Optimization and Modeling of Ammonia Nitrogen Removal from High Strength Synthetic Wastewater Using Vacuum Thermal Stripping
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Composition
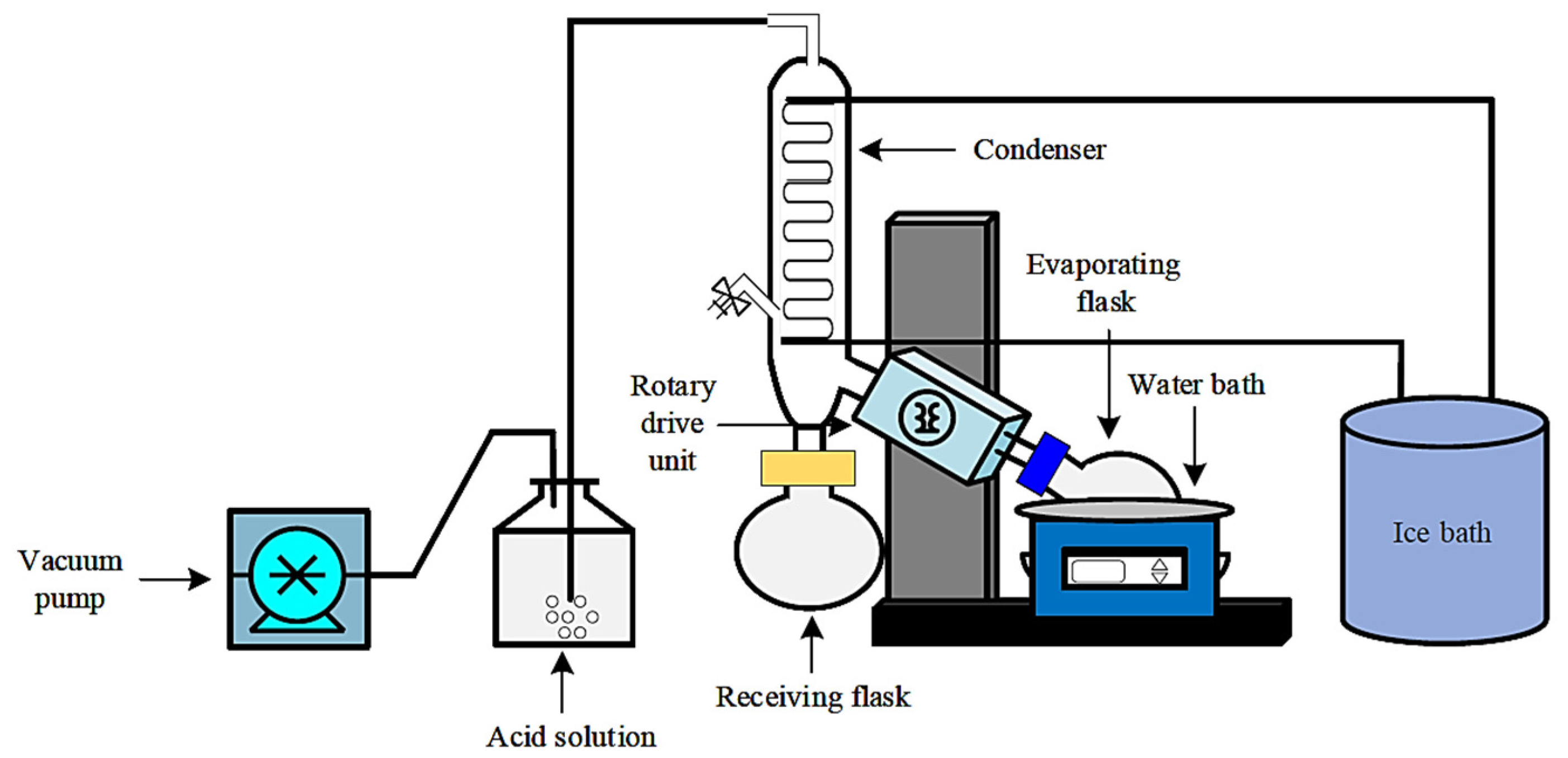
2.2. Experimental Setup
2.3. Experimental Design
2.3.1. Response Surface Methodology (RSM)
2.3.2. Artificial Neural Network (ANN)
2.4. Statistical Comparison between the Developed Models
2.5. Sampling and Analysis
3. Results and Discussion
3.1. Response Surface Methodology (RSM) Model
3.2. Effects of Operational Parameters on Ammonia Removal
3.3. Process Optimization and Validation
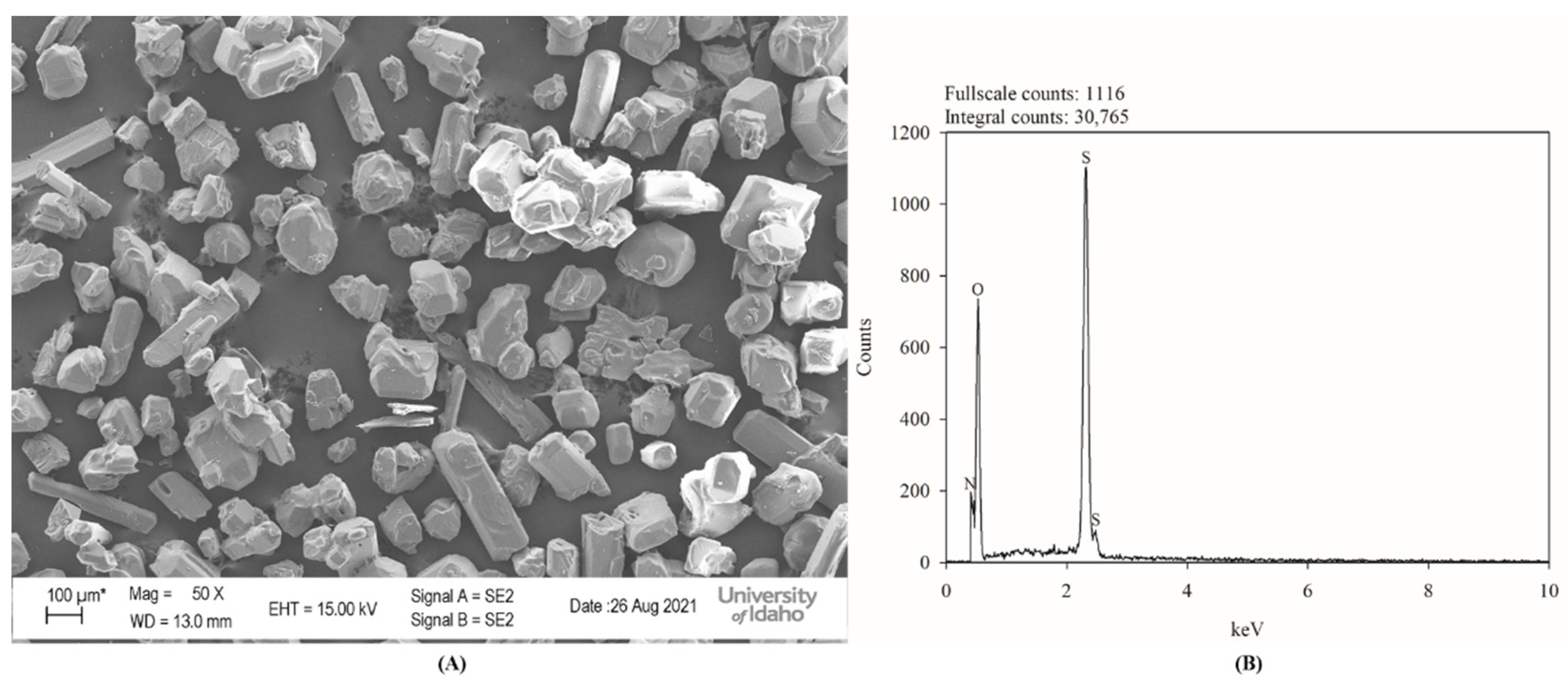
3.4. Ammonium Sulphate Recovery and Characterization
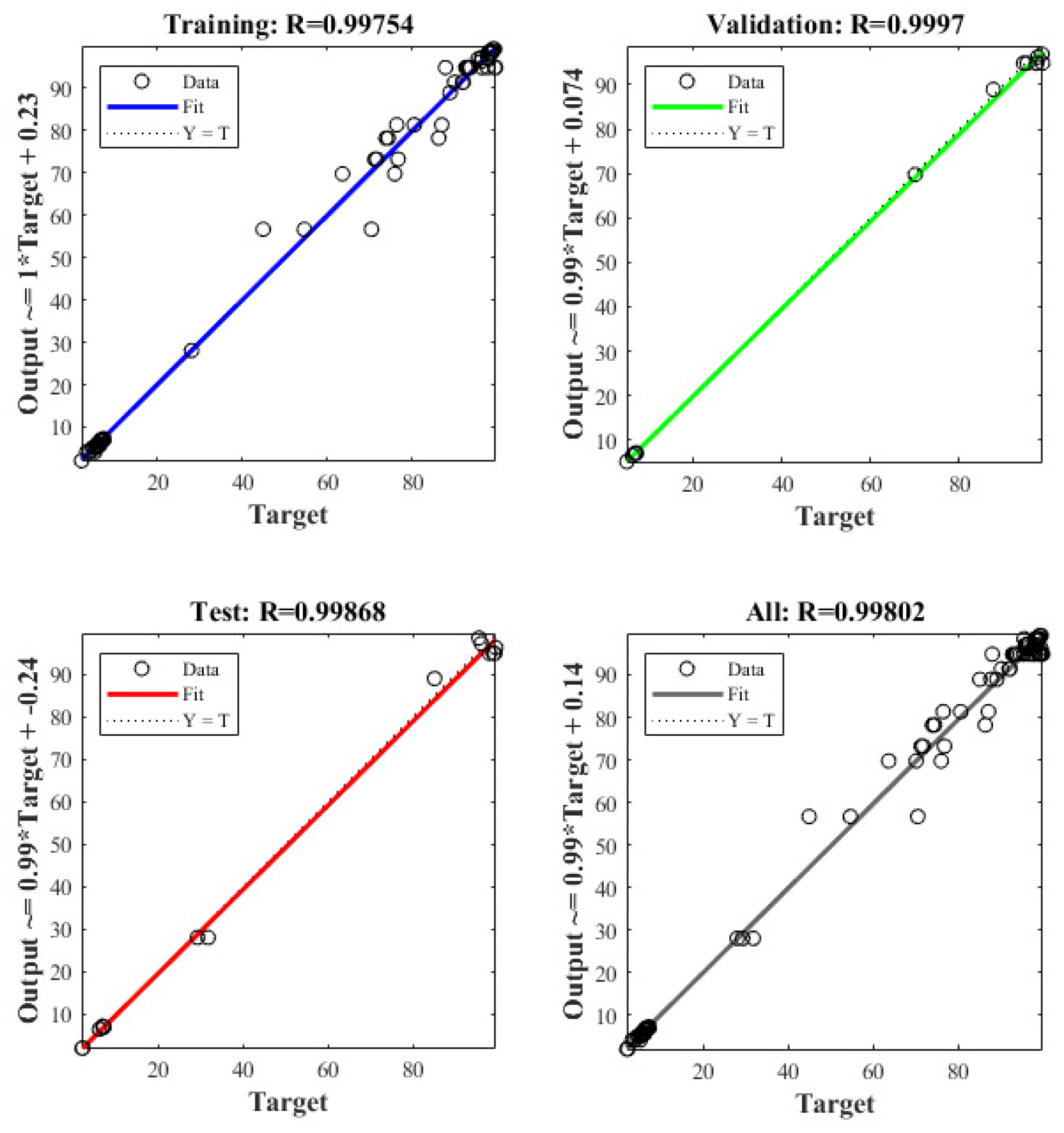
3.5. Response Surface Methodology (RSM)–Artificial Neural Network (ANN) Model
3.6. Model Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of US Freshwaters: Analysis of Potential Economic Damages. Environ. Sci. Technol. 2009, 45, 12–19. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, Y.; Zhang, G.; Ruan, R.; Wang, Y.; Wu, X.; Zheng, H.; Zhang, Q.; Cao, L. New Progress of Ammonia Recovery during Ammonia Nitrogen Removal from Various Wastewaters. World J. Microbiol. Biotechnol. 2020, 36, 1–20. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, J.; Waite, T.D. The Impact of Absorbents on Ammonia Recovery in a Capacitive Membrane Stripping System. Chem. Eng. J. 2020, 382, 122851. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.D.; Samah, R.A.; Puteh, M.H.; Ismail, A.F.; Mustafa, A.; Rahman, M.A.; Jaafar, J. Current Trends and Future Prospects of Ammonia Removal in Wastewater: A Comprehensive Review on Adsorptive Membrane Development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Ren, Z.; Jia, B.; Zhang, G.; Fu, X.; Wang, Z.; Wang, P.; Lv, L. Study on Adsorption of Ammonia Nitrogen by Iron-Loaded Activated Carbon from Low Temperature Wastewater. Chemosphere 2021, 262, 127895. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Altaf, M.M.; Khan, N.A.; Khan, A.H.; Khan, A.A.; Ahmed, S.; Kumar, P.S.; Naushad, M.; Rajapaksha, A.U.; Iqbal, J.; et al. Recent Technologies for Nutrient Removal and Recovery from Wastewaters: A Review. Chemosphere 2021, 277, 130328. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Chen, X.; Chen, J.; Feng, X.; Peng, X. Nitrogen Removal via Nitritation Pathway for Low-Strength Ammonium Wastewater by Adsorption, Biological Desorption and Denitrification. Bioresour. Technol. 2018, 267, 541–549. [Google Scholar] [CrossRef]
- Yang, H.; Li, D.; Zeng, H.; Zhang, J. Impact of Mn and Ammonia on Nitrogen Conversion in Biofilter Coupling Nitrification and ANAMMOX That Simultaneously Removes Fe, Mn and Ammonia. Sci. Total Environ. 2019, 648, 955–961. [Google Scholar] [CrossRef]
- Zubair, M.; Wang, S.; Zhang, P.; Ye, J.; Liang, J.; Nabi, M.; Zhou, Z.; Tao, X.; Chen, N.; Sun, K. Biological Nutrient Removal and Recovery from Solid and Liquid Livestock Manure: Recent Advance and Perspective. Bioresour. Technol. 2020, 301, 122823. [Google Scholar] [CrossRef]
- Jeong, G.; Jung, J.-H.; Lim, J.-H.; Won, Y.S.; Lee, J.-K. A Computational Mechanistic Study of Breakpoint Chlorination for the Removal of Ammonia Nitrogen from Water. J. Chem. Eng. Jpn. 2014, 47, 225–229. [Google Scholar] [CrossRef]
- Stefán, D.; Erdélyi, N.; Izsák, B.; Záray, G.; Vargha, M. Formation of Chlorination By-Products in Drinking Water Treatment Plants Using Breakpoint Chlorination. Microchem. J. 2019, 149, 104008. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Blatchley III, E.R.; Wang, X.; Ren, P. UV/Chlorine Process for Ammonia Removal and Disinfection By-Product Reduction: Comparison with Chlorination. Water Res. 2015, 68, 804–811. [Google Scholar] [CrossRef]
- Jorgensen, T.C.; Weatherley, L.R. Ammonia Removal from Wastewater by Ion Exchange in the Presence of Organic Contaminants. Water Res. 2003, 37, 1723–1728. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Ding, L. Recovery of Phosphate and Ammonia Nitrogen from the Anaerobic Digestion Supernatant of Activated Sludge by Chemical Precipitation. J. Clean. Prod. 2015, 102, 437–446. [Google Scholar] [CrossRef]
- Chai, L.; Cong, P.; Min, X.; Tang, C.; Song, Y.; Zhang, Y.; Zhang, J.; Mohammad, A.L.I. Two-Sectional Struvite Formation Process for Enhanced Treatment of Copper–Ammonia Complex Wastewater. Trans. Nonferrous Met. Soc. China 2017, 27, 457–466. [Google Scholar] [CrossRef]
- Reza, A.; Shim, S.; Kim, S.; Ahmed, N.; Won, S.; Ra, C. Nutrient Leaching Loss of Pre-Treated Struvite and Its Application in Sudan Grass Cultivation as an Eco-Friendly and Sustainable Fertilizer Source. Sustainability 2019, 11, 4204. [Google Scholar] [CrossRef]
- Zhao, Q.-B.; Ma, J.; Zeb, I.; Yu, L.; Chen, S.; Zheng, Y.-M.; Frear, C. Ammonia Recovery from Anaerobic Digester Effluent through Direct Aeration. Chem. Eng. J. 2015, 279, 31–37. [Google Scholar] [CrossRef]
- Serna-Maza, A.; Heaven, S.; Banks, C.J. Biogas Stripping of Ammonia from Fresh Digestate from a Food Waste Digester. Bioresour. Technol. 2015, 190, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Sotoft, L.F.; Pryds, M.B.; Nielsen, A.K.; Norddahl, B. Process Simulation of Ammonia Recovery from Biogas Digestate by Air Stripping with Reduced Chemical Consumption. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2015; Volume 37, pp. 2465–2470. [Google Scholar]
- Ji, Y.; Bai, J.; Li, J.; Luo, T.; Qiao, L.; Zeng, Q.; Zhou, B. Highly Selective Transformation of Ammonia Nitrogen to N2 Based on a Novel Solar-Driven Photoelectrocatalytic-Chlorine Radical Reactions System. Water Res. 2017, 125, 512–519. [Google Scholar] [CrossRef]
- Mao, X.; Xiong, L.; Hu, X.; Yan, Z.; Wang, L.; Xu, G. Remediation of Ammonia-Contaminated Groundwater in Landfill Sites with Electrochemical Reactive Barriers: A Bench Scale Study. Waste Manag. 2018, 78, 69–78. [Google Scholar] [CrossRef]
- Lee, G.; Kim, K.; Chung, J.; Han, J.-I. Electrochemical Ammonia Accumulation and Recovery from Ammonia-Rich Livestock Wastewater. Chemosphere 2021, 270, 128631. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, T. A Novel Selective Hybrid Cation Exchanger for Low-Concentration Ammonia Nitrogen Removal from Natural Water and Secondary Wastewater. Chem. Eng. J. 2015, 281, 295–302. [Google Scholar] [CrossRef]
- Qiang, J.; Zhou, Z.; Wang, K.; Qiu, Z.; Zhi, H.; Yuan, Y.; Zhang, Y.; Jiang, Y.; Zhao, X.; Wang, Z. Coupling Ammonia Nitrogen Adsorption and Regeneration Unit with a High-Load Anoxic/Aerobic Process to Achieve Rapid and Efficient Pollutants Removal for Wastewater Treatment. Water Res. 2020, 170, 115280. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Ukwuani, A.T. Coupling Thermal Stripping and Acid Absorption for Ammonia Recovery from Dairy Manure: Ammonia Volatilization Kinetics and Effects of Temperature, PH and Dissolved Solids Content. Chem. Eng. J. 2015, 280, 188–196. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Kang, J.-H.; Ahn, J.-H. Ammonia Removal from Swine Wastewater by Microwave-Assisted Stripping. J. Environ. Eng. 2020, 146, 04020089. [Google Scholar] [CrossRef]
- Melgaço, L.A.; Meers, E.; Mota, C.R. Ammonia Recovery from Food Waste Digestate Using Solar Heat-Assisted Stripping-Absorption. Waste Manag. 2020, 113, 244–250. [Google Scholar] [CrossRef]
- Bower, C.E.; Bidwell, J.P. Ionization of Ammonia in Seawater: Effects of Temperature, pH, and Salinity. J. Fish. Res. Board Can. 1978, 35, 1012–1016. [Google Scholar] [CrossRef]
- Anwar, S.W.; Tao, W. Cost Benefit Assessment of a Novel Thermal Stripping–Acid Absorption Process for Ammonia Recovery from Anaerobically Digested Dairy Manure. Water Pract. Technol. 2016, 11, 355–364. [Google Scholar] [CrossRef][Green Version]
- Ukwuani, A.T.; Tao, W. Developing a Vacuum Thermal Stripping–Acid Absorption Process for Ammonia Recovery from Anaerobic Digester Effluent. Water Res. 2016, 106, 108–115. [Google Scholar] [CrossRef]
- Haaz, E.; Fozer, D.; Nagy, T.; Valentinyi, N.; Andre, A.; Matyasi, J.; Balla, J.; Mizsey, P.; Toth, A.J. Vacuum Evaporation and Reverse Osmosis Treatment of Process Wastewaters Containing Surfactant Material: COD Reduction and Water Reuse. Clean Technol. Environ. Policy 2019, 21, 861–870. [Google Scholar] [CrossRef]
- Akinapally, S.; Dheeravath, B.; Panga, K.K.; Saranga, V.K.; Golla, S.; Vurimindi, H.; Sanaga, S. Treatment of Pesticide Intermediate Industrial Wastewater Using Different Advanced Treatment Processes. Sustain. Water Resour. Manag. 2021, 7, 74. [Google Scholar] [CrossRef]
- Staal, L.B.; Petersen, A.B.; Jørgensen, C.A.; Nielsen, U.G.; Nielsen, P.H.; Reitzel, K. Extraction and Quantification of Polyphosphates in Activated Sludge from Wastewater Treatment Plants by 31P NMR Spectroscopy. Water Res. 2019, 157, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, X.; Gouda, S.G.; Yuan, Q. Humidification-Dehumidification Process Used for the Concentration and Nutrient Recovery of Biogas Slurry. J. Clean. Prod. 2020, 247, 119142. [Google Scholar] [CrossRef]
- Nair, A.T.; Makwana, A.R.; Ahammed, M.M. The Use of Response Surface Methodology for Modelling and Analysis of Water and Wastewater Treatment Processes: A Review. Water Sci. Technol. 2013, 69, 464–478. [Google Scholar] [CrossRef]
- Ye, Z.-L.; Chen, S.-H.; Wang, S.-M.; Lin, L.-F.; Yan, Y.-J.; Zhang, Z.-J.; Chen, J.-S. Phosphorus Recovery from Synthetic Swine Wastewater by Chemical Precipitation Using Response Surface Methodology. J. Hazard. Mater. 2010, 176, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Won, S.G.; Baldwin, S.A.; Lau, A.K.; Rezadehbashi, M. Optimal Operational Conditions for Biohydrogen Production from Sugar Refinery Wastewater in an ASBR. Int. J. Hydrogen Energy 2013, 38, 13895–13906. [Google Scholar] [CrossRef]
- Shim, S.; Won, S.; Reza, A.; Kim, S.; Ahmed, N.; Ra, C. Design and Optimization of Fluidized Bed Reactor Operating Conditions for Struvite Recovery Process from Swine Wastewater. Processes 2020, 8, 422. [Google Scholar] [CrossRef]
- Gadekar, M.R.; Ahammed, M.M. Modelling Dye Removal by Adsorption onto Water Treatment Residuals Using Combined Response Surface Methodology-Artificial Neural Network Approach. J. Environ. Manag. 2019, 231, 241–248. [Google Scholar] [CrossRef]
- Fan, M.; Hu, J.; Cao, R.; Ruan, W.; Wei, X. A Review on Experimental Design for Pollutants Removal in Water Treatment with the Aid of Artificial Intelligence. Chemosphere 2018, 200, 330–343. [Google Scholar] [CrossRef]
- Yu, A.; Liu, Y.; Li, X.; Yang, Y.; Zhou, Z.; Liu, H. Modeling and Optimizing of NH4+ Removal from Stormwater by Coal-Based Granular Activated Carbon Using RSM and ANN Coupled with GA. Water 2021, 13, 608. [Google Scholar] [CrossRef]
- Uslu, S.; Celik, M.B. Performance and Exhaust Emission Prediction of a SI Engine Fueled with I-Amyl Alcohol-Gasoline Blends: An ANN Coupled RSM Based Optimization. Fuel 2020, 265, 116922. [Google Scholar] [CrossRef]
- Ong, M.Y.; Nomanbhay, S.; Kusumo, F.; Raja Shahruzzaman, R.M.H.; Shamsuddin, A.H. Modeling and Optimization of Microwave-Based Bio-Jet Fuel from Coconut Oil: Investigation of Response Surface Methodology (RSM) and Artificial Neural Network Methodology (ANN). Energies 2021, 14, 295. [Google Scholar] [CrossRef]
- Rathankumar, A.K.; Vaithyanathan, V.K.; Saikia, K.; Anand, S.S.; Vaidyanathan, V.K.; Cabana, H. Effect of Alkaline Treatment on the Removal of Contaminants of Emerging Concern from Municipal Biosolids: Modelling and Optimization of Process Parameters Using RSM and ANN Coupled GA. Chemosphere 2022, 286, 131847. [Google Scholar] [CrossRef]
- O’Flaherty, E.; Gray, N.F. A Comparative Analysis of the Characteristics of a Range of Real and Synthetic Wastewaters. Environ. Sci. Pollut. Res. 2013, 20, 8813–8830. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mazumder, D. Kinetic Study on Nitrification of Ammonium Nitrogen-Enriched Synthetic Wastewater Using Activated Sludge. Water Sci. Technol. 2020, 81, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Liu, H.; Yan, Y. Improvement of the Activity of Anaerobic Sludge by Low-Intensity Ultrasound. J. Environ. Manag. 2009, 90, 260–264. [Google Scholar] [CrossRef]
- Fuchs, W.; Drosg, B. Assessment of the State of the Art of Technologies for the Processing of Digestate Residue from Anaerobic Digesters. Water Sci. Technol. 2013, 67, 1984–1993. [Google Scholar] [CrossRef]
- Kim, S.; Reza, A.; Shim, S.; Won, S.; Ra, C. Development of a Real-Time Controlled Bio-Liquor Circulation System for Swine Farms: A Lab-Scale Study. Animals 2021, 11, 311. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Nazari, S. Applying Response Surface Methodology to Optimize the Fenton Oxidation Process in the Removal of Reactive Red 2. Pol. J. Environ. Stud. 2017, 26, 765–772. [Google Scholar] [CrossRef]
- Behera, S.K.; Meena, H.; Chakraborty, S.; Meikap, B.C. Application of Response Surface Methodology (RSM) for Optimization of Leaching Parameters for Ash Reduction from Low-Grade Coal. Int. J. Min. Sci. Technol. 2018, 28, 621–629. [Google Scholar] [CrossRef]
- Ameer, K.; Bae, S.-W.; Jo, Y.; Lee, H.-G.; Ameer, A.; Kwon, J.-H. Optimization of Microwave-Assisted Extraction of Total Extract, Stevioside and Rebaudioside-A from Stevia Rebaudiana (Bertoni) Leaves, Using Response Surface Methodology (RSM) and Artificial Neural Network (ANN) Modelling. Food Chem. 2017, 229, 198–207. [Google Scholar] [CrossRef]
- Kıranşan, M.; Khataee, A.; Karaca, S.; Sheydaei, M. Artificial Neural Network Modeling of Photocatalytic Removal of a Disperse Dye Using Synthesized of ZnO Nanoparticles on Montmorillonite. Spectroc. Acta Part A Mol. Biomol. Spectr. 2015, 140, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudi, M.; Ghaedi, M.; Zinali, A.; Ghaedi, A.M.; Habibi, M.H. Artificial Neural Network (ANN) Method for Modeling of Sunset Yellow Dye Adsorption Using Zinc Oxide Nanorods Loaded on Activated Carbon: Kinetic and Isotherm Study. Spectroc. Acta Part A Mol. Biomol. Spectr. 2015, 134, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dil, E.A.; Ghaedi, M.; Asfaram, A.; Mehrabi, F.; Bazrafshan, A.A.; Ghaedi, A.M. Trace Determination of Safranin O Dye Using Ultrasound Assisted Dispersive Solid-Phase Micro Extraction: Artificial Neural Network-Genetic Algorithm and Response Surface Methodology. Ultrason. Sonochem. 2016, 33, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Karri, R.R.; Tanzifi, M.; Tavakkoli Yaraki, M.; Sahu, J.N. Optimization and Modeling of Methyl Orange Adsorption onto Polyaniline Nano-Adsorbent through Response Surface Methodology and Differential Evolution Embedded Neural Network. J. Environ. Manag. 2018, 223, 517–529. [Google Scholar] [CrossRef]
- Yildiz, Y.Ş.; Şenyiğit, E.; İrdemez, Ş. Optimization of Specific Energy Consumption for Bomaplex Red CR-L Dye Removal from Aqueous Solution by Electrocoagulation Using Taguchi-Neural Method. Neural Comput. Appl. 2013, 23, 1061–1069. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Chin, N.L.; Yusof, Y.A.; Talib, R.A.; Law, C.L. Optimization of Total Phenolic Content Extracted from Garcinia Mangostana Linn. Hull Using Response Surface Methodology versus Artificial Neural Network. Ind. Crops Prod. 2012, 40, 247–253. [Google Scholar] [CrossRef]
- Geyikçi, F.; Kılıç, E.; Çoruh, S.; Elevli, S. Modelling of Lead Adsorption from Industrial Sludge Leachate on Red Mud by Using RSM and ANN. Chem. Eng. J. 2012, 183, 53–59. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmed, A.; Ahmad, A.; Ahmad, M.S.; Sandhu, M.A. Optimization of Mixed Surfactants-Based β-Carotene Nanoemulsions Using Response Surface Methodology: An Ultrasonic Homogenization Approach. Food Chem. 2018, 253, 179–184. [Google Scholar] [CrossRef]
- Naseem, Z.; Zahid, M.; Hanif, M.A.; Shahid, M. Green Extraction of Ethnomedicinal Compounds from Cymbopogon Citratus Stapf Using Hydrogen-Bonded Supramolecular Network. Sep. Purif. Technol. 2021, 56, 1520–1533. [Google Scholar] [CrossRef]
- Bilici Baskan, M.; Pala, A. A Statistical Experiment Design Approach for Arsenic Removal by Coagulation Process Using Aluminum Sulfate. Desalination 2010, 254, 42–48. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response Surface Methodology (RSM) as a Tool for Optimization in Analytical Chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Shojaeimehr, T.; Rahimpour, F.; Khadivi, M.A.; Sadeghi, M. A Modeling Study by Response Surface Methodology (RSM) and Artificial Neural Network (ANN) on Cu2+ Adsorption Optimization Using Light Expended Clay Aggregate (LECA). J. Ind. Eng. Chem. 2014, 20, 870–880. [Google Scholar] [CrossRef]
- Arogo, J.; Zhang, R.H.; Riskowski, G.L.; Christianson, L.L.; Day, D.L. Mass Transfer Coefficient of Ammonia in Liquid Swine Manure and Aqueous Solutions. J. Agric. Eng. Res. 1999, 73, 77–86. [Google Scholar] [CrossRef]
- Vaddella, V.K.; Ndegwa, P.M.; Ullman, J.L.; Jiang, A. Mass Transfer Coefficients of Ammonia for Liquid Dairy Manure. Atmos. Environ. 2013, 66, 107–113. [Google Scholar] [CrossRef]
- Tao, W.; Ukwuani, A.T.; Agyeman, F. Recovery of Ammonia in Anaerobic Digestate Using Vacuum Thermal Stripping—Acid Absorption Process: Scale-up Considerations. Water Sci. Technol. 2018, 78, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Ledda, C.; Schievano, A.; Salati, S.; Adani, F. Nitrogen and Water Recovery from Animal Slurries by a New Integrated Ultrafiltration, Reverse Osmosis and Cold Stripping Process: A Case Study. Water Res. 2013, 47, 6157–6166. [Google Scholar] [CrossRef]
- Zarebska, A.; Nieto, D.R.; Christensen, K.V.; Norddahl, B. Ammonia Recovery from Agricultural Wastes by Membrane Distillation: Fouling Characterization and Mechanism. Water Res. 2014, 56, 1–10. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003; ISBN 978-0-07-049439-8. [Google Scholar]
- Mohod, A.V.; Gogate, P.R. Improved Crystallization of Ammonium Sulphate Using Ultrasound Assisted Approach with Comparison with the Conventional Approach. Ultrason. Sonochem. 2018, 41, 310–318. [Google Scholar] [CrossRef]
- Karri, R.R.; Sahu, J.N. Modeling and Optimization by Particle Swarm Embedded Neural Network for Adsorption of Zinc (II) by Palm Kernel Shell Based Activated Carbon from Aqueous Environment. J. Environ. Manag. 2018, 206, 178–191. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Mohmmadi, L.; Ahmadi, S.; Rahdar, A.; Khadkhodaiy, D.; Dehghani, R.; Rahdar, S. Modeling of Adsorption of Methylene Blue Dye on Ho-CaWO4 Nanoparticles Using Response Surface Methodology (RSM) and Artificial Neural Network (ANN) Techniques. MethodsX 2019, 6, 1779–1797. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Das, P.; Sinha, K. Modeling of Biosorption of Cu(II) by Alkali-Modified Spent Tea Leaves Using Response Surface Methodology (RSM) and Artificial Neural Network (ANN). Appl. Water Sci. 2015, 5, 191–199. [Google Scholar] [CrossRef]
- Betiku, E.; Okunsolawo, S.S.; Ajala, S.O.; Odedele, O.S. Performance Evaluation of Artificial Neural Network Coupled with Generic Algorithm and Response Surface Methodology in Modeling and Optimization of Biodiesel Production Process Parameters from Shea Tree (Vitellaria Paradoxa) Nut Butter. Renew. Energy 2015, 76, 408–417. [Google Scholar] [CrossRef]
- Sinha, K.; Chowdhury, S.; Saha, P.D.; Datta, S. Modeling of Microwave-Assisted Extraction of Natural Dye from Seeds of Bixa Orellana (Annatto) Using Response Surface Methodology (RSM) and Artificial Neural Network (ANN). Ind. Crops Prod. 2013, 41, 165–171. [Google Scholar] [CrossRef]





| Parameters | Coded Levels | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |
| pH (x1) | 9 | 9.5 | 10 | 10.5 | 11 |
| Temperature (x2) (°C) | 58 | 61 | 64 | 67 | 70 |
| Stripping time (x3) (min) | 30 | 45 | 60 | 75 | 90 |
| Vacuum pressure (kPa) | 73.3 | ||||
| Rotation speed (rpm) | 80 | ||||
| Parameters | Details |
|---|---|
| Network | Two-layer feed forward; three inputs, one output and one hidden layer with five hidden neurons |
| Data | 60; training: 70%, validation: 15%, testing: 15% (all data are selected randomly) |
| Transfer | Tangent sigmoid (tansig) (between input and hidden layers) Linear (purelin) (between hidden and output layers) |
| Training | Levenberg–Marquardt backpropagation algorithm (trainlm) |
| Performance | Mean Squared Error (MSE) |
| Run | Independent Variables 1 | Points | Response (y: NH3-N Removal Efficiency (%)) | ||||
|---|---|---|---|---|---|---|---|
| x1 | x2 (°C) | x3 (min) | Experimental Data | Predicted Value | |||
| RSM | RSM–ANN | ||||||
| 1 | 9.5 (−1) | 67 (+1) | 45 (−1) | Factorial | 91.35 | 94.96 | 91.99 |
| 2 | 10.5 (+1) | 67 (+1) | 45(−1) | Factorial | 97.42 | 98.95 | 97.02 |
| 3 | 9.5 (−1) | 61 (−1) | 45(−1) | Factorial | 56.64 | 55.56 | 56.64 |
| 4 | 9.5 (−1) | 61 (−1) | 75 (+1) | Factorial | 69.86 | 70.58 | 69.85 |
| 5 | 10.5 (+1) | 61 (−1) | 45 (−1) | Factorial | 73.21 | 70.40 | 72.81 |
| 6 | 10.5 (+1) | 67 (+1) | 75 (+1) | Factorial | 97.73 | 101.07 | 98.05 |
| 7 | 10.5 (+1) | 61 (−1) | 75 (+1) | Factorial | 81.32 | 79.97 | 80.49 |
| 8 | 9.5 (−1) | 67 (+1) | 75 (+1) | Factorial | 97.46 | 102.53 | 97.03 |
| 9 | 11 (+α) | 64 (0) | 60 (0) | Axial | 96.77 | 97.54 | 97.13 |
| 10 | 10 (0) | 58 (−α) | 60 (0) | Axial | 29.69 | 33.08 | 29.70 |
| 11 | 10 (0) | 64 (0) | 30 (−α) | Axial | 78.18 | 78.68 | 78.19 |
| 12 | 10 (0) | 70 (+α) | 60 (0) | Axial | 99.22 | 93.57 | 98.10 |
| 13 | 9 (−α) | 64 (0) | 60 (0) | Axial | 87.20 | 84.16 | 88.25 |
| 14 | 10 (0) | 64 (0) | 90 (+α) | Axial | 98.58 | 95.82 | 98.56 |
| 15 | 10 (0) | 64 (0) | 60 (0) | Central | 94.18 | 95.58 | 96.48 |
| 16 | 10 (0) | 64 (0) | 60 (0) | Central | 93.03 | 95.58 | 96.48 |
| 17 | 10 (0) | 64 (0) | 60 (0) | Central | 94.38 | 95.58 | 96.48 |
| 18 | 10 (0) | 64 (0) | 60 (0) | Central | 92.02 | 95.58 | 96.48 |
| 19 | 10 (0) | 64 (0) | 60 (0) | Central | 93.31 | 95.58 | 96.48 |
| 20 | 10 (0) | 64 (0) | 60 (0) | Central | 92.63 | 95.58 | 96.48 |
| Source | SS 1 | df 2 | MS 3 | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 5881.82 | 9 | 653.54 | 38.79 | <0.001 | significant |
| x1 (pH) | 179.04 | 1 | 179.04 | 10.63 | 0.009 | |
| x2 (Temperature) | 3659.69 | 1 | 3659.69 | 217.20 | <0.001 | |
| x3 (Time) | 293.82 | 1 | 293.82 | 17.44 | 0.002 | |
| x12 | 35.03 | 1 | 35.03 | 2.08 | 0.179 | |
| x22 | 1634.45 | 1 | 1634.45 | 97.00 | < 0.001 | |
| x32 | 108.91 | 1 | 108.91 | 6.46 | 0.029 | |
| x1x2 | 58.76 | 1 | 58.76 | 3.49 | 0.091 | |
| x1x3 | 14.87 | 1 | 14.87 | 0.883 | 0.369 | |
| x2x3 | 27.77 | 1 | 27.77 | 1.65 | 0.228 | |
| Residual | 168.49 | 10 | 16.85 | |||
| Lack of Fit | 125.53 | 5 | 25.11 | 2.92 | 0.132 | not significant |
| Pure Error | 42.96 | 5 | 8.59 | |||
| R2 | 0.972 | |||||
| Adjusted R2 | 0.947 | |||||
| Predicted R2 | 0.818 | |||||
| Coefficient of variation (%) | 4.74 | |||||
| Adequate precision | 23.927 |
| Parameters 1 | Optimum Conditions | Response (NH3-N Removal Efficiency (%)) | ||||||
|---|---|---|---|---|---|---|---|---|
| Predicted Values | Observed Value | 95% CI Low | 95% CI High | |||||
| RSM | RSM–ANN | RSM | RSM–ANN | RSM | RSM–ANN | |||
| x1 | 9.6 | 99.44 | 97.39 | 97.84 ± 1.86 | 93.91 | 89.76 | 104.99 | 105.02 |
| x2 (°C) | 65.5 | |||||||
| x3 (min) | 59.6 | |||||||
| Parameters | RSM | RSM–ANN |
|---|---|---|
| Coefficient of determination (R2) | 0.972 | 0.998 |
| Root mean square error (RMSE) | 4.215 | 1.221 |
| Absolute average deviation (ADD) | 0.340 | 0.143 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reza, A.; Chen, L. Optimization and Modeling of Ammonia Nitrogen Removal from High Strength Synthetic Wastewater Using Vacuum Thermal Stripping. Processes 2021, 9, 2059. https://doi.org/10.3390/pr9112059
Reza A, Chen L. Optimization and Modeling of Ammonia Nitrogen Removal from High Strength Synthetic Wastewater Using Vacuum Thermal Stripping. Processes. 2021; 9(11):2059. https://doi.org/10.3390/pr9112059
Chicago/Turabian StyleReza, Arif, and Lide Chen. 2021. "Optimization and Modeling of Ammonia Nitrogen Removal from High Strength Synthetic Wastewater Using Vacuum Thermal Stripping" Processes 9, no. 11: 2059. https://doi.org/10.3390/pr9112059
APA StyleReza, A., & Chen, L. (2021). Optimization and Modeling of Ammonia Nitrogen Removal from High Strength Synthetic Wastewater Using Vacuum Thermal Stripping. Processes, 9(11), 2059. https://doi.org/10.3390/pr9112059







