholar] [CrossRef]
- Sasmito, A.P.; Birgersson, E.; Lum, K.; Mujumdar, A. Fan selection and stack design for open-cathode polymer electrolyte fuel cell stacks. Renew. Energy 2012, 37, 325–332. [Google Scholar] [CrossRef]
- Akbari, M.; Tamayol, A.; Bahrami, M. Thermal assessment of convective heat transfer in air-cooled PEMFC stacks: An experimental study. Energy Procedia 2012, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shahsavari, S.; Desouza, A.; Bahrami, M.; Kjeang, E. Thermal analysis of air-cooled PEM fuel cells. Int. J. Hydrogen Energy 2012, 37, 18261–18271. [Google Scholar] [CrossRef]
- Ko, J.; Ju, H. Comparison of numerical simulation results and experimental data during cold-start of polymer electrolyte fuel cells. Appl. Energy 2012, 94, 364–374. [Google Scholar] [CrossRef]
- Chippar, P.; Ju, H. Evaluating cold-start behaviors of end and intermediate cells in a polymer electrolyte fuel cell (PEFC) stack. Solid State Ion. 2012, 225, 85–91. [Google Scholar] [CrossRef]
- Tabe, Y.; Saito, M.; Fukui, K.; Chikahisa, T. Cold start characteristics and freezing mechanism dependence on start-up temperature in a polymer electrolyte membrane fuel cell. J. Power Sources 2012, 208, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J. (Ed.) PEM Fuel Cell Electrocatalysts and Catalyst Layers: Fundamentals and Applications; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Chavan, S.L.; Talange, D.B. Modeling and performance evaluation of PEM fuel cell by controlling its input parameters. Energy 2017, 138, 437–445. [Google Scholar] [CrossRef]
- Newman, J.; Tiedemann, W. Porous-electrode theory with battery applications. AIChE J. 1975, 21, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer electrolyte fuel cell model. J. Electrochem. Soc. 1991, 138, 2334. [Google Scholar] [CrossRef]
- Bhaiya, M.; Putz, A.; Secanell, M. Analysis of non-isothermal effects on polymer electrolyte fuel cell electrode assemblies. Electrochim. Acta 2014, 147, 294–309. [Google Scholar] [CrossRef]
- Natarajan, D.; Van Nguyen, T. A two-dimensional, two-phase, multicomponent, transient model for the cathode of a proton exchange membrane fuel cell using conventional gas distributors. J. Electrochem. Soc. 2001, 148, A1324. [Google Scholar] [CrossRef]
- Plawsky, J.L. Transport Phenomena Fundamentals; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Vetter, R.; Schumacher, J.O. Free open reference implementation of a two-phase PEM fuel cell model. Comput. Phys. Commun. 2019, 234, 223–234. [Google Scholar] [CrossRef]
- Burheim, O.S.; Pharoah, J.G.; Lampert, H.; Vie, P.J.S.; Kjelstrup, S. Through-plane thermal conductivity of PEMFC porous transport layers. J. Fuel Cell Sci. Technol. 2011, 8, 2. [Google Scholar] [CrossRef]
- Khandelwal, M.; Mench, M.M. Direct measurement of through-plane thermal conductivity and contact resistance in fuel cell materials. J. Power Sources 2006, 161, 1106–1115. [Google Scholar] [CrossRef]
- El-Kharouf, A.; Mason, T.J.; Brett, D.; Pollet, B.G. Ex-situ characterisation of gas diffusion layers for proton exchange membrane fuel cells. J. Power Sources 2012, 218, 393–404. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.S.; Van Nguyen, T. Multicomponent transport in porous electrodes of proton exchange membrane fuel cells using the interdigitated gas distributors. J. Electrochem. Soc. 1999, 146, 38. [Google Scholar] [CrossRef]
- Gode, P.; Jaouen, F.; Lindbergh, G.; Lundblad, A.; Sundholm, G. Influence of the composition on the structure and electrochemical characteristics of the PEFC cathode. Electrochim. Acta 2003, 48, 4175–4187. [Google Scholar] [CrossRef]
- Weber, A.Z.; Borup, R.L.; Darling, R.M.; Das, P.K.; Dursch, T.J.; Gu, W.; Harvey, D.; Kusoglu, A.; Litster, S.; Mench, M.M.; et al. A critical review of modeling transport phenomena in polymer-electrolyte fuel cells. J. Electrochem. Soc. 2014, 161, F1254. [Google Scholar] [CrossRef] [Green Version]
- Holzer, L.; Pecho, O.; Schumacher, J.; Marmet, P.; Büchi, F.; Lamibrac, A.; Münch, B. Microstructure-property relationships in a gas diffusion layer (GDL) for Polymer Electrolyte Fuel Cells, Part II: Pressure-induced water injection and liquid permeability. Electrochim. Acta 2017, 241, 414–432. [Google Scholar] [CrossRef]
- Holzer, L.; Pecho, O.; Schumacher, J.; Marmet, P.; Stenzel, O.; Büchi, F.; Lamibrac, A.; Münch, B. Microstructure-property relationships in a gas diffusion layer (GDL) for Polymer Electrolyte Fuel Cells, Part I: Effect of compression and anisotropy of dry GDL. Electrochim. Acta 2017, 227, 419–434. [Google Scholar] [CrossRef]
- Vetter, R.; Schumacher, J.O. Experimental parameter uncertainty in proton exchange membrane fuel cell modeling. Part II: Sensitivity analysis and importance ranking. J. Power Sources 2019, 439, 126529. [Google Scholar] [CrossRef] [Green Version]
- Anisimov, E.; Faddeev, N.; Smirnova, N. Data on the thermo-fluid simulation of open-cathode fuel cell stack depending on the location of the oxidizer/cooling supply system. Data Brief 2020, 31, 105771. [Google Scholar] [CrossRef]
- Tsilingiris, P.T. Thermophysical and transport properties of humid air at temperature range between 0 and 100 °C. Energy Convers. Manag. 2008, 49, 1098–1110. [Google Scholar] [CrossRef]
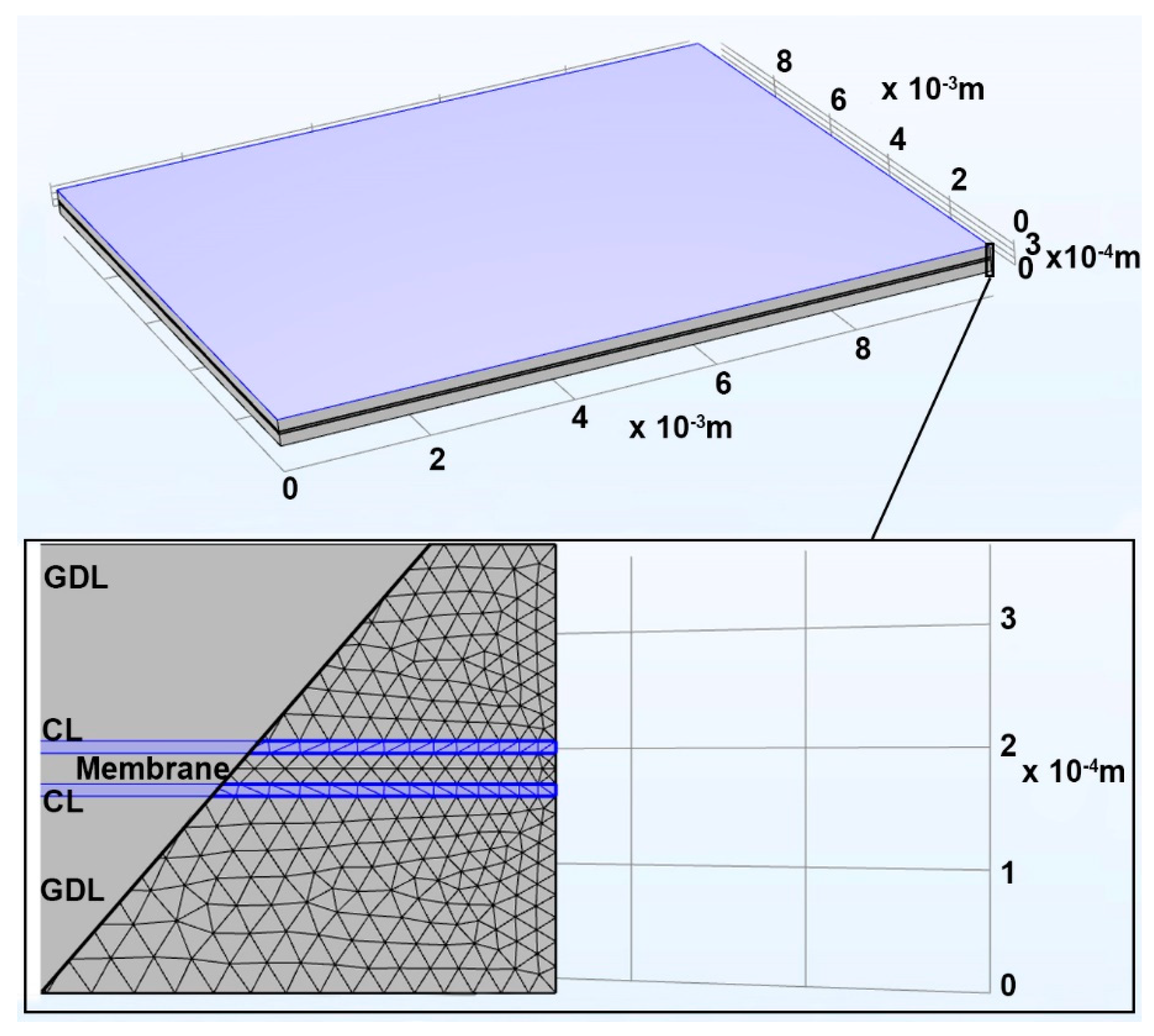

| Name | Flux | Continuity Equation |
|---|---|---|
| Ohm’s law for electrons (𝜙e) | ||
| Ohm’s law for protons (𝜙p) | ||
| Water transport in ionomer (λ) | ||
| Fourier heat conduction (T) | k∇T | |
| Darcy’s law (liquid water transport) (s) | ||
| Fickean water vapor diffusion ) | ||
| Fickean hydrogen diffusion ) | ||
| Fickean oxygen diffusion ) |
| Source | AGDL | ACL | PEM | CCL | CGDL |
|---|---|---|---|---|---|
| 0 | -i | i | 0 | ||
| i | 0 | -i | |||
| 0 | |||||
| 0 | |||||
| 0 | |||||
| 0 |
| Variable | AGDL/AGC | ACL/AGDL | PEM/ACL | CCL/PEM | CGDL/CCL | CGC/CGDL |
|---|---|---|---|---|---|---|
| incessancy | incessancy | |||||
| incessancy | incessancy | |||||
| λ | incessancy | incessancy | ||||
| T | incessancy | incessancy | incessancy | incessancy | ||
| s | incessancy | |||||
| incessancy | incessancy | |||||
| incessancy | ||||||
| incessancy |
| Description | Value |
|---|---|
| CL thickness | 10 μm |
| GDL thickness | 160 μm |
| PEM thickness | 25 μm |
| Ionomer volume fraction in CL | 0.3 |
| Ionomer volume fraction in PEM | 1 |
| Pore volume fraction GDL | 0.76 |
| Pore volume fraction CL | 0.4 |
| Thermal conductivity GDL | 1.6 W/m∙K [22] |
| Thermal conductivity CL | 0.27 W/m∙K [23] |
| Thermal conductivity PEM | 0.3 W/m∙K [23] |
| Pore tortuosity GDL | 1.6 |
| Pore tortuosity CL | 1.6 |
| Absolute permeability GDL | 6.15 × 10−12 m2 [24] |
| Absolute permeability CL | 10−13 m2 [25] |
| Electrical conductivity GDL | 1250 S/m [26] |
| Electrical conductivity CL | 350 S/m [26] |
| Gas Pressure in Cathode and Anode | 1.5 atm. |
| Relative Humidity in Cathode and Anode GC | 100% |
| Liquid Saturation at GC/CGDL Interface | 0.12 |
| Ambient Temperature | −50–+50 °C |
| H2 Mole Fraction in Fuel | 1.00 |
| O2 Mole Fraction in Oxidant | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faddeev, N.; Anisimov, E.; Belichenko, M.; Kuriganova, A.; Smirnova, N. Investigation of the Ambient Temperature Influence on the PEMFC Characteristics: Modeling from a Single Cell to a Stack. Processes 2021, 9, 2117. https://doi.org/10.3390/pr9122117
Faddeev N, Anisimov E, Belichenko M, Kuriganova A, Smirnova N. Investigation of the Ambient Temperature Influence on the PEMFC Characteristics: Modeling from a Single Cell to a Stack. Processes. 2021; 9(12):2117. https://doi.org/10.3390/pr9122117
Chicago/Turabian StyleFaddeev, Nikita, Evgeny Anisimov, Maxim Belichenko, Alexandra Kuriganova, and Nina Smirnova. 2021. "Investigation of the Ambient Temperature Influence on the PEMFC Characteristics: Modeling from a Single Cell to a Stack" Processes 9, no. 12: 2117. https://doi.org/10.3390/pr9122117
APA StyleFaddeev, N., Anisimov, E., Belichenko, M., Kuriganova, A., & Smirnova, N. (2021). Investigation of the Ambient Temperature Influence on the PEMFC Characteristics: Modeling from a Single Cell to a Stack. Processes, 9(12), 2117. https://doi.org/10.3390/pr9122117






