Investigation of Effects of Copper, Zinc, and Strontium Doping on Electrochemical Properties of Titania Nanotube Arrays for Neural Interface Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Nanotube Arrays
2.2. Characterization of the Annealed Nanotube Arrays
2.3. Electrochemical Properties of the Fabricated Nanotube Arrays
2.4. Contact Angle Measurements
2.5. Vero Cell Culture on the Fabricated Nanotube Arrays
2.6. Mouse Embryo Dissection Procedure
2.7. Cortical Neurons Culture on the Fabricated Nanotube Arrays
2.8. Cell Counting
3. Results and Discussion
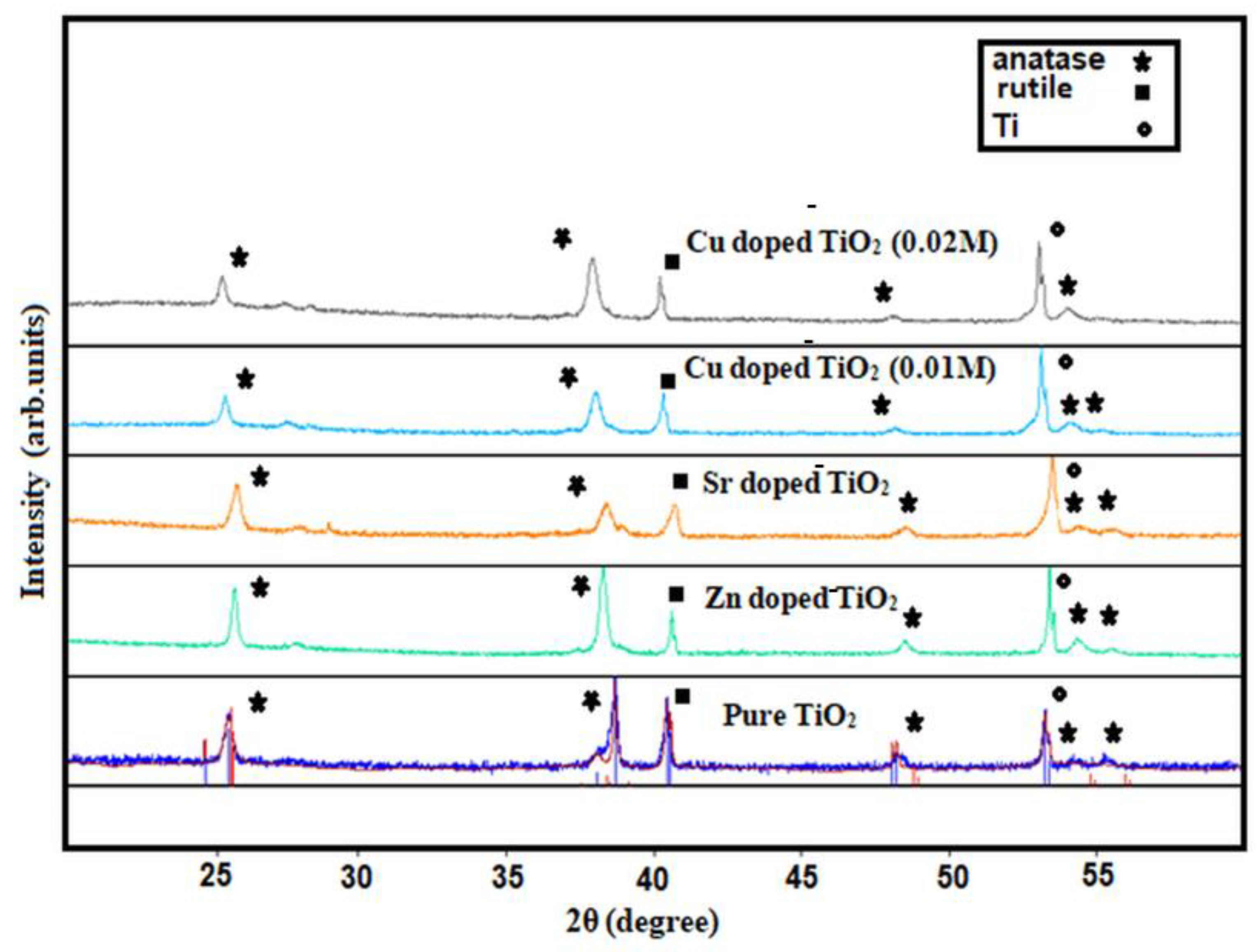
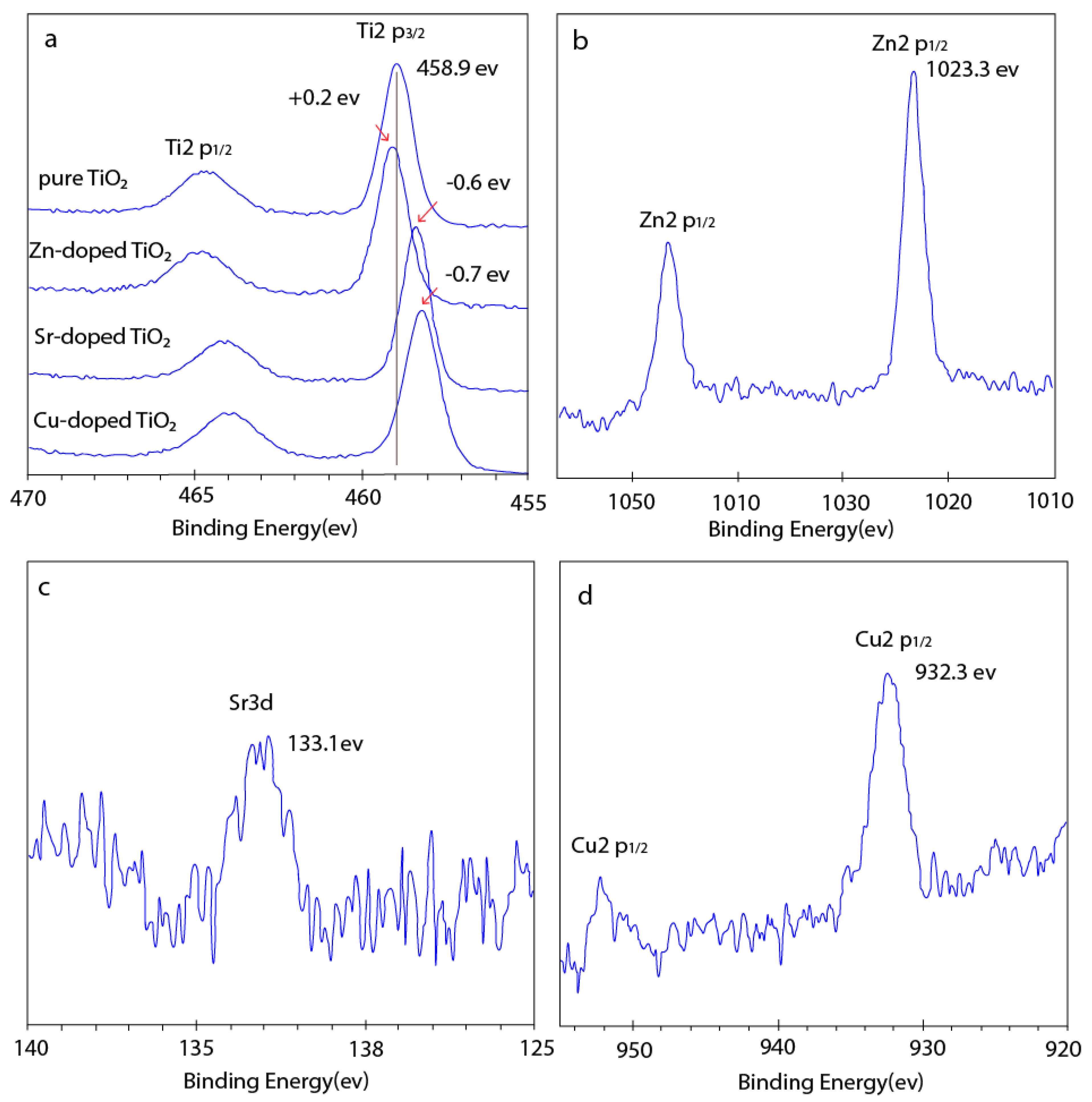
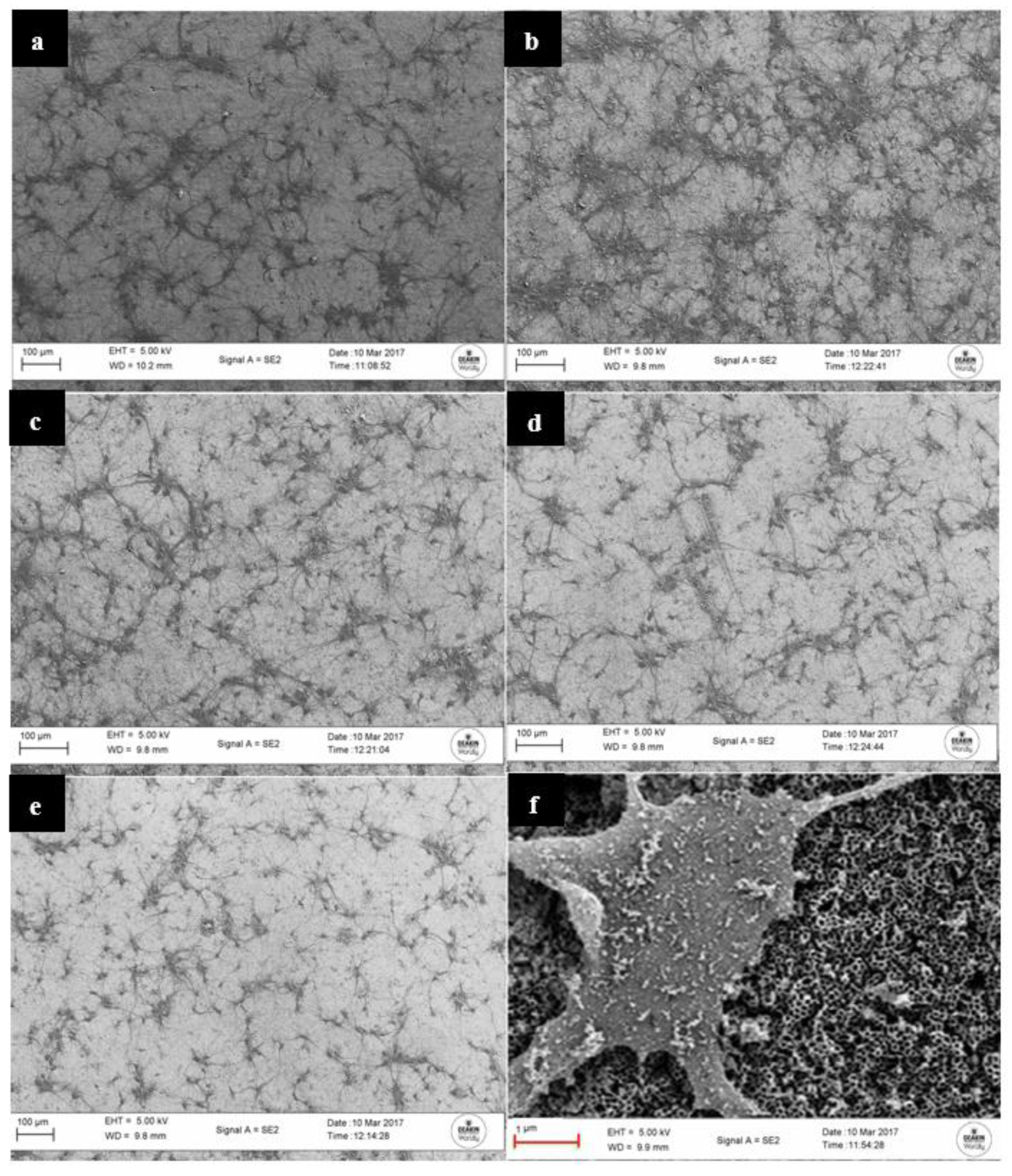
3.1. Structure and Morphology of the Fabricated Nanotube Arrays
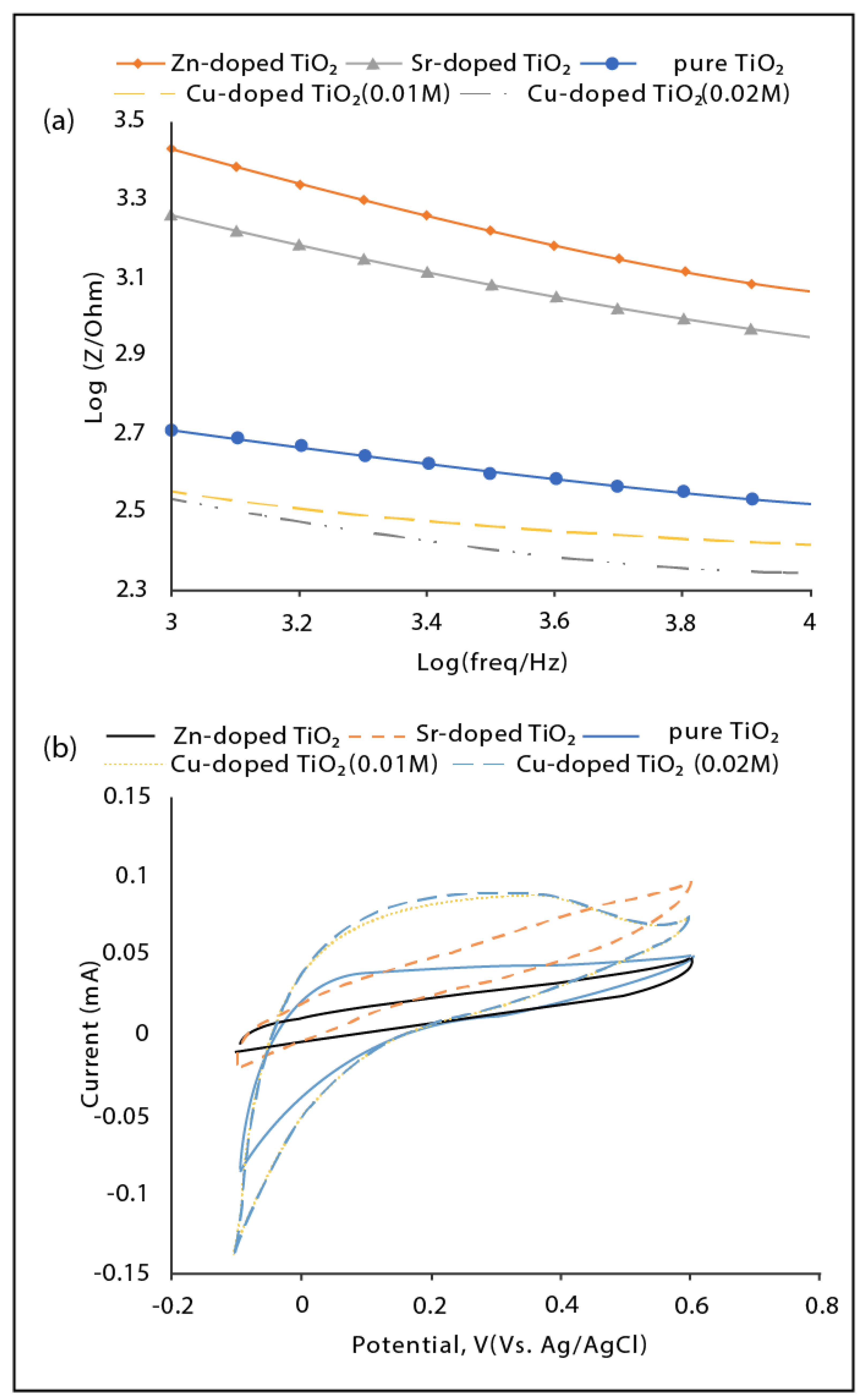
3.2. Electrochemical Performance of the Fabricated Nanotube Arrays
3.3. Wettability of the Fabricated Nanotube Arrays
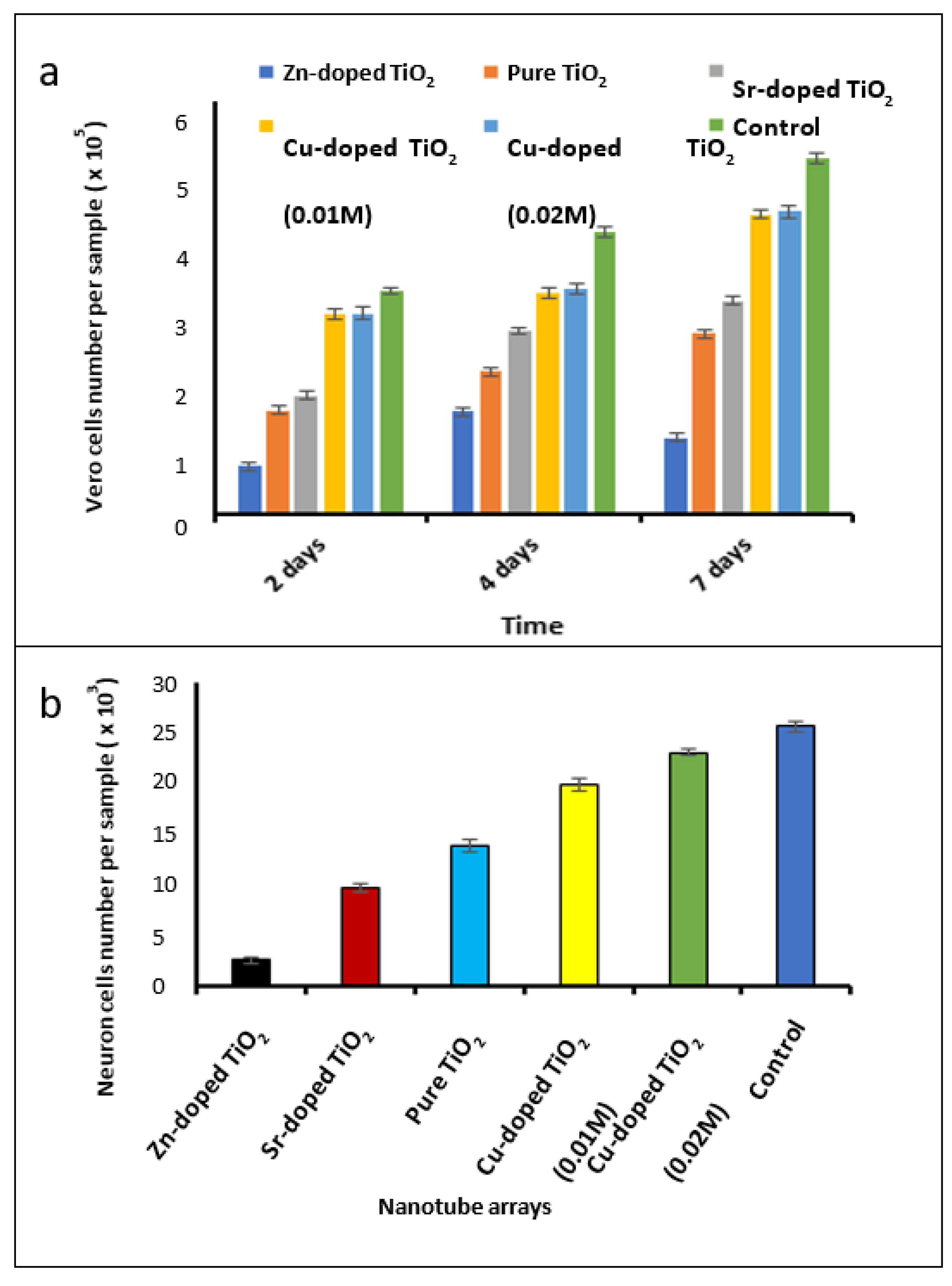
3.4. Investigation of Biocompatibility of the Fabricated Nanotube Arrays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angotzi, G.N.; Boi, F.; Lecomte, A.; Miele, E.; Malerba, M.; Zucca, S.; Casile, A.; Berdondini, L. Sinaps: An implantable active pixel sensor CMOS-probe for simultaneous large-scale neural recordings. Biosens. Bioelectron. 2019, 126, 355–364. [Google Scholar] [CrossRef]
- Carli, S.; Lambertini, L.; Zucchini, E.; Ciarpella, F.; Scarpellini, A.; Prato, M.; Castagnola, E.; Fadiga, L.; Ricci, D. Single walled carbon nanohorns composite for neural sensing and stimulation. Sens. Actuators B Chem. 2018, 271, 280–288. [Google Scholar] [CrossRef]
- Kotov, N.A.; Winter, J.O.; Clements, I.P.; Jan, E.; Timko, B.P.; Campidelli, S.; Pathak, S.; Mazzatenta, A.; Lieber, C.M.; Prato, M.; et al. Nanomaterials for neural interfaces. Adv. Mater. 2009, 21, 3970–4004. [Google Scholar] [CrossRef] [Green Version]
- Khudhair, D.; Nahavandi, S.; Garmestani, H.; Bhatti, A. Microelectrode arrays: Architecture, challenges and engineering solutions. In Emerging Trends in Neuro Engineering and Neural Computation; Springer: Cham, Switzerland, 2017; pp. 41–59. [Google Scholar]
- Xie, C.; Lin, Z.; Hanson, L.; Cui, Y.; Cui, B. Intracellular recording of action potentials by nanopillar electroporation. Nat. Nanotechnol. 2012, 7, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Brüggemann, D.; Wolfrum, B.; Maybeck, V.; Mourzina, Y.; Jansen, M.; Offenhäusser, A. Nanostructured gold microelectrodes for extracellular recording from electrogenic cells. Nanotechnology 2011, 22, 265104. [Google Scholar] [CrossRef]
- Suyatin, D.B.; Wallman, L.; Thelin, J.; Prinz, C.N.; Jörntell, H.; Samuelson, L.; Montelius, L.; Schouenborg, J. Nanowire-based electrode for acute in vivo neural recordings in the brain. PLoS ONE 2013, 8, e56673. [Google Scholar] [CrossRef]
- Heo, D.N.; Kim, H.-J.; Lee, Y.J.; Heo, M.; Lee, S.J.; Lee, D.; Do, S.H.; Lee, S.H.; Kwon, I.K. Flexible and highly biocompatible nanofiber-based electrodes for neural surface interfacing. ACS Nano 2017, 11, 2961–2971. [Google Scholar] [CrossRef]
- Yu, Z.; McKnight, T.E.; Ericson, M.N.; Melechko, A.V.; Simpson, M.L.; Morrison, B. Vertically aligned carbon nanofiber neural chip for interfacing with neurological system. In Proceedings of the 4th IEEE International Conference on Nano/Molecular Medicine and Engineering (IEEE-NANOMED), Hong Kong, China, 5–9 December 2010; pp. 188–191. [Google Scholar]
- Lin, Z.; Xie, C.; Osakada, Y.; Cui, Y.; Cui, B. Iridium oxide nanotube electrodes for sensitive and prolonged intracellular measurement of action potentials. Nat. Commun. 2014, 5, 3206. [Google Scholar] [CrossRef]
- Richards, C.I.; Luong, K.; Srinivasan, R.; Turner, S.W.; Dougherty, D.A.; Korlach, J.; Lester, H.A. Live-Cell Imaging of Single Receptor Composition Using Zero-Mode Waveguide Nanostructures. Nano Lett. 2012, 12, 3690–3694. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Fishman, H.A.; Dai, H.; Harris, J.S. Neural Stimulation with a Carbon Nanotube Microelectrode Array. Nano Lett. 2006, 6, 2043–2048. [Google Scholar] [CrossRef]
- Khudhair, D.; Hamedani, H.A.; Gaburro, J.; Shafei, S.; Nahavandi, S.; Garmestani, H.; Bhatti, A. Enhancement of electro-chemical properties of TiO2 nanotubes for biological interfacing. Mater. Sci. Eng. C 2017, 77, 111–120. [Google Scholar] [CrossRef]
- Khudhair, D.; Gaburro, J.; Shafei, S.; Barlow, A.; Nahavandi, S.; Bhatti, A. Enhancement of electrochemical properties of micro/nano electrodes based on TiO2 nanotube arrays. J. Phys. Conf. Ser. 2017, 829, 012010. [Google Scholar] [CrossRef]
- Grimes, C.A.; Mor, G.K. TiO2 Nanotube Arrays: Synthesis, Properties, and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Zhou, M.; Glushenkov, A.M.; Kartachova, O.; Li, Y.; Chen, Y. Titanium dioxide nanotube films for electrochemical supercapacitors: Biocompatibility and operation in an electrolyte based on a physiological fluid. J. Electrochem. Soc. 2015, 162, A5065–A5069. [Google Scholar] [CrossRef]
- Khudhair, D.; Bhatti, A.; Li, Y.; Hamedani, H.; Garmestani, H.; Hodgson, P.; Nahavandi, S. Anodization parameters influencing the morphology and electrical properties of TiO2 nanotubes for living cell interfacing and investigations. Mater. Sci. Eng. C 2016, 59, 1125–1142. [Google Scholar] [CrossRef]
- Shim, S.C.; Choe, B.H.; Jang, I.S.; Choi, D.S.; Lee, J.K.; Cha, B.K.; Choi, W.Y. Effect of TiO2 Nanotube Arrays on Osseointegration for Dental Implant. Adv. Mater. Res. 2014, 922, 71–74. [Google Scholar] [CrossRef]
- Lai, Y.-K.; Wang, Q.; Huang, J.-Y.; Li, H.-Q.; Chen, Z.; Zhao, A.Z.-J.; Wang, Y.; Zhang, K.-Q.; Sun, H.-T.; Al-Deyab, S.S. TiO2 nanotube platforms for smart drug delivery: A review. Int. J. Nanomed. 2016, 11, 4819–4834. [Google Scholar] [CrossRef] [Green Version]
- Camargo, S.E.A.; Xia, X.; Fares, C.; Ren, F.; Hsu, S.-M.; Budei, D.; Aravindraja, C.; Kesavalu, L.; Esquivel-Upshaw, J.F. Nanostructured Surfaces to Promote Osteoblast Proliferation and Minimize Bacterial Adhesion on Titanium. Materials 2021, 14, 4357. [Google Scholar] [CrossRef]
- Oh, S.; Brammer, K.S.; Li, Y.S.J.; Teng, D.; Engler, A.J.; Chien, S.; Jin, S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. USA 2009, 106, 2130–2135. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Bauer, S.; Schmuki, P.; von der Mark, K. Narrow Window in Nanoscale Dependent Activation of Endothelial Cell Growth and Differentiation on TiO2 Nanotube Surfaces. Nano Lett. 2009, 9, 3157–3164. [Google Scholar] [CrossRef]
- Szkoda, M.; Lisowska-Oleksiak, A.; Siuzdak, K. Optimization of boron-doping process of titania nanotubes via electrochemical method toward enhanced photoactivity. J. Solid State Electrochem. 2016, 20, 1765–1774. [Google Scholar] [CrossRef] [Green Version]
- Shikandar, D.; Shetti, N.; Kulkarni, R.; Kulkarni, S. Silver-doped titania modified carbon electrode for electrochemical studies of Furantril. ECS J. Solid State Sci. Technol. 2018, 7, Q3215–Q3220. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Kakarla, R.R.; Reddy, C.; Aminabhavi, T.M. ZnO-based nanostructured electrodes for electrochemical sensors and biosensors in biomedical applications. Biosens. Bioelectron. 2019, 141, 111–417. [Google Scholar] [CrossRef]
- Bukkitgar, S.D.; Shetti, N.P.; Kulkarni, R.M.; Reddy, K.R.; Shukla, S.S.; Saji, V.S.; Aminabhavi, T.M. Electro-catalytic behavior of Mg-doped ZnO nano-flakes for oxidation of anti-inflammatory drug. J. Electrochem. Soc. 2019, 166, B3072–B3078. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Wen, C.; Li, Y. Processing and characterization of SrTiO3–TiO2 nanoparticle–nanotube heterostructures on titanium for biomedical applications. ACS Appl. Mater. Interfaces 2015, 7, 16018–16026. [Google Scholar] [CrossRef]
- Hamedani, H.A.; Allam, N.K.; Garmestani, H.; El-Sayed, M.A. Electrochemical fabrication of strontium-doped TiO2 nanotube array electrodes and investigation of their photoelectrochemical properties. J. Phys. Chem. C 2011, 115, 13480–13486. [Google Scholar] [CrossRef]
- Wang, S.; Meng, K.; Zhao, L.; Jiang, Q.; Lian, J. Superhydrophilic Cu-doped TiO2 thin film for solar-driven photocatalysis. Ceram. Int. 2014, 40, 5107–5110. [Google Scholar] [CrossRef]
- Wers, E.; Bunetel, L.; Oudadesse, H.; Lefeuvre, B.; Lucas-Girot, A.; Mostafa, A.; Pellen, P. Effect of Copper and Zinc on the Bioactivity and Cells Viability of Bioactive Glasses. Bioceram. Dev. Appl. 2013, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Ghane-Motlagh, B.; Sawan, M. A review of Microelectrode Array technologies: Design and implementation challenges. In Proceedings of the 2nd International Conference on Advances in Biomedical Engineering, Beirut, Lebanon, 11–13 September 2013; pp. 38–41. [Google Scholar]
- Shedid, S.A.; Ghannam, M.T. Influences of Droplet Volume on Contact Angle of Reservoir Rocks. Energy Sources 2005, 27, 1085–1097. [Google Scholar] [CrossRef]
- Kirkpatrick, C. Biological testing of materials and medical devices-a critical view of current and proposed methodologies for biocompatibility testing: Cytotoxicity in vitro. Regul. Affairs 1992, 4, 13–32. [Google Scholar]
- Hamedani, H.A.; Allam, N.K.; El-Sayed, M.A.; Khaleel, M.A.; Garmestani, H.; Alamgir, F.M. An Experimental Insight into the Structural and Electronic Characteristics of Strontium-Doped Titanium Dioxide Nanotube Arrays. Adv. Funct. Mater. 2014, 24, 6783–6796. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Ghonchegi, Z. Fabrication and characterization of copper doped TiO2 nanotube arrays by in situ electrochemical method as efficient visible-light photocatalyst. Ceram. Int. 2015, 41, 8735–8741. [Google Scholar] [CrossRef]
- Heckelsberg, L.F.; Calrk, A.; Bailey, G.C. Electrical Conductivity and Catalytic Activity of Zinc Oxide. J. Phys. Chem. 1956, 60, 559–561. [Google Scholar] [CrossRef]
- Chao, S.; Petrovsky, V.; Dogan, F. Complex Impedance Study of Fine and Coarse Grain TiO2 Ceramics. J. Am. Ceram. Soc. 2010, 93, 3031–3034. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, Y.; Garcia, B.B.; Sepehri, S.; Liu, D.; Cao, G. Nanostructured electrode with titania nanotube arrays: Fabrication, electrochemical properties, and applications for biosensing. J. Nanosci. Nanotechnol. 2009, 9, 2426–2436. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.; Sun, X.; He, X. Effect of the geometry of the anodized titania nanotube array on the performance of dye-sensitized solar cells. J. Nanosci. Nanotechnol. 2010, 10, 4551–4561. [Google Scholar] [CrossRef]
- Mohajernia, S.; Hejazi, S.; Andryskova, P.; Zoppellaro, G.; Tomanec, O.; Zboril, R.; Schmuki, P. Conductive Cu doped TiO2 nanotubes for enhanced photoelectrochemical methanol oxidation and concomitant hydrogen generation. ChemElectroChem 2019, 6, 1244–1249. [Google Scholar] [CrossRef]
- Salari, M.; Konstantinov, K.; Liu, H.K. Enhancement of the capacitance in TiO2 nanotubes through controlled introduction of oxygen vacancies. J. Mater. Chem. 2011, 21, 5128–5133. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Wang, S.; Li, W.; Yuan, X.; Bai, Y. Hydrophobic ZnO-TiO2 nanocomposite with photocatalytic promoting self-cleaning surface. Int. J. Photoenergy 2015, 2015, 925638. [Google Scholar] [CrossRef] [Green Version]
- Brammer, K.S.; Oh, S.; Frandsen, C.J.; Jin, S. Biomaterials and biotechnology schemes utilizing TiO2 nanotube arrays. Biomater. Sci. Eng. 2011, 193–210. [Google Scholar]
- Sorkin, J.A. Titania Nanotube Arrays as Potential Interfaces for Neurological Prostheses. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2014. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khudhair, D.; Gaburro, J.; Hamedani, H.A.; Barlow, A.; Garmestani, H.; Bhatti, A. Investigation of Effects of Copper, Zinc, and Strontium Doping on Electrochemical Properties of Titania Nanotube Arrays for Neural Interface Applications. Processes 2021, 9, 2099. https://doi.org/10.3390/pr9122099
Khudhair D, Gaburro J, Hamedani HA, Barlow A, Garmestani H, Bhatti A. Investigation of Effects of Copper, Zinc, and Strontium Doping on Electrochemical Properties of Titania Nanotube Arrays for Neural Interface Applications. Processes. 2021; 9(12):2099. https://doi.org/10.3390/pr9122099
Chicago/Turabian StyleKhudhair, Dhurgham, Julie Gaburro, Hoda Amani Hamedani, Anders Barlow, Hamid Garmestani, and Asim Bhatti. 2021. "Investigation of Effects of Copper, Zinc, and Strontium Doping on Electrochemical Properties of Titania Nanotube Arrays for Neural Interface Applications" Processes 9, no. 12: 2099. https://doi.org/10.3390/pr9122099
APA StyleKhudhair, D., Gaburro, J., Hamedani, H. A., Barlow, A., Garmestani, H., & Bhatti, A. (2021). Investigation of Effects of Copper, Zinc, and Strontium Doping on Electrochemical Properties of Titania Nanotube Arrays for Neural Interface Applications. Processes, 9(12), 2099. https://doi.org/10.3390/pr9122099






