Manual Application versus Autonomous Release of Water Repellent Agent to Prevent Reinforcement Corrosion in Cracked Concrete
Abstract
:1. Introduction
2. Materials
2.1. Healing Agent
2.2. Capsules
2.3. Concrete
3. Methods
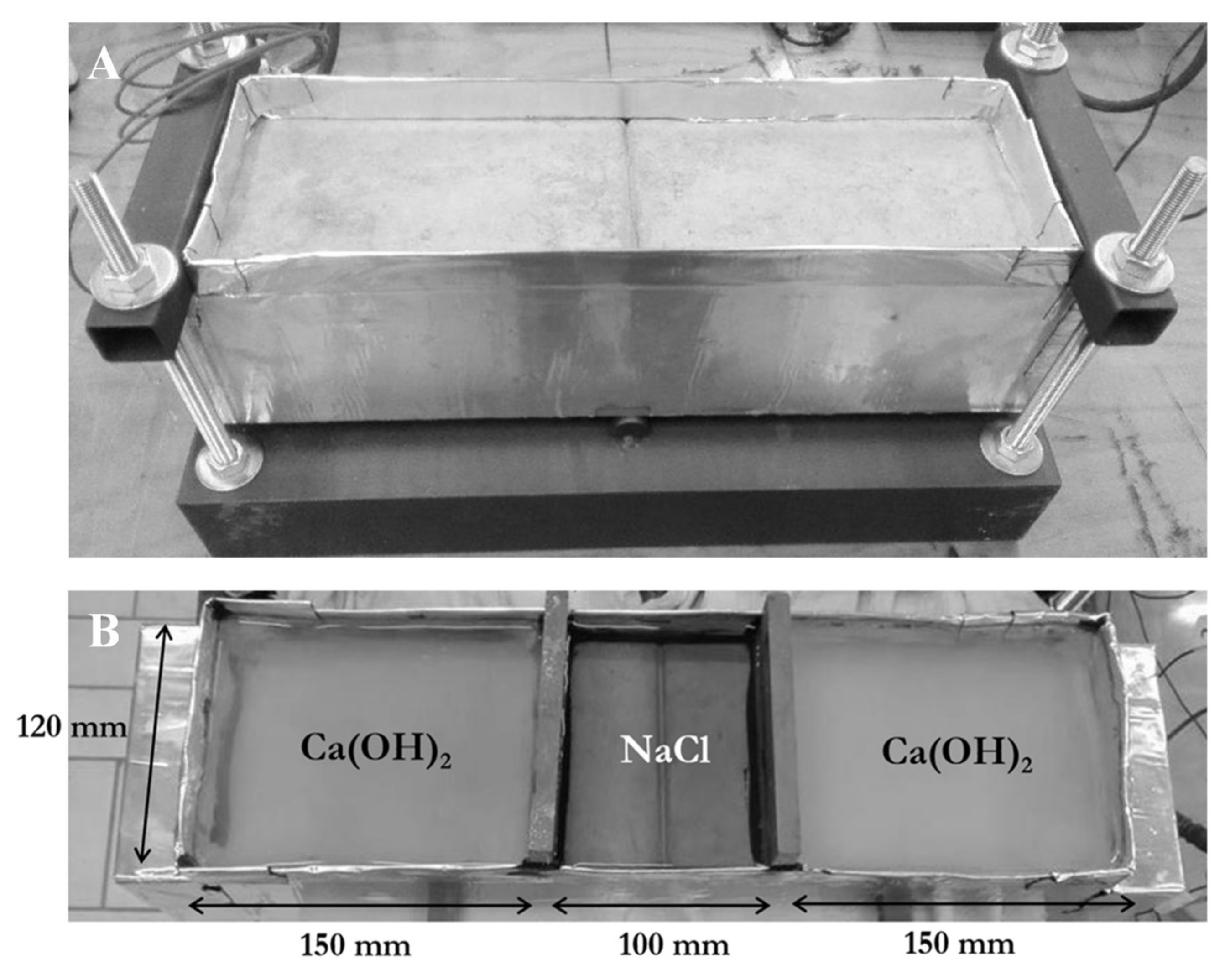
3.1. Preparation of Concrete Samples
3.2. Crack Creation
3.3. Crack Healing
3.4. Crack Width Measurement
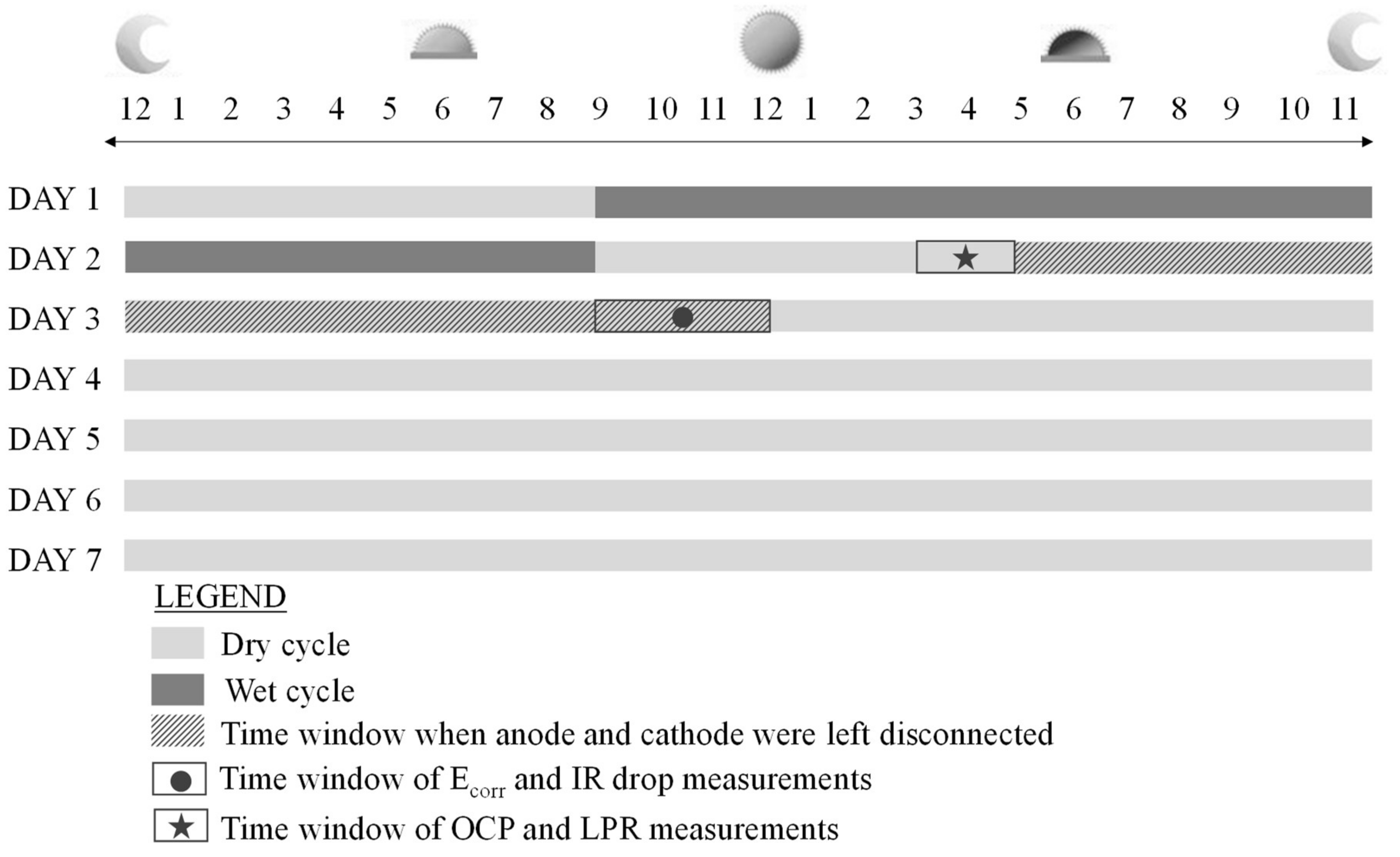
3.5. Cyclic Exposure Regime
3.6. Corrosion Monitoring
3.6.1. Measurement of the Macro-Cell Corrosion Current
3.6.2. Electrochemical Corrosion Measurements
3.6.3. Determination of the Mass and Volume Loss of the Anode
3.7. Determination of the Chloride Profile
3.7.1. Chloride Concentration Determined by Potentiometric Titration
3.7.2. Visual Evaluation by Spraying Silver Nitrate Solution
3.8. Visual Inspection of the (Corroded) Reinforcement Bars
3.8.1. Visual Inspection through Photography
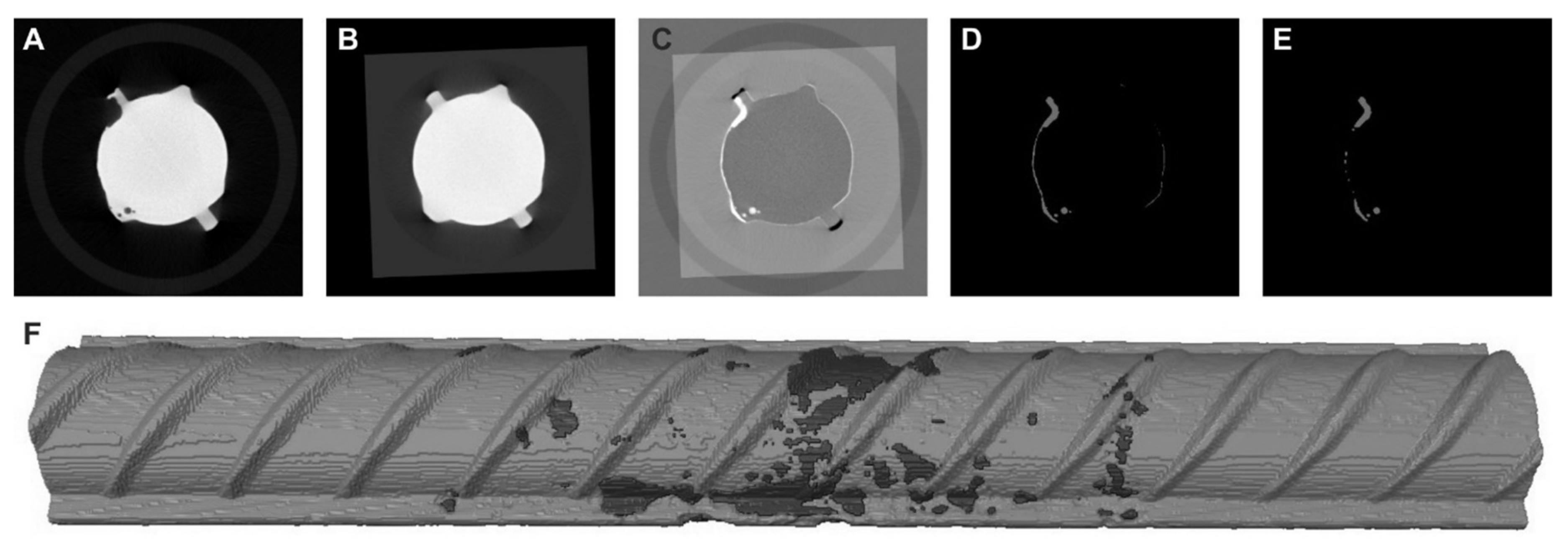
3.8.2. X-ray Computed Microtomography Acquisition, Analysis and Visualization
3.9. Statistical Analysis
4. Results and Discussion
4.1. Crack Widths
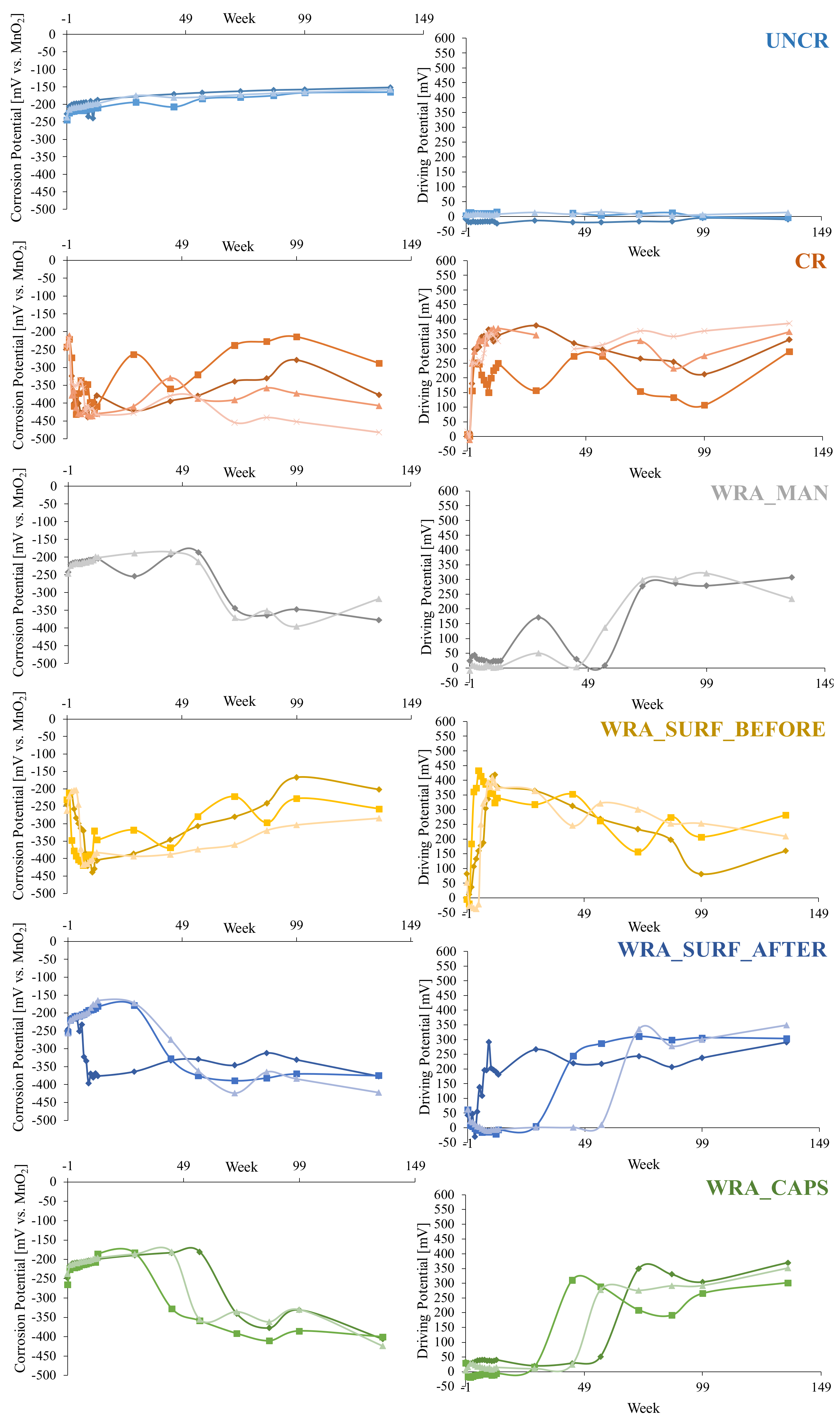
4.2. Corrosion Monitoring
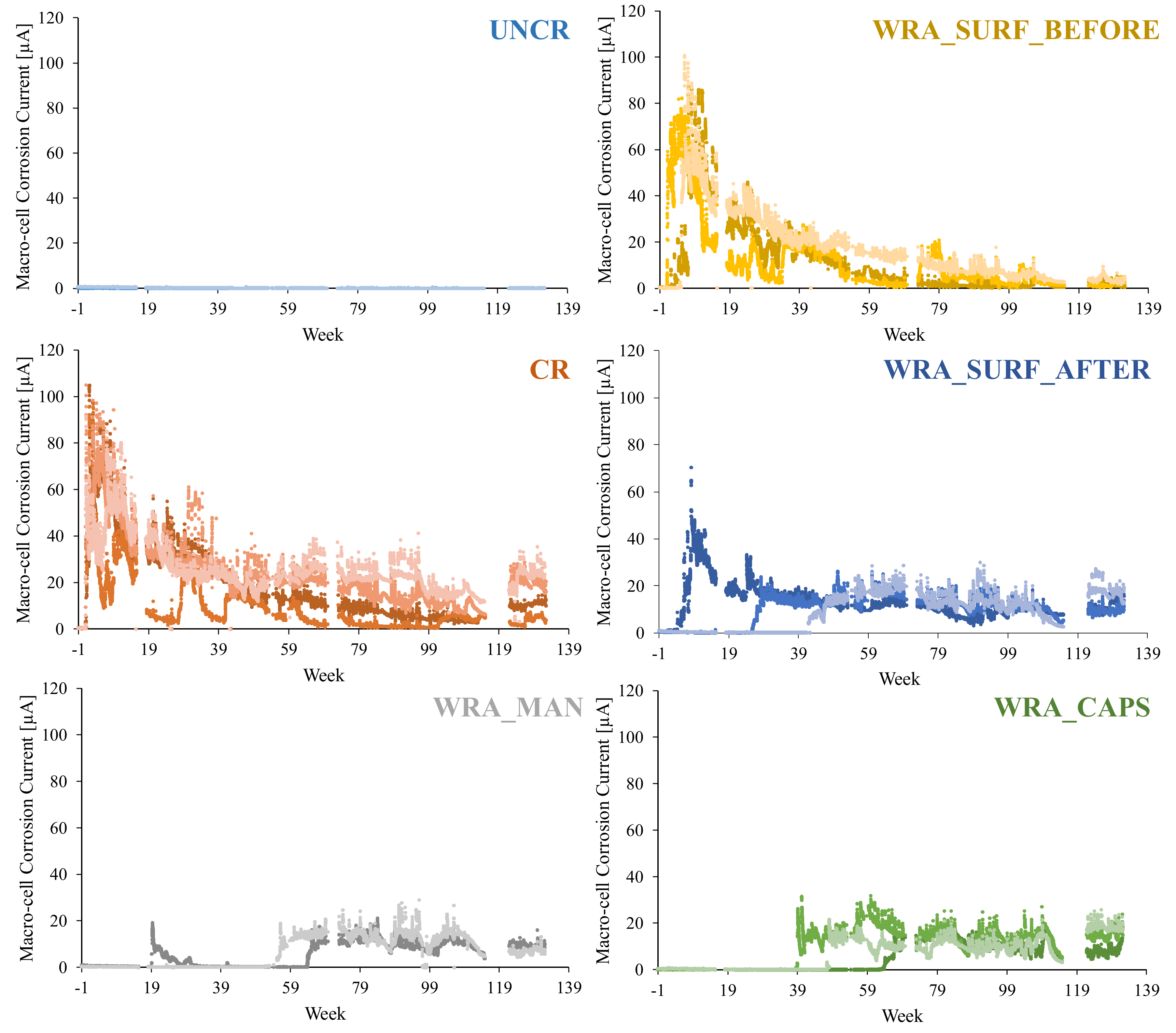
4.2.1. Measurement of the Macro-Cell Corrosion Current
4.2.2. Electrochemical Corrosion Measurements
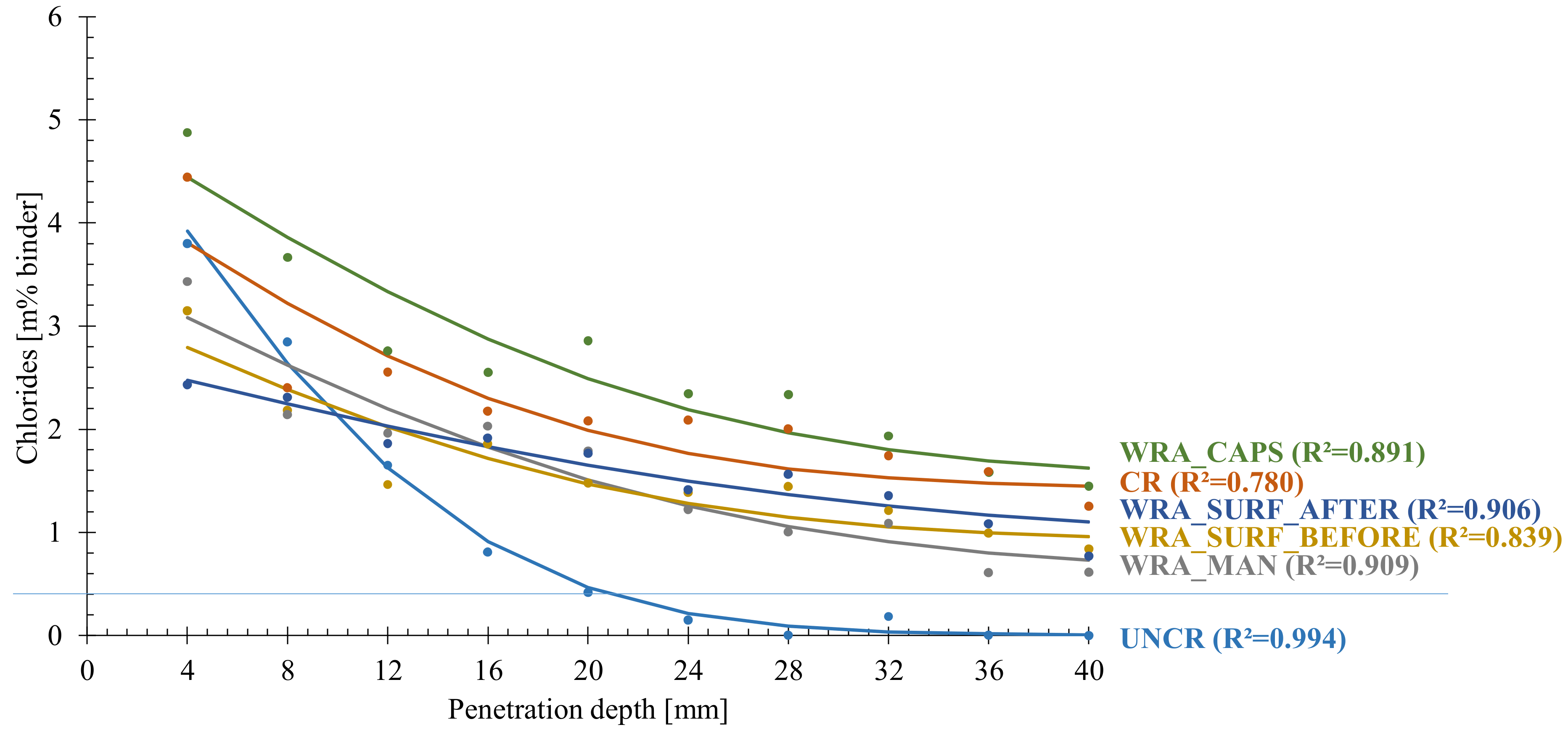
4.3. Chloride Ingress Profile
4.4. Chloride Ingress Front
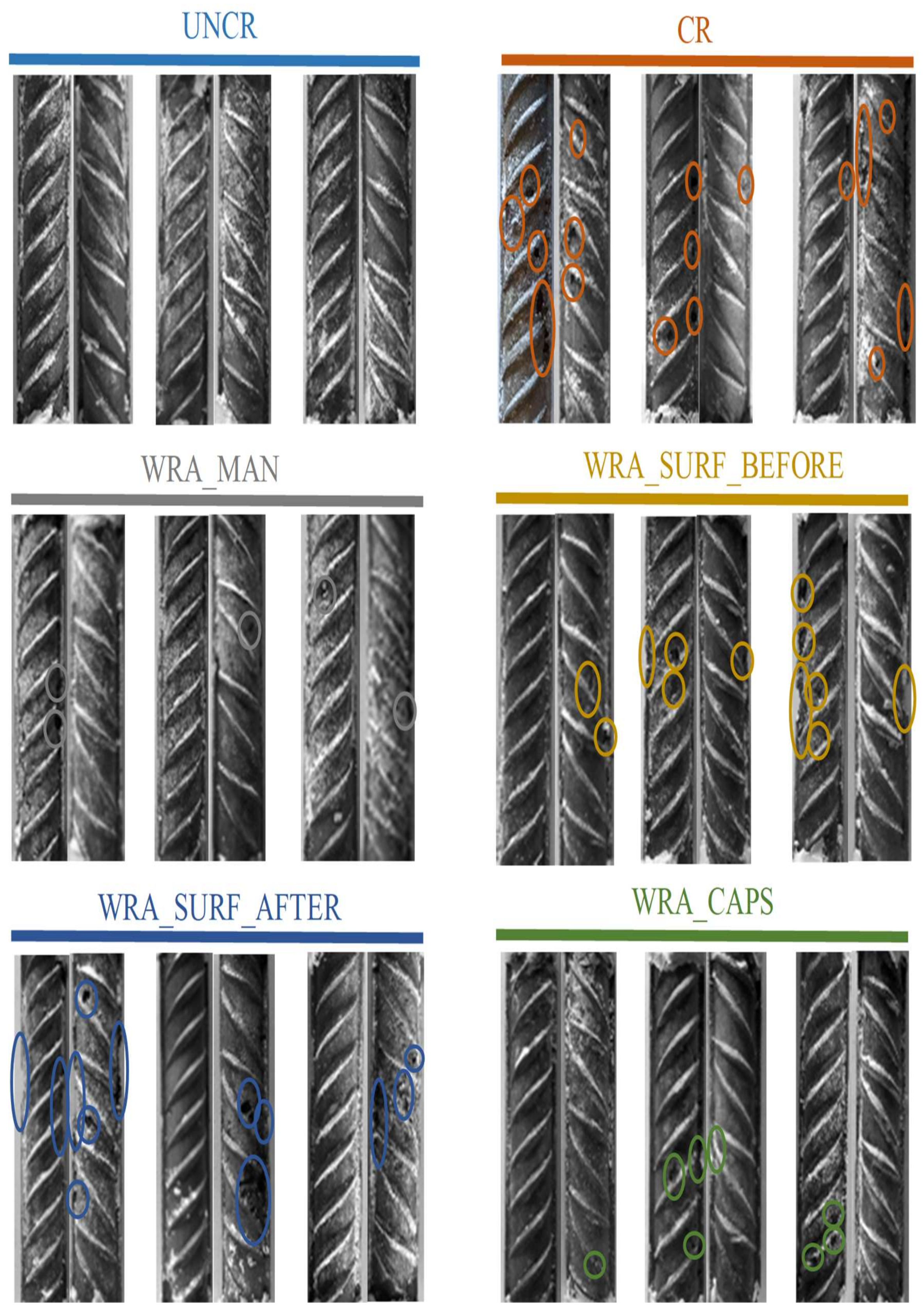
4.5. Visual Inspection Reinforcement Bars
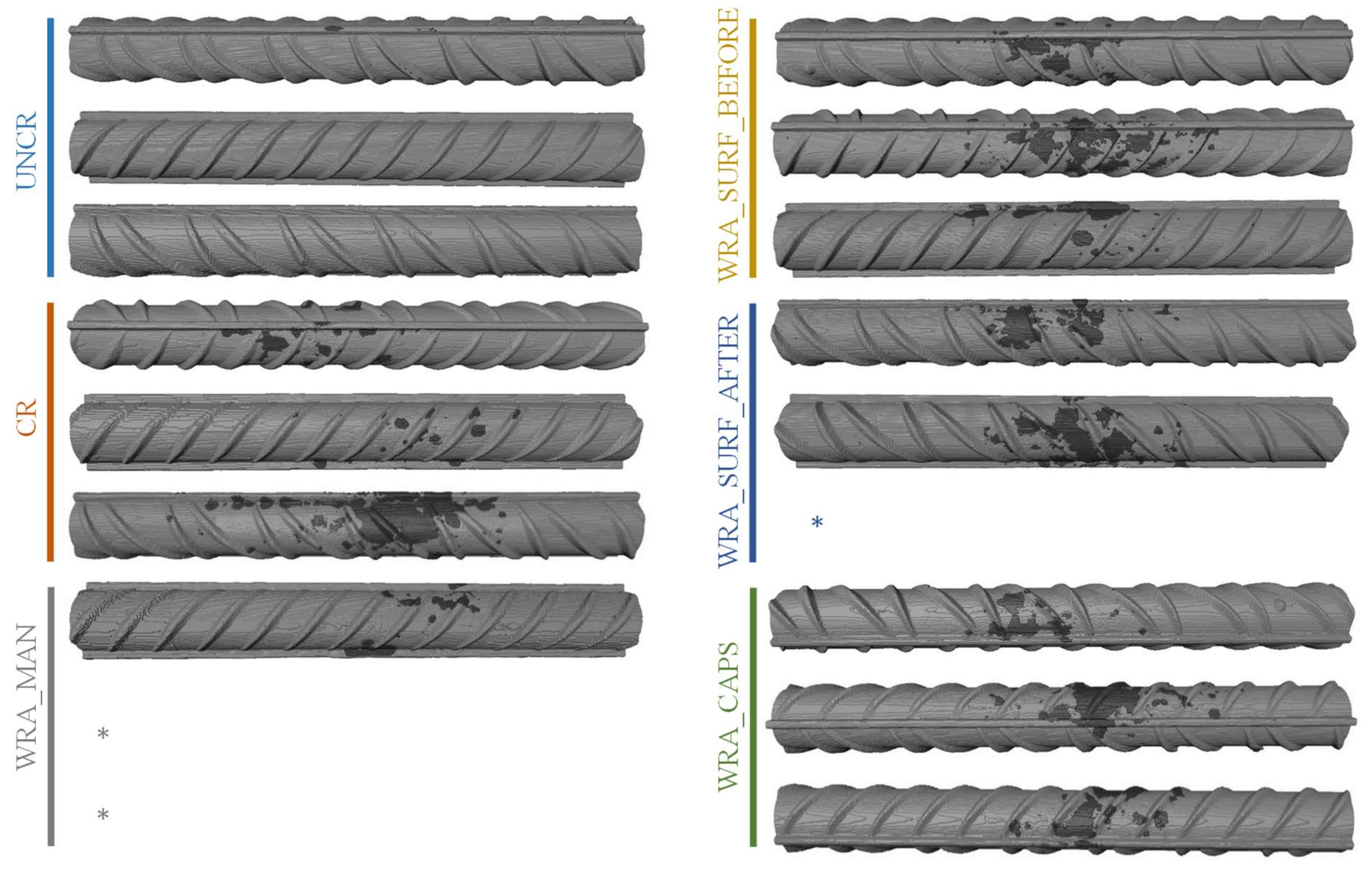
4.6. Inspection of Reinforcement Bars through X-ray μCT
5. Conclusions and Future Recommendations
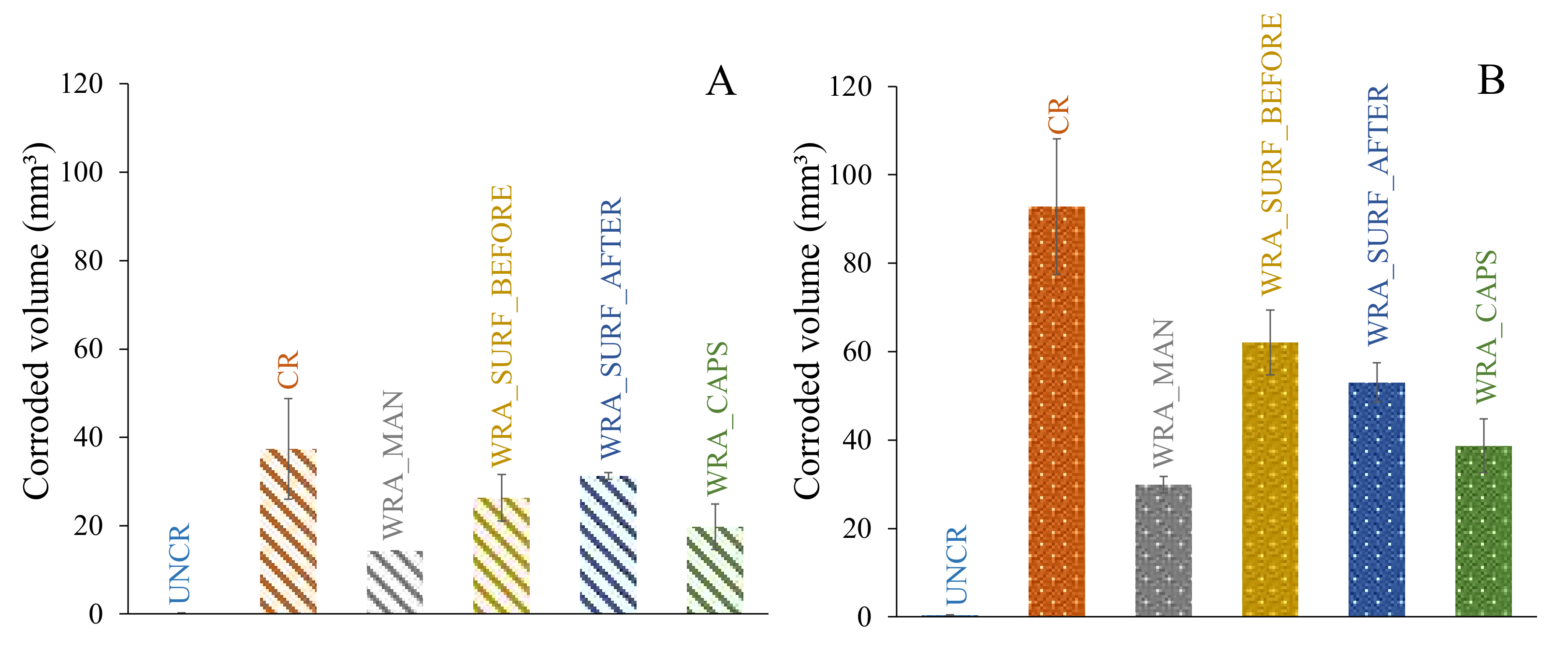
- It was clear that only the uncracked test series was able to completely prevent the occurrence of rebar corrosion after 135 weekly cycles of chloride exposure.
- In case untreated cracked samples were submitted to a cyclic chloride exposure, all samples showed immediate initiation of corrosion.
- When the WRA was applied onto the surface before crack creation, a small delay in corrosion onset was seen; however, after 4–6 weeks, the first signs of corrosion became visible and when considering the corrosion propagation, not that much improvement compared to the untreated samples was noticed.
- When the WRA was applied on the surface after crack formation, one sample already showed signs of corrosion after 5 weeks, but the two other samples were able to resist corrosion for 26–42 weeks.
- A real improvement in terms of delay of corrosion initiation and corrosion propagation was noticed when the healing agent was injected into the crack. When the agent was manually injected some signs of corrosion were seen after 19 weeks; however, only after 64 weeks a clear onset of corrosion occurred. When the agent was released through embedded capsules, clear signs of corrosion onset were noticed after 39–64 weeks. Moreover, for both of the latter test series, the measured macro-cell corrosion current was clearly lower compared to the other series, which was a proof that the corrosion propagated slower.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Verma, S.K.; Bhadauria, S.S.; Akhtar, S. Monitoring Corrosion of Steel Bars in Reinforced Concrete Structures. Sci. World J. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bargaheiser, K.; Butalia, T.S. Prevention of corrosion in concrete using fly ash concrete mixes. In Proceedings of the Concrete Technology Forum, Dallas, TX, USA, 22–25 May 2007. [Google Scholar]
- Kaur, K.; Goyal, S.; Bhattacharjee, B.; Kumar, M. Efficiency of Migratory-Type Organic Corrosion Inhibitors in Carbonated Environment. J. Adv. Concr. Technol. 2016, 14, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Kaur, K.; Goyal, S.; Bhattacharjee, B.; Kumar, M. Electrochemical Impedance Spectroscopy to Study the Carbonation Behavior of Concrete Treated with Corrosion Inhibitors. J. Adv. Concr. Technol. 2017, 15, 738–748. [Google Scholar] [CrossRef] [Green Version]
- Söylev, T.A.; Richardson, M. Corrosion inhibitors for steel in concrete: State-of-the-art report. Constr. Build. Mater. 2008, 22, 609–622. [Google Scholar] [CrossRef]
- Okeniyi, J.; Omotosho, O.; Ajayi, O.; Loto, C.A. Effect of potassium-chromate and sodium-nitrite on concrete steel-rebar degradation in sulphate and saline media. Constr. Build. Mater. 2014, 50, 448–456. [Google Scholar] [CrossRef]
- Garcés, P.; Saura, P.; Zornoza, E.; Andrade, C. Influence of pH on the nitrite corrosion inhibition of reinforcing steel in simulated concrete pore solution. Corros. Sci. 2011, 53, 3991–4000. [Google Scholar] [CrossRef]
- Brenna, A.; Bolzoni, F.; Beretta, S.; Ormellese, M. Long-term chloride-induced corrosion monitoring of reinforced concrete coated with commercial polymer-modified mortar and polymeric coatings. Constr. Build. Mater. 2013, 48, 734–744. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, S.; Sharma, S.K.; Mehta, R. Evaluation of corrosion inhibition and self healing capabilities of nanoclay and tung oil microencapsulated epoxy coatings on rebars in concrete. Constr. Build. Mater. 2020, 259, 120278. [Google Scholar] [CrossRef]
- Dong, B.; Ding, W.; Qin, S.; Fang, G.; Liu, Y.; Dong, P.; Hong, S. 3D visualized tracing of rebar corro-sion-inhibiting features in concrete with a novel chemical self-healing system. Constr. Build. Mater. 2018, 168, 11–20. [Google Scholar] [CrossRef]
- Dong Biqin, D.; Weijian, Q.; Shaofeng, H.; Ningxu, F.; Guohao, L.; Yuqing, X.; Feng, H.; Shuxian, H. Chemical self-healing system with novel microcapsules for corrosion inhibition of rebar in concrete. Cem. Concr. Compos. 2017, 85. [Google Scholar]
- Çopuroğlu, O.; Fraaij, A.; Bijen, J. Effect of sodium monofluorophosphate treatment on microstructure and frost salt scaling durability of slag cement paste. Cem. Concr. Res. 2006, 36, 1475–1482. [Google Scholar] [CrossRef]
- Xu, J.; Tang, Y.; Wang, X.; Wang, Z.; Yao, W. Application of ureolysis-based microbial CaCO3 precipitation in self-healing of concrete and inhibition of reinforcement corrosion. Constr. Build. Mater. 2020, 265, 120364. [Google Scholar] [CrossRef]
- Erşan, Y.; Van Tittelboom, K.; Boon, N.; De Belie, N. Nitrite producing bacteria inhibit reinforcement bar corrosion in cementitious materials. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, K.M.; Taher, F.M.; El-Tawab, A.A.; Faried, A.S. Role of different microorganisms on the mechanical characteristics, self-healing efficiency, and corrosion protection of concrete under different curing conditions. J. Build. Eng. 2021, 41, 102414. [Google Scholar] [CrossRef]
- Van Belleghem, B.; Kessler, S.; Heede, P.V.D.; Van Tittelboom, K.; De Belie, N. Chloride induced reinforcement corrosion behavior in self-healing concrete with encapsulated polyurethane. Cem. Concr. Res. 2018, 113, 130–139. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; Van Belleghem, B.; Boone, M.N.; Van Hoorebeke, L.; De Belie, N. X-ray radiography to visualize the re-bar-cementitious matrix interface and judge the delay in corrosion through self-repair by encapsulated polyurethane. Adv. Mater. Interfaces 2018, 5, 1701021. [Google Scholar] [CrossRef]
- Van Belleghem, B. Effect of Capsule-Based Self-Healing on Chloride Induced Corrosion of Reinforced Concrete. Ph.D. Thesis, Ghent University, Ghen, Belgium, 2018; p. 328. [Google Scholar]
- Van Tittelboom, K.; De Maesschalck, C.; Van Belleghem, B.; Van den Heede, P.; Kessler, S.; De Belie, N. Self-Healing of Concrete Cracks by the Release of Embedded Water Repellent Agents and Corrosion Inhibitors to Reduce the Risk of Reinforcement Corrosion. In Proceedings of the IV DBMC 14th International Conference on Durability of Building Materials and Components (XIV DBMC 2017), Paris, France, 29–31 May 2017; De Schutter, G., De Belie, N., Janssens, A., Van Den Bossche, N., Eds.; RILEM Publications: Paris, France, 2017; pp. 1–10. [Google Scholar]
- Dai, J.; Akira, Y.; Kato, E.; Yokota, H. Investigation of Chloride Ingress in Cracked Concrete Treated with Water Repellent Agents. In Proceedings of the Hydrophobe V, Fifth International Conference on Water Repellent Treatment of Building Materials, Brussels, Belgium, 15–16 April 2008; Aedificatio Publishers: Freiburg, Germany, 2008; pp. 299–310. [Google Scholar]
- Dai, J.-G.; Akira, Y.; Wittmann, F.; Yokota, H.; Zhang, P. Water repellent surface impregnation for extension of service life of reinforced concrete structures in marine environments: The role of cracks. Cem. Concr. Compos. 2010, 32, 101–109. [Google Scholar] [CrossRef]
- NBN. EN 12350-2 Testing Fresh Concrete—Part 2: Slump-Test 2009; NBN: Brussels, Belgium, 2009. [Google Scholar]
- NBN. EN 12350-5 Testing Fresh Concrete—Part 5: Flow Table Test; NBN: Brussels, Belgium, 2019. [Google Scholar]
- NBN. EN 12350-7 Testing Fresh Concrete—Part 7: Air Content-Pressure Methods; NBN: Brussels, Belgium, 2009. [Google Scholar]
- NBN. EN 12390-1 Testing Hardened Concrete—Part 1: Shape, Dimensions and other Requirements for Specimens and Moulds; NBN: Brussels, Belgium, 2012. [Google Scholar]
- Hiemer, F.; Keßler, S.; Gehlen, C.; Wiedmann, A.; Kotan, E.; Müller, H. Reinforcement corrosion behavior in bending cracks after short-time chloride exposure. In Proceedings of the 4th International Conference on Concrete Repair, Rehabilitation and Retrofitting, Leipzig, Germany, 5–7 October 2015. [Google Scholar] [CrossRef]
- Hiemer, F.; Keßler, S.; Gehlen, C. Development of chloride induced reinforcement corrosion in cracked concrete after Application of a Surface Protection System. In Proceedings of the Concrete Solutions 6th International Conference on Concrete Repair, Thessaloniki, Greece, 20–23 June 2016. [Google Scholar]
- Keßler, S.; Hiemer, F.; Gehlen, C. Einfluss einer Betonbeschichtung auf die Mechanismen der Bewehrungkorrosion in gerissenem Stahlbeton. Beton Stahlbetonbau 2017, 112, 198–206. [Google Scholar] [CrossRef]
- Van Steen, C.; Wevers, M.; Verstrynge, E. X-Ray Computed Tomography for the Detection of Corrosion-Induced Damage at the Reinforcement-Concrete Interface. In Proceedings of the 7th Conference on Industrial Computed Tomography, Leuven, Belgium, 7–9 February 2017. [Google Scholar]
- Michel, A.; Pease, B.J.; Geiker, M.R.; Stang, H.; Olesen, J.F. Monitoring reinforcement corrosion and corrosion-induced cracking using non-destructive x-ray attenuation measurements. Cem. Concr. Res. 2011, 41, 1085–1094. [Google Scholar] [CrossRef]
- Masschaele, B.; Dierick, M.; Van Loo, D.; Boone, M.N.; Brabant, L.; Pauwels, E.; Cnudde, V.; Van Hoorebeke, L. HECTOR: A 240kV Micro-CT Setup Optimized for Research. J. Phys. Conf. Ser. 2013, 463, 012012. [Google Scholar] [CrossRef]
- Vlassenbroeck, M.J.; Dierick, M.; Masschaele, B.; Cnudde, V.; Van Hoorebeke, L.; Jacobs, P. Software tools for quantification of X-ray microtomography at the UGCT. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2007, 580, 442–445. [Google Scholar] [CrossRef]
- Boone, M.; De Kock, T.; Bultreys, T.; De Schutter, G.; Vontobel, P.; Van Hoorebeke, L.; Cnudde, V. 3D mapping of water in oolithic limestone at atmospheric and vacuum saturation using X-ray micro-CT differential imaging. Mater. Charact. 2014, 97, 150–160. [Google Scholar] [CrossRef]
- Marcotte, T.D.; Hansson, C.M. The influence of silica fume on the corrosion resistance of steel in high performance concrete exposed to simulated sea water. J. Mater. Sci. 2003, 38, 4765–4776. [Google Scholar] [CrossRef]





| Component | Amount |
|---|---|
| Sand 0/4 (natural river sand) | 696 kg/m3 |
| Aggregates 2/8 (rounded gravel) | 502 kg/m3 |
| Aggregates 8/16 (rounded gravel) | 654 kg/m3 |
| Cement | 317.6 kg/m3 |
| Fly ash | 56 kg/m3 |
| Water | 153 kg/m3 |
| Superplasticizer | 3 mL/kg binder |
| Property | Batch 1 | Batch 2 |
|---|---|---|
| Slump class | S3 | S3 |
| Flow class Density [kg/m3] | F3 2371 ± 6.6 | F3 2363 ± 10.7 |
| Compressive strength [N/mm2] | 61.3 ± 0.6 | 63.4 ± 3.6 |
| Series Code | Cracked | Healed | Further Details |
|---|---|---|---|
| UNCR | Uncracked beams | ||
| CR | √ | Cracked beams of which the crack was left untreated | |
| WRA_MAN | √ | √ | Cracked beams of which the crack was manually injected with WRA |
| WRA_SURF_BEFORE | √ | √ | Cracked beams of which the test surface was sprayed with WRA before cracking |
| WRA_SURF_AFTER | √ | √ | Cracked beams of which the test surface was sprayed with WRA after cracking |
| WRA_CAPS | √ | √ | Cracked beams with embedded capsules filled with WRA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Tittelboom, K.; Van Belleghem, B.; Van den Heede, P.; Van Der Putten, J.; Callens, R.; Van Stappen, J.; Deprez, M.; Cnudde, V.; De Belie, N. Manual Application versus Autonomous Release of Water Repellent Agent to Prevent Reinforcement Corrosion in Cracked Concrete. Processes 2021, 9, 2101. https://doi.org/10.3390/pr9122101
Van Tittelboom K, Van Belleghem B, Van den Heede P, Van Der Putten J, Callens R, Van Stappen J, Deprez M, Cnudde V, De Belie N. Manual Application versus Autonomous Release of Water Repellent Agent to Prevent Reinforcement Corrosion in Cracked Concrete. Processes. 2021; 9(12):2101. https://doi.org/10.3390/pr9122101
Chicago/Turabian StyleVan Tittelboom, Kim, Bjorn Van Belleghem, Philip Van den Heede, Jolien Van Der Putten, Renaat Callens, Jeroen Van Stappen, Maxim Deprez, Veerle Cnudde, and Nele De Belie. 2021. "Manual Application versus Autonomous Release of Water Repellent Agent to Prevent Reinforcement Corrosion in Cracked Concrete" Processes 9, no. 12: 2101. https://doi.org/10.3390/pr9122101
APA StyleVan Tittelboom, K., Van Belleghem, B., Van den Heede, P., Van Der Putten, J., Callens, R., Van Stappen, J., Deprez, M., Cnudde, V., & De Belie, N. (2021). Manual Application versus Autonomous Release of Water Repellent Agent to Prevent Reinforcement Corrosion in Cracked Concrete. Processes, 9(12), 2101. https://doi.org/10.3390/pr9122101









