Increase of Cycling Stability in Pilot-Scale 21700 Format Li-Ion Cells by Foil Tab Design
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- -
- A 22% higher resistance, which can be explained by the lower cross-sections of the foil tabs (10–15 µm) compared to the welded tabs (130–150 µm).
- -
- A marginally lower C-rate capability for charging and discharging. The reason is most likely the increased resistance (see Equation (1)).
- -
- Most remarkably, an increase of cycle stability as shown by 14.4% more cycles and by 11.2% more total charge throughput until 80% capacity retention.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sturm, J.; Frank, A.; Rheinfeld, A.; Erhard, S.V.; Jossen, A. Impact of Electrode and Cell Design on Fast Charging Capabilities of Cylindrical Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 130505. [Google Scholar] [CrossRef]
- Waldmann, T.; Gorse, S.; Samtleben, T.; Schneider, G.; Knoblauch, V.; Wohlfahrt-Mehrens, M. A Mechanical Aging Mechanism in Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A1742–A1747. [Google Scholar] [CrossRef]
- Gorse, S.; Kugler, B.; Samtleben, T.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Schneider, G.; Knoblauch, V. An Explanation of the Ageing Mechanism of Li-Ion Batteries by Metallographic and Material Analysis. Pract. Metallogr. 2014, 51, 829–848. [Google Scholar] [CrossRef]
- Quinn, J.B.; Waldmann, T.; Richter, K.; Kasper, M.; Wohlfahrt-Mehrens, M. Energy Density of Cylindrical Li-Ion Cells: A Comparison of Commercial 18650 to the 21700 Cells. J. Electrochem. Soc. 2018, 165, A3284–A3291. [Google Scholar] [CrossRef]
- Waldmann, T.; Scurtu, R.-G.; Richter, K.; Wohlfahrt-Mehrens, M. 18650 vs. 21700 Li-Ion Cells—A Direct Comparison of Electrochemical, Thermal, and Geometrical Properties. J. Power Sources 2020, 472, 228614. [Google Scholar] [CrossRef]
- McCleary, D.A.H.; Meyers, J.P.; Kim, B. Three-Dimensional Modeling of Electrochemical Performance and Heat Generation of Spirally and Prismatically Wound Lithium-Ion Batteries. J. Electrochem. Soc. 2013, 160, A1931–A1943. [Google Scholar] [CrossRef]
- Kosch, S.; Rheinfeld, A.; Erhard, S.V.; Jossen, A. An Extended Polarization Model to Study the Influence of Current Collector Geometry of Large-Format Lithium-Ion Pouch Cells. J. Power Sources 2017, 342, 666–676. [Google Scholar] [CrossRef]
- Samba, A.; Omar, N.; Gualous, H.; Capron, O.; Van den Bossche, P.; Van Mierlo, J. Impact of Tab Location on Large Format Lithium-Ion Pouch Cell Based on Fully Coupled Tree-Dimensional Electrochemical-Thermal Modeling. Electrochim. Acta 2014, 147, 319–329. [Google Scholar] [CrossRef]
- Waldmann, T.; Geramifard, G.; Wohlfahrt-Mehrens, M. Influence of Current Collecting Tab Design on Thermal and Electrochemical Performance of Cylindrical Lithium-Ion Cells during High Current Discharge. J. Energy Storage 2016, 5, 163–168. [Google Scholar] [CrossRef]
- Osswald, P.J.; Erhard, S.V.; Wilhelm, J.; Hoster, H.E.; Jossen, A. Simulation and Measurement of Local Potentials of Modified Commercial Cylindrical Cells: I. Cell Preparation and Measurements. J. Electrochem. Soc. 2015, 162, A2099–A2105. [Google Scholar] [CrossRef]
- Cheon, S.-E.; Kim, K.-H. Secondary Battery with Collector Plate and Electrode Package Thereof. U.S. Patent No. 20050277020A1, 15 June 2010. [Google Scholar]
- Tsuruta, K.; Dermer, M.E.; Dhiman, R. A Cell with a Tabless Electrode. U.S. Patent No. 20200144676A1, 7 May 2020. [Google Scholar]
- Sumihara, M.; Imai, T. Secondary Battery. International Patent No. 2009096188A1, 6 August 2009. [Google Scholar]
- Waldmann, T.; Rössler, S.; Blessing, M.; Schäfer, R.; Scurtu, R.-G.; Braunwarth, W.; Wohlfahrt-Mehrens, M. A Direct Comparison of Pilot-Scale Li-Ion Cells in the Formats PHEV1, Pouch, and 21700. J. Electrochem. Soc. 2021, 168, 090519. [Google Scholar] [CrossRef]
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Levartovsky, Y.; Levy, N.R.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D.; et al. Fast Charging of Lithium-Ion Batteries: A Review of Materials Aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Moulton, H.F. Current Flow in Rectangular Conductors. Proc. Lond. Math. Soc. 1905, 2, 104–110. [Google Scholar] [CrossRef]
- Newman, J.S.; Thomas-Alyea, K.E. Electrochemical Systems; Wiley-Interscience: Hoboken, NJ, USA, 2004; ISBN 0-471-47756-7. [Google Scholar]
- Meaden, G.T. Electrical Resistance of Metals; Springer: Berlin/Heidelberg, Germany, 1965; ISBN 978-1-4899-5717-7. [Google Scholar]
- Serway, R.A. Principles of Physics, 2nd ed.; Saunders Golden Sunburst Series; Saunders College Pub: Fort Worth, TX, USA, 1998; ISBN 978-0-03-020457-9. [Google Scholar]
- Piłatowicz, G.; Marongiu, A.; Drillkens, J.; Sinhuber, P.; Sauer, D.U. A Critical Overview of Definitions and Determination Techniques of the Internal Resistance Using Lithium-Ion, Lead-Acid, Nickel Metal-Hydride Batteries and Electrochemical Double-Layer Capacitors as Examples. J. Power Sources 2015, 296, 365–376. [Google Scholar] [CrossRef]
- Scipioni, R.; Jørgensen, P.S.; Graves, C.; Hjelm, J.; Jensen, S.H. A Physically-Based Equivalent Circuit Model for the Impedance of a LiFePO 4 /Graphite 26650 Cylindrical Cell. J. Electrochem. Soc. 2017, 164, A2017–A2030. [Google Scholar] [CrossRef]
- Broussely, M.; Herreyre, S.; Biensan, P.; Kasztejna, P.; Nechev, K.; Staniewicz, R. Aging Mechanism in Li Ion Cells and Calendar Life Predictions. J. Power Sources 2001, 97–98, 13–21. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W.; et al. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Smart, M.C.; Ratnakumar, B.V.; Whitcanack, L.; Chin, K.; Rodriguez, M.; Surampudi, S. Performance Characteristics of Lithium Ion Cells at Low Temperatures; IEEE: Piscataway Township, NJ, USA, 2002; pp. 41–46. [Google Scholar]
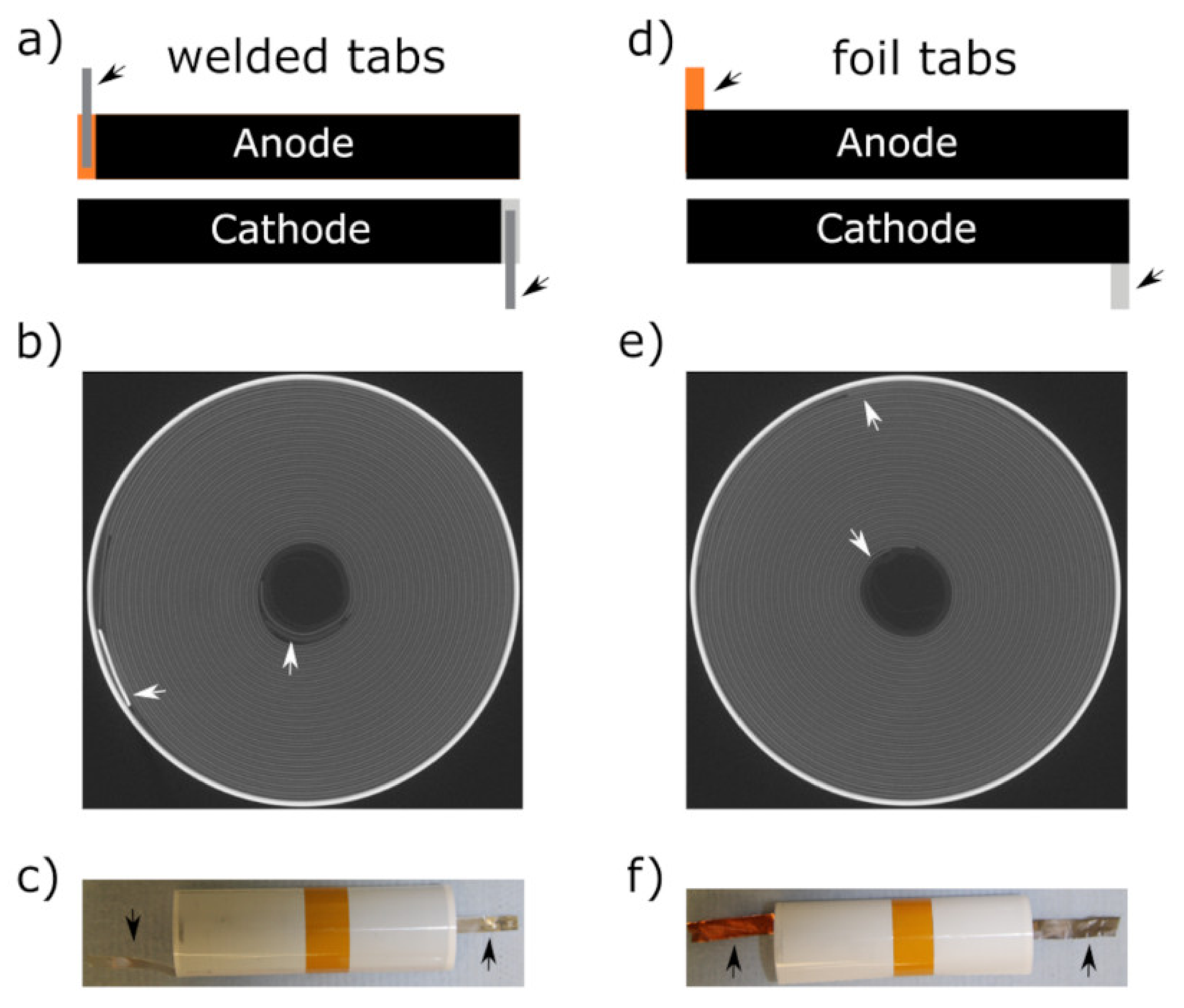

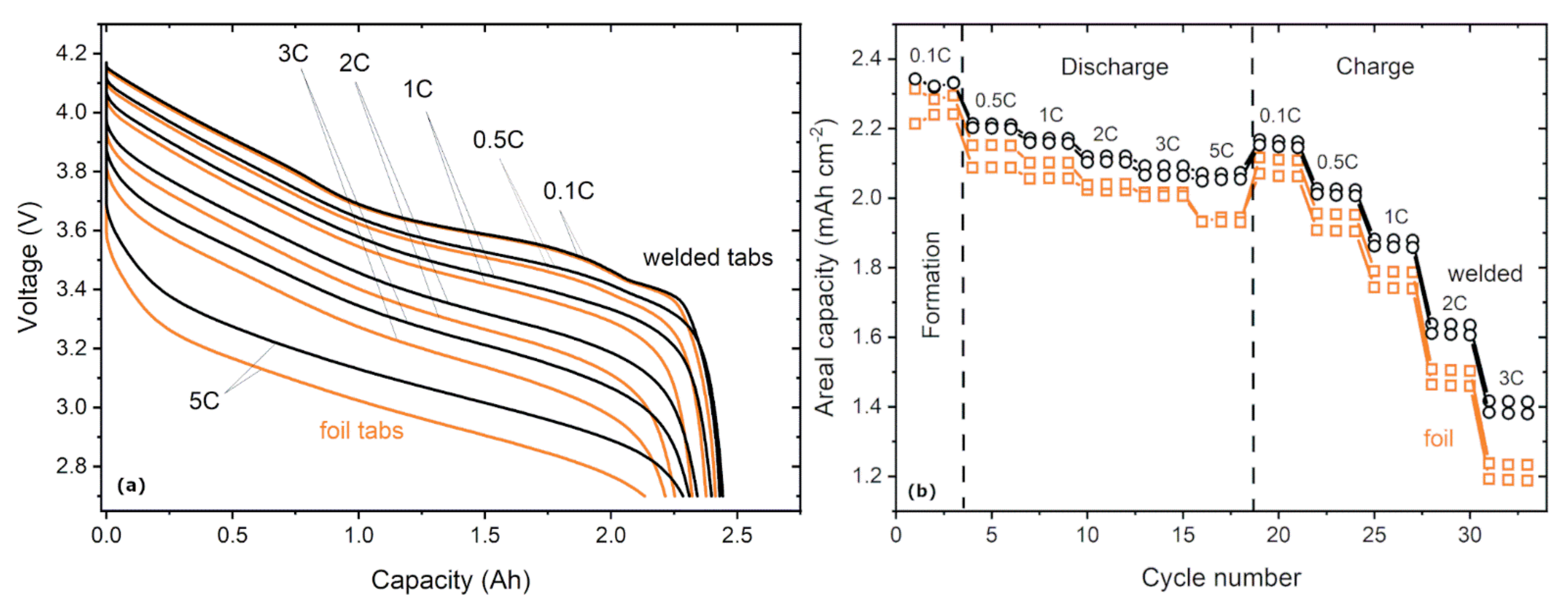
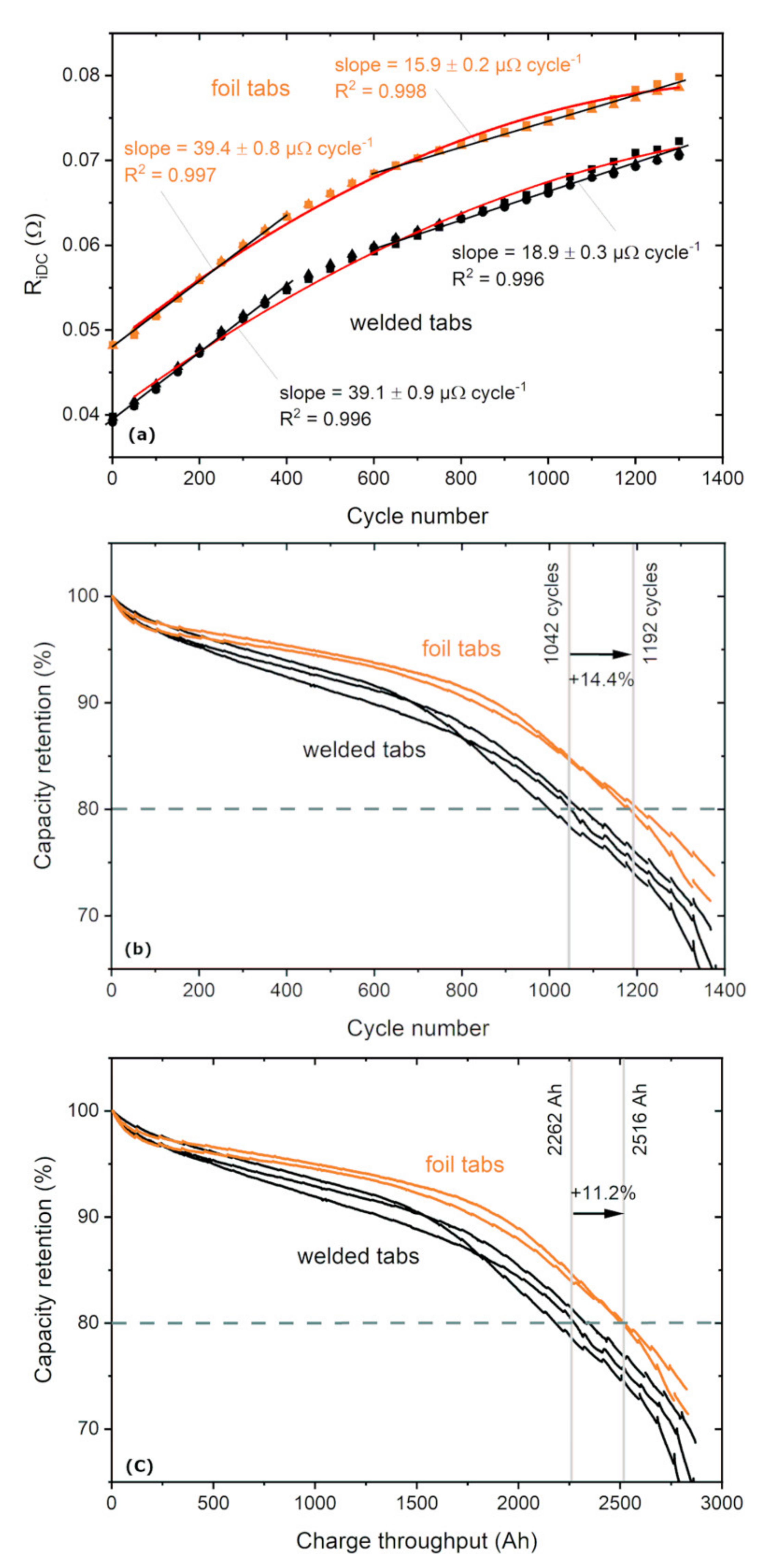

| Welded Tabs | Foil Tabs | |||
|---|---|---|---|---|
| Anode | Cathode | Anode | Cathode | |
| material | Ni | Al | Cu | Al |
| thickness/mm | 0.15 | 0.13 | 0.010 | 0.015 |
| Atab/mm2 | 4 × 0.15 = 0.6 | 4 × 0.13 = 0.52 | 6 × 0.01 = 0.06 | 6 × 0.15 = 0.09 |
| ltab/mm | 15 | 15 | 15 | 15 |
| ρ/µΩ cm | 7 [18] | 2.82 [19] | 1.7 [18,19] | 2.82 [19] |
| Rtab/mΩ | 1.75 | 0.81 | 4.25 | 4.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waldmann, T.; Scurtu, R.-G.; Brändle, D.; Wohlfahrt-Mehrens, M. Increase of Cycling Stability in Pilot-Scale 21700 Format Li-Ion Cells by Foil Tab Design. Processes 2021, 9, 1908. https://doi.org/10.3390/pr9111908
Waldmann T, Scurtu R-G, Brändle D, Wohlfahrt-Mehrens M. Increase of Cycling Stability in Pilot-Scale 21700 Format Li-Ion Cells by Foil Tab Design. Processes. 2021; 9(11):1908. https://doi.org/10.3390/pr9111908
Chicago/Turabian StyleWaldmann, Thomas, Rares-George Scurtu, Daniel Brändle, and Margret Wohlfahrt-Mehrens. 2021. "Increase of Cycling Stability in Pilot-Scale 21700 Format Li-Ion Cells by Foil Tab Design" Processes 9, no. 11: 1908. https://doi.org/10.3390/pr9111908
APA StyleWaldmann, T., Scurtu, R.-G., Brändle, D., & Wohlfahrt-Mehrens, M. (2021). Increase of Cycling Stability in Pilot-Scale 21700 Format Li-Ion Cells by Foil Tab Design. Processes, 9(11), 1908. https://doi.org/10.3390/pr9111908






