Conversion of Organic Matter of Carbonate Deposits in the Hydrothermal Fluid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
2.3. Analysis
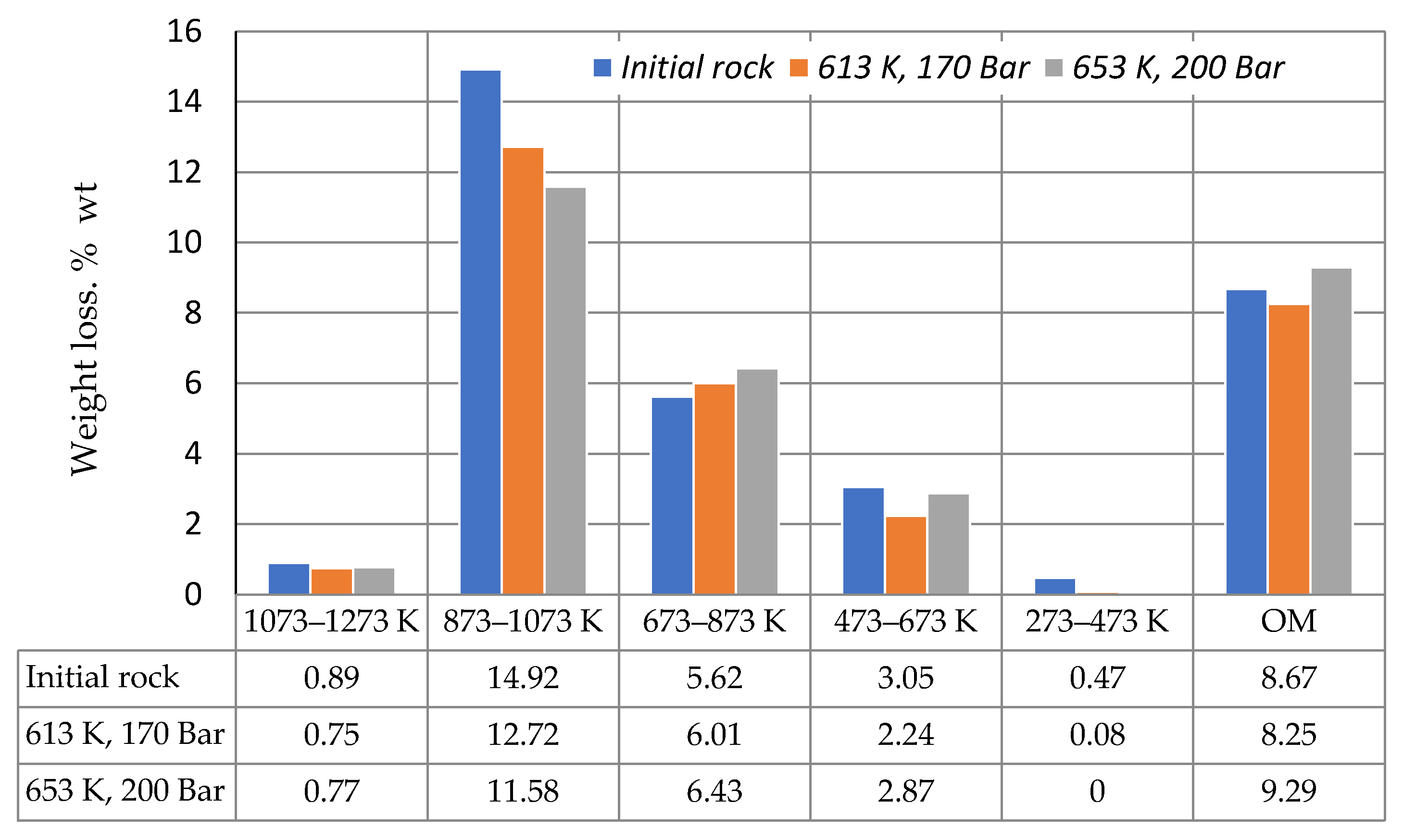
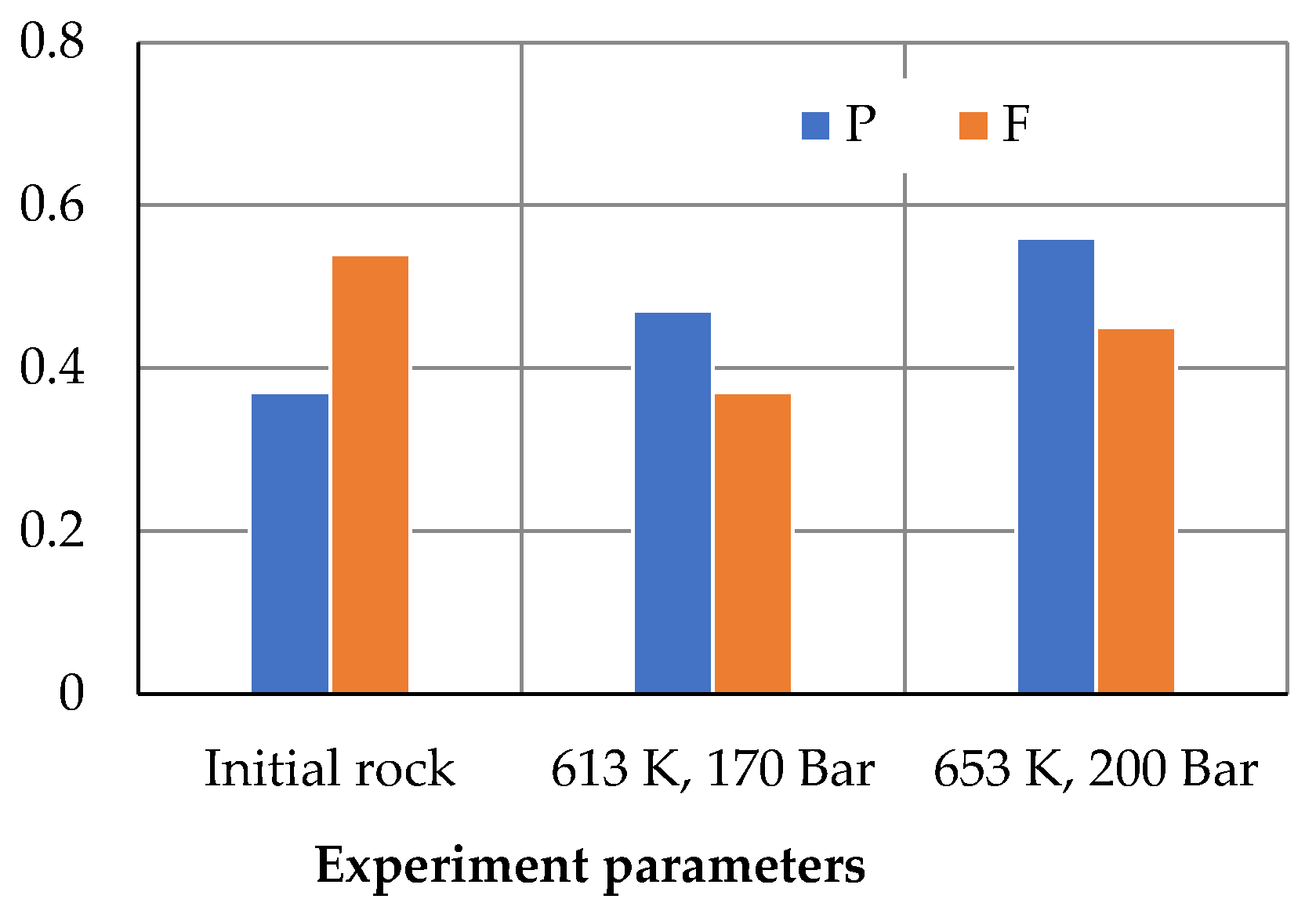
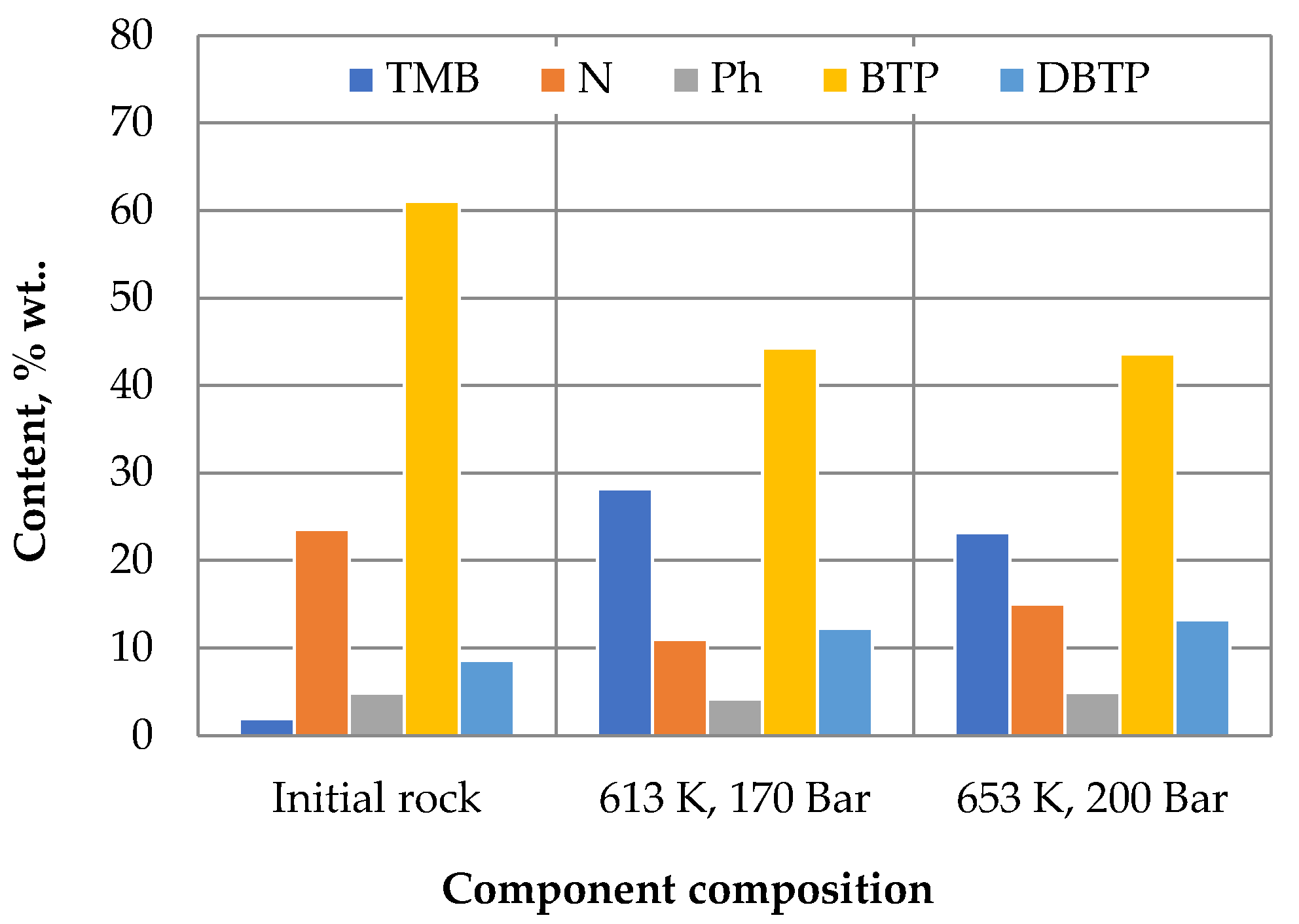
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Owen, N.A.; Inderwildi, O.R.; King, D.A. The status of conventional world oil reserves-Hype or cause for concern? Energy Policy 2010, 38, 4743–4749. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, G.; Wang, Z.; Wen, Z.; Tian, Z.; Wang, H.; Ma, F.; Wu, Y. Distribution and potential of global oil and gas resources. Pet. Explor. Dev. 2018, 45, 779–789. [Google Scholar] [CrossRef]
- Liu, Z. Innovation and practice of petroleum engineering synergetic management for the development of difficult-to-produce reserves. Pet. Explor. Dev. 2020, 47, 1316–1324. [Google Scholar] [CrossRef]
- Bentley, R.; Mannan, S.; Wheeler, S. Assessing the date of the global oil peak: The need to use 2P reserves. Energy Policy 2007, 35, 6364–6382. [Google Scholar] [CrossRef]
- Al-Fattah, S.M. Non-OPEC conventional oil: Production decline, supply outlook and key implications. J. Pet. Sci. Eng. 2020, 189, 107049. [Google Scholar] [CrossRef]
- Satter, A.; Iqbal, G.M. Conventional and unconventional petroleum reserves-definitions and world outlook. In Reservoir Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 427–438. [Google Scholar]
- Kaul, S.; Edinger, R. Efficiency versus cost of alternative fuels from renewable resources: Outlining decision parameters. Energy Policy 2004, 32, 929–935. [Google Scholar] [CrossRef]
- Priya; Deora, P.S.; Verma, Y.; Muhal, R.A.; Goswami, C.; Singh, T. Biofuels: An alternative to conventional fuel and energy source. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Khusnutdinov, I.; Goncharova, I.; Safiulina, A. A study on the reaction capacity of normal structure α-olefins for synthesis of oxygen-containing fuel additives. Fuel 2021, 298, 120863. [Google Scholar] [CrossRef]
- Chandrasekar, K.; Sudhakar, S.; Rajappan, R.; Senthil, S.; Balu, P. Present developments and the reach of alternative fuel: A review. Mater. Today: Proc. 2021. [Google Scholar] [CrossRef]
- Perkins, G.; Batalha, N.; Kumar, A.; Bhaskar, T.; Konarova, M. Recent advances in liquefaction technologies for production of liquid hydrocarbon fuels from biomass and carbonaceous wastes. Renew. Sustain. Energy Rev. 2019, 115, 109400. [Google Scholar] [CrossRef]
- Ghodke, P.K. High-quality hydrocarbon fuel production from municipal mixed plastic waste using a locally available low-cost catalyst. Fuel Commun. 2021, 8, 100022. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Speight, J.G. Feedstock types and properties. Refin. Futur. 2020, 1–42. [Google Scholar] [CrossRef]
- Khusnutdinov, I.S.; Safiulina, A.G.; Zabbarov, R.R.; Dubovikov, O.A.; Khusnutdinov, S.I.; Khaldarov, N.K. Influence of Physicochemical Properties of Highly Organized Oil Disperse Systems on Efficiency of Thermomechanical Dehydration. Chem. Technol. Fuels Oils 2017, 52, 779–784. [Google Scholar] [CrossRef]
- Fahim, M.A.; Alsahhaf, T.A.; Elkilani, A. Refinery Feedstocks and Products. Fundam. Pet. Refin. 2010, 11–31. [Google Scholar] [CrossRef]
- Alaseeva, A.A.; Khusnutdinov, S.I.; Petrov, S.M.; Khusnutdinov, I.S.; Safiulina, A.G.; Bashkirtseva, N.Y. Properties and Applications of Distillate Fractions from Highly Stable Dispersions of Liquid Pyrolysis Products. Chem. Technol. Fuels Oils 2018, 54, 271–277. [Google Scholar] [CrossRef]
- Khusnutdinov, I.; Goncharova, I.; Safiulina, A. Extractive deasphalting as a method of obtaining asphalt binders and low-viscosity deasphalted hydrocarbon feedstock from natural bitumen. Egypt. J. Pet. 2021, 30, 69–73. [Google Scholar] [CrossRef]
- Goncharova, I.; Safiulina, A.; Khusnutdinov, I.; Alawode, E.; Skvortsova, G. The effect of feedstock treatment on the deasphalting process. Pet. Sci. Technol. 2018, 36, 1405–1411. [Google Scholar] [CrossRef]
- Safiulina, A.G.; Zabbarov, R.R.; Khusnutdinov, S.I.; Alekseeva, A.; Khusnutdinov, I.S.; Petrov, S.M. Thermomechanical Dehydration of Highly-Stable Dispersions of Liquid Pyrolysis Products. Chem. Technol. Fuels Oils 2018, 54, 265–270. [Google Scholar] [CrossRef]
- Petrov, S.M.; Kayukova, G.P.; Goncharova, I.N.; Safiulina, A.G.; Lakhova, A.I. High-quality asphalt binders produced by deasphalting of natural bitumen. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, Albena, Bulgaria, 2–8 July 2018; Volume 18, pp. 455–460. [Google Scholar]
- Hosseini, S.H.; Shakouri, H.; Kazemi, A. Oil price future regarding unconventional oil production and its near-term deployment: A system dynamics approach. Energy 2021, 222, 119878. [Google Scholar] [CrossRef]
- E Gwyn, J. Oil from shale as a viable replacement of depleted crude reserves: Processes and challenges. Fuel Process. Technol. 2001, 70, 27–40. [Google Scholar] [CrossRef]
- Kryukov, V.; Moe, A. Does Russian unconventional oil have a future? Energy Policy 2018, 119, 41–50. [Google Scholar] [CrossRef]
- Crawford, P.; Biglarbigi, K.; Dammer, A.; Knaus, E. Advances in World Oil-Shale Production Technologies. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008; pp. 4101–4111. [Google Scholar]
- Petrov, S.M.; Safiulina, A.G.; Bashkirtseva, N.Y.; Lakhova, A.I.; Islamova, G.G. Influence of Metal Oxides and Their Precursors on the Composition of Final Products of Aquathermolysis of Raw Ashalchin Oil. Processes 2021, 9, 256. [Google Scholar] [CrossRef]
- Kayukova, G.; Gubaidullin, A.; Petrov, S.; Romanov, G.; Petrukhina, N.N.; Vakhin, A.V. Changes of Asphaltenes’ Structural Phase Characteristics in the Process of Conversion of Heavy Oil in the Hydrothermal Catalytic System. Energy Fuels 2016, 30, 773–783. [Google Scholar] [CrossRef]
- Lakhova, A.; Petrov, S.; Ibragimova, D.; Kayukova, G.; Safiulina, A.; Shinkarev, A.; Okekwe, R. Aquathermolysis of heavy oil using nano oxides of metals. J. Pet. Sci. Eng. 2017, 153, 385–390. [Google Scholar] [CrossRef]
- Strelets, L.A.; Ilyin, S.O. Effect of enhanced oil recovery on the composition and rheological properties of heavy crude oil. J. Pet. Sci. Eng. 2021, 203, 108641. [Google Scholar] [CrossRef]
- Khisamov, R.S.; Bazarevskaya, V.G.; Tarasova, T.I.; Mikhailova, O.V.; Mikhailov, S.N. Geochemical evidence for petroleum potential of Domanic deposits in the Republic of Tatarstan (Russian). Neftyanoe Khozyaystvo Oil Ind. 2016, 2016, 10–13. [Google Scholar]
- Vakhin, A.V.; Cherkasova, E.I.; Safiulina, A.G.; Islamova, G.G.; Petrov, S.M.; Bashkirtseva, N.Y. Catalytic Oxidation of Heavy Residual Oil by Pulsed Nuclear Magnetic Resonance. Processes 2021, 9, 158. [Google Scholar] [CrossRef]
- Mikhailova, A.N.; Kayukova, G.; Vakhin, A.V.; Eskin, A.A.; Vandyukova, I.I. Composition features of hydrocarbons and rocks of Domanic deposits of different oil fields in the Tatarstan territory. Pet. Sci. Technol. 2019, 37, 374–381. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Khasanova, N.M.; Morozov, V.P.; Vakhin, A.V.; Nazimov, N.A.; Sotnikov, O.S.; Khisamov, R.S. Influence of Hydrothermal and Pyrolysis Processes on the Transformation of Organic Matter of Dense Low-Permeability Rocks from Domanic Formations of the Romashkino Oil Field. Geofluids 2018, 2018, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Petrov, S.; Ibragimova, D.; Safiulina, A.; Kalorazi, B.T.; Vakhin, A.; Okekwe, R.; Karalin, E. Conversion of organic matter in the carbonaceous medium in the supercritical water. J. Pet. Sci. Eng. 2017, 159, 497–505. [Google Scholar] [CrossRef]
- Muslimov, R.K.; Plotnikova, I.N. Consideration of the processes of oil deposit reformation during long-term operation and deep feeding in modeling the development of oil fields. Georesursy 2018, 20, 186–192. [Google Scholar] [CrossRef]
- Jiu, B.; Huang, W.; Li, Y. The effect of hydrothermal fluids on Ordovician carbonate rocks, southern Ordos Basin, China. Ore Geol. Rev. 2020, 126, 103803. [Google Scholar] [CrossRef]
- Wei, X.; Ren, J.; Zhao, J.; Zhang, D.; Luo, S.; Wei, L.; Chen, J. Paleogeomorphy evolution of the Ordovician weathering crust and its implication for reservoir development, eastern Ordos Basin. Pet. Res. 2018, 3, 77–89. [Google Scholar] [CrossRef]
- Wagner, T.; Jochum, J. Fluid–rock interaction processes related to hydrothermal vein-type mineralization in the Siegerland district, Germany: Implications from inorganic and organic alteration patterns. Appl. Geochem. 2002, 17, 225–243. [Google Scholar] [CrossRef]
- Teng, J.; Mastalerz, M.; Liu, B.; Gognat, T.; Hauser, E.; McLaughlin, P. Variations of organic matter transformation in response to hydrothermal fluids: Example from the Indiana part of the Illinois Basin. Int. J. Coal Geol. 2020, 219, 103410. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Zhu, D.; Meng, Q.; Liu, W.; Qiu, D.; Huang, Z. The role of deep fluid in the formation of organic-rich source rocks☆. J. Nat. Gas Geosci. 2018, 3, 171–180. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Zhu, D.; Meng, Q.; Huang, X. The function and impact of deep fluid on the organic matter during the hydrogeneration and evolution process. J. Nat. Gas Geosci. 2019, 4, 231–244. [Google Scholar] [CrossRef]
- Lakhova, A.; Soldatova, R.; Petrov, S.; Nosova, A.; Safiulina, A. Transformation of heavy oil in hydrothermal impact. Pet. Sci. Technol. 2019, 37, 611–616. [Google Scholar] [CrossRef]
- Petrov, S.M.; Zakiyeva, R.R.; Ibrahim, A.Y.; Baybekova, L.R.; Gussamov, I.I.; Sitnov, S.A.; Vakhin, A.V. Upgrading of high-viscosity naphtha in the super-critical water environment. Int. J. Appl. Eng. Res. 2015, 10, 44656–44661. [Google Scholar]
- Gudiyella, S.; Lai, L.; Borne, I.H.; Tompsett, G.A.; Timko, M.T.; Choi, K.-H.; Alabsi, M.H.; Green, W.H. An experimental and modeling study of vacuum residue upgrading in supercritical water. AIChE J. 2018, 64, 1732–1743. [Google Scholar] [CrossRef]
- Petrov, S.M.; Kayukova, G.P.; Vakhin, A.V.; Petrova, A.N.; Ibrahim, A.Y.I.; Onishchenko, Y.V.; Nurgaliyev, D.K. Catalytic effects research of carbonaceous rock under conditions of in-situ oxidation of super-sticky naphtha. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1624–1629. [Google Scholar]
- Canıaz, R.O.; Arca, S.; Yaşar, M.; Erkey, C. Refinery bitumen and domestic unconventional heavy oil upgrading in supercritical water. J. Supercrit. Fluids 2019, 152, 104569. [Google Scholar] [CrossRef]
- Petrov, S.; Abdelsalam, Y.I.I.; Vakhin, A.V.; Baibekova, L.R.; Kayukova, G.; Karalin, E.A. Study of the Rheological Properties of Heat-Treatment Products of Asphaltic Oils in the Presence of Rock-Forming Minerals. Chem. Technol. Fuels Oils 2015, 51, 133–139. [Google Scholar] [CrossRef]
- Petrov, S.; Nosova, A.; Bashkirtseva, N.; Fakhrutdinov, R. Features of heavy oil spraying with single evaporation. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Chengdu, China, 15–19 October 2018; Institute of Physics Publishing: Sydney, UK, 2019; Volume 282, p. 012004. [Google Scholar]
- Nosova, A.; Petrov, S.; Safiulina, A.; Kayukova, G.P.; Bashkirceva, N. The transformation of high-viscosity oil of carbonate rock in the presence of CO[AcAc]3 catalyst in a vapor-air medium. Pet. Sci. Technol. 2018, 36, 1001–1006. [Google Scholar] [CrossRef]
- Lakhova, A.; Safiulina, A.; Islamova, G.; Amansaryev, A.; Salakhov, I.; Petrov, S.; Bashkirtseva, N. Study of the Impact of Nonionic Additives on the Composition and Structure of Petroleum Dispersed Systems by IR Spectroscopy. Processes 2021, 9, 553. [Google Scholar] [CrossRef]
- Petrov, S.M.; Ibragimova, D.A.; Abdelsalam, Y.I.I.; Kayukova, G.P. Effect of rock-forming and catalytic additives on transformation of heavy high-viscosity oil. Petrochemistry 2016, 56, 24–29. [Google Scholar]
- Lakhova, A.I.; Petrov, S.M.; Zakieva, R.R.; Ibragimova, D.A.; Baibekova, L.R.; Isakov, D.R. Impact of the Group Content on the Properties of Bitumen of Different Grades. Res. J. Appl. Sci. 2015, 10, 917–921. [Google Scholar] [CrossRef]
- Zakieva, R.R.; Gussamov, I.I.; Gadel’Shin, R.M.; Petrov, S.M.; Ibragimova, D.A.; Fakhrutdinov, R.Z. Effect of Modification of a Copolymer of Ethylene with Vinyl Acetate on the Performance of Cement and Asphalt Concrete Based on It. Chem. Technol. Fuels Oils 2015, 51, 480–486. [Google Scholar] [CrossRef]
- Baibekova, L.R.; Petrov, S.M.; Mukhamatdinov, I.I.; Burnina, M.A. Polymer Additive Influence on Composition and Properties of Bitumen Polymer Compound. Int. J. Appl. Chem. 2015, 11, 593–599. [Google Scholar]
- Mahzari, P.; Jones, A.P.; Oelkers, E.H. An integrated evaluation of enhanced oil recovery and geochemical processes for carbonated water injection in carbonate rocks. J. Pet. Sci. Eng. 2019, 181, 106188. [Google Scholar] [CrossRef]
- Gentzis, T.; Carvajal-Ortiz, H.; Xie, Z.H.; Hackley, P.C.; Fowler, H. An integrated geochemical, spectroscopic, and petrographic approach to examining the producibility of hydrocarbons from liquids-rich unconventional formations. Fuel 2021, 298, 120357. [Google Scholar] [CrossRef]
- Monin, J.; Barth, D.; Perrut, M.; Espitalié, M.; Durand, B. Extraction of hydrocarbons from sedimentary rocks by supercritical carbon dioxide. Org. Geochem. 1988, 13, 1079–1086. [Google Scholar] [CrossRef]
- Manshad, A.K.; Ali, J.A.; Haghighi, O.M.; Sajadi, S.M.; Keshavarz, A. Oil recovery aspects of ZnO/SiO2 nano-clay in carbonate reservoir. Fuel 2021, 307, 121927. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Morozov, V.P.; Islamova, R.R.; Nosova, F.F.; Plotnikova, I.N.; Petrov, S.M.; Vakhin, A.V. Composition of oils of carbonate reservoirs in current and ancient water-oil contact zones. Chem. Technol. Fuels Oils 2015, 51, 117–126. [Google Scholar] [CrossRef]

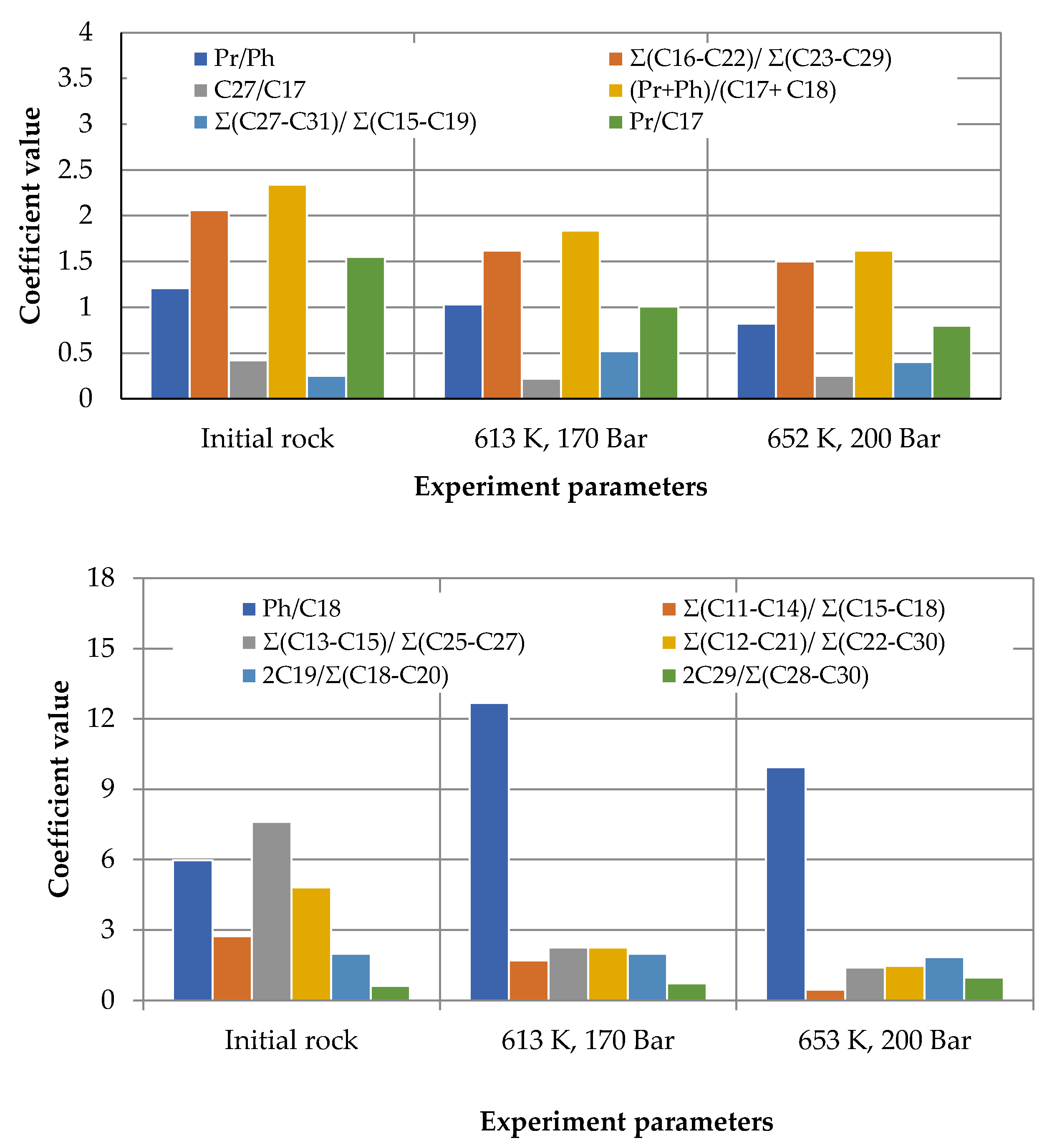
| IR Spectral Coefficients | Initial Rock | 613 K, 170 Bar | 653 K, 200 Bar | |||
|---|---|---|---|---|---|---|
| Resins in Extracts | Asphaltenes in Extracts | Resins in Extracts | Asphaltenes in Extracts | Resins in Extracts | Asphaltenes in Extracts | |
| Ar | 1.02 | 0.49 | 1.02 | 0.56 | 0.99 | 0.55 |
| Ox | 0.36 | 0.20 | 0.4 | 0.24 | 0.39 | 0.21 |
| C | 0.85 | 0.16 | 0.48 | 0.20 | 0.42 | 0.18 |
| Br | 0.70 | 0.60 | 0.60 | 0.60 | 0.61 | 0.61 |
| Al | 0.84 | 0.78 | 0.89 | 0.79 | 0.87 | 0.78 |
| Structural Parameters | Initial Rock | 613 K, 170 Bar | 653 K, 200 Bar |
|---|---|---|---|
| BET specific surface, m2/g | 1.569 | 3.491 | 0.752 |
| BJH Total pore volume, sm3/g | 0.1204 | 0.1248 | 0.1164 |
| BJH Average pore diameter, nm | 4.209 | 4.228 | 4.221 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, S.M.; Lakhova, A.I.; Safiulina, A.G.; Ignashev, N.E.; Khelkhal, M.A.; Vakhin, A.V. Conversion of Organic Matter of Carbonate Deposits in the Hydrothermal Fluid. Processes 2021, 9, 1893. https://doi.org/10.3390/pr9111893
Petrov SM, Lakhova AI, Safiulina AG, Ignashev NE, Khelkhal MA, Vakhin AV. Conversion of Organic Matter of Carbonate Deposits in the Hydrothermal Fluid. Processes. 2021; 9(11):1893. https://doi.org/10.3390/pr9111893
Chicago/Turabian StylePetrov, Sergey M., Alfiya I. Lakhova, Aliya G. Safiulina, Nikita E. Ignashev, Mohammed A. Khelkhal, and Alexey V. Vakhin. 2021. "Conversion of Organic Matter of Carbonate Deposits in the Hydrothermal Fluid" Processes 9, no. 11: 1893. https://doi.org/10.3390/pr9111893
APA StylePetrov, S. M., Lakhova, A. I., Safiulina, A. G., Ignashev, N. E., Khelkhal, M. A., & Vakhin, A. V. (2021). Conversion of Organic Matter of Carbonate Deposits in the Hydrothermal Fluid. Processes, 9(11), 1893. https://doi.org/10.3390/pr9111893









