The Search for the Elixir of Life: On the Therapeutic Potential of Alkaline Reduced Water in Metabolic Syndromes
Abstract
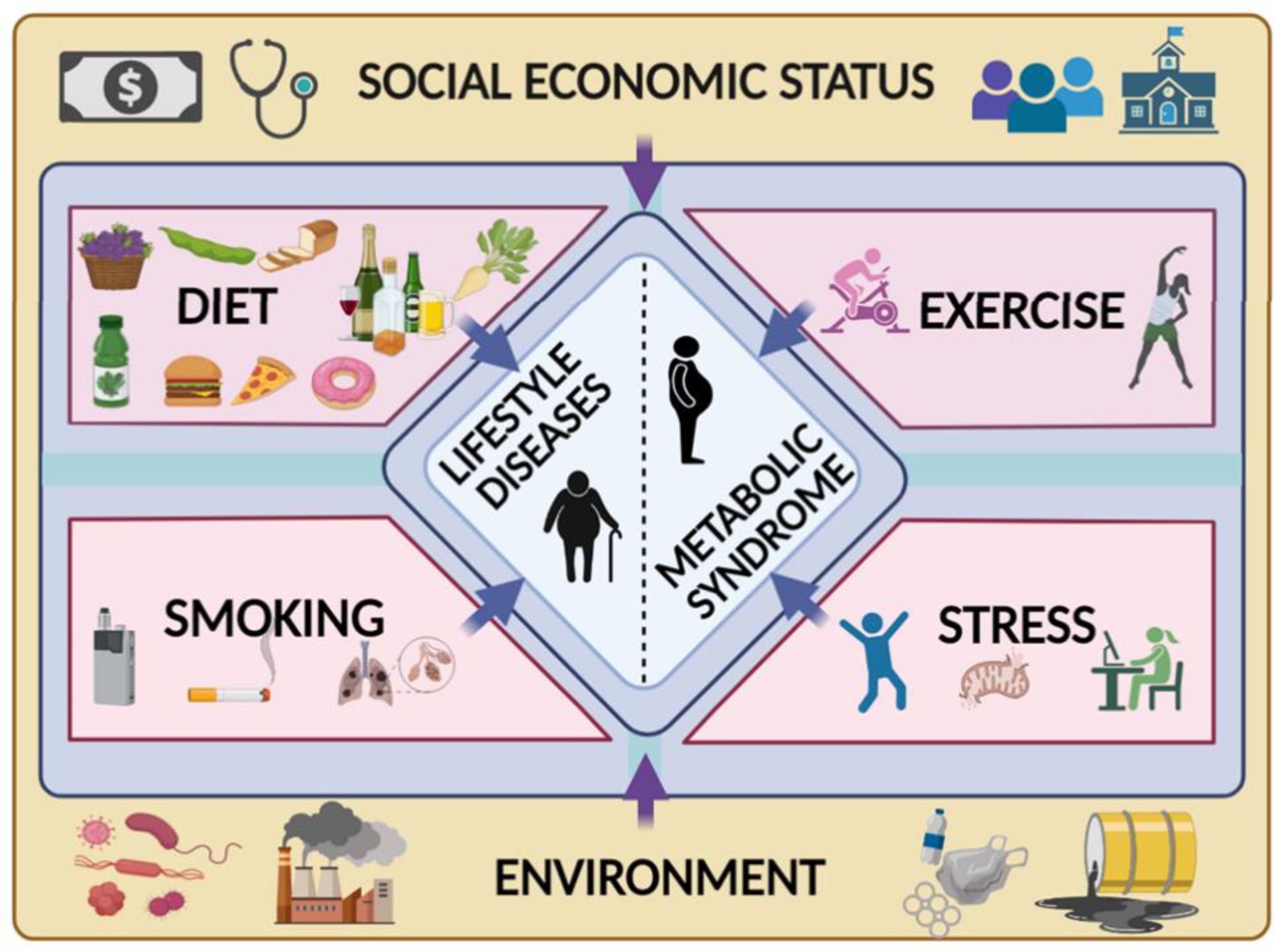
:1. Introduction
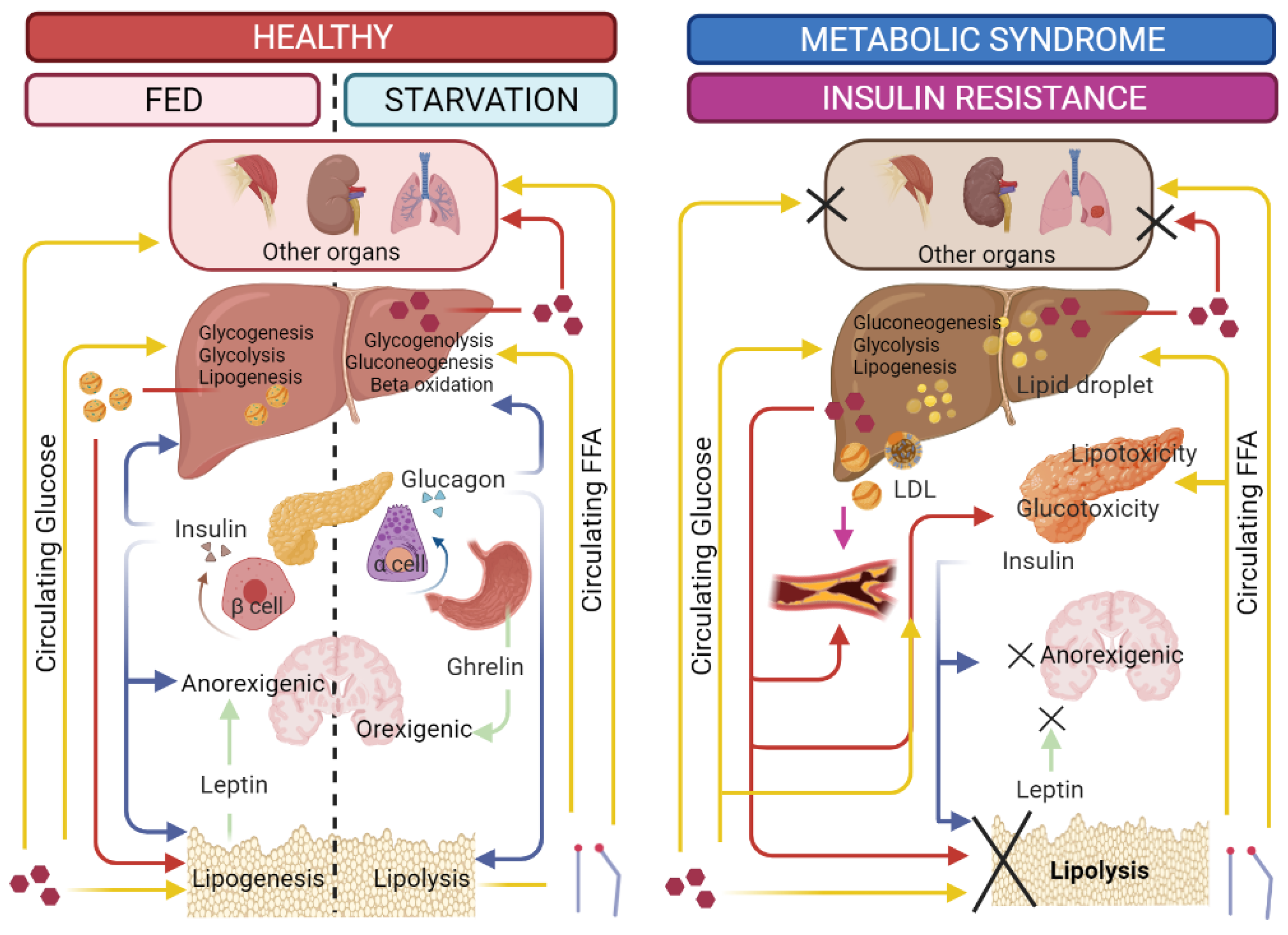
2. Metabolic Syndrome and ARW
2.1. Obesity
2.2. Diabetes mellitus
2.3. Non-Alcoholic Fatty Liver Disease
2.4. Cancer
3. Nutritional and Therapeutic Interventions for Metabolic Syndrome
3.1. Effects of ARW on Diabetes, Obesity, and Exercise
3.2. ARW Mechanism of Action
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEW | Alkaline Electrolyzed Water |
| AIW | Alkaline Induced Water |
| ARW | Alkaline Reduced Water |
| DM | Diabetes Mellitus |
| ERW | Electrolyzed Reduced Water |
| FA | Fatty Acid |
| FFA | Free Fatty Acid |
| FGF | Fibroblast Growth Factor |
| GPT | Glutamic Pyruvate Transaminase |
| GOT | Glutamic Oxaloacetate Transaminase |
| HDL | High-density Lipoproteins |
| HRW | Hydrogen-rich Water |
| IFN | Interferon |
| LDL | Low-density Lipoprotein |
| MRW | Mineral-induced Alkaline-reduced Water |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NASH | Non-alcoholic Steatohepatitis |
| NCD | Non-communicable Diseases |
| OLETF | Otsuka Long-Evans Tokushima Fatty |
| ORP | Oxidation-reduction Potential |
| QoL | Quality of Life |
| ROS | Reactive Oxygen Species |
| SCFAs | Short Chain Fatty Acids |
| SOD | Superoxide Dismutase |
| T1DM | Type 1 Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| TLC | Therapeutic Lifestyle Changes |
| TG | Triglycerides |
| TNF | Tumor Necrosis Factor |
| VEGF | Vascular Endothelial Growth Factor |
| WHO | World Health Organization |
References
- World Health Organization (WHO). Non-Communicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 19 September 2021).
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [Green Version]
- Al-Maskari, F. Lifestyle Diseases: An Economic Burden on the Health Services. Available online: https://www.un.org/en/chronicle/article/lifestyle-diseases-economic-burden-health-services (accessed on 19 September 2021).
- Koh, E.J.; Hwang, S.Y. Multi-omics approaches for understanding environmental exposure and human health. Mol. Cell. Toxicol. 2019, 15, 1–7. [Google Scholar] [CrossRef]
- Angeles-Agdeppa, I.; Sun, Y.; Tanda, K.V. Dietary pattern and nutrient intakes in association with non-communicable disease risk factors among filipino adults: A cross-sectional study. Nutr. J. 2020, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K. Challenges in the Treatment of cardiometabolic syndrome. Indian J. Pharmacol. 2012, 44, 155–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-K.; Park, S.; Chang, S.-J.; Kim, S.-K.; Song, J.S.; Kim, H.-R.; Oh, S.-S.; Koh, S.-B. Pesticides as a risk factor for metabolic syndrome: Population-based longitudinal study in Korea. Mol. Cell. Toxicol. 2019, 15, 431–441. [Google Scholar] [CrossRef]
- Pate, R.R. Physical activity and public health. A recommendation from the centers for disease control and prevention and the American College of Sports Medicine. JAMA 1995, 273, 402–407. [Google Scholar] [CrossRef]
- Hackshaw, A.K.; Law, M.R.; Wald, N.J. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ 1997, 315, 980–988. [Google Scholar] [CrossRef] [Green Version]
- West, R. The multiple facets of cigarette addiction and what they mean for encouraging and helping smokers to stop. COPD J. Chronic Obstr. Pulm. Dis. 2009, 6, 277–283. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The metabolic syndrome—What is it and how should it be managed? Eur. J. Prev. Cardiol. 2019, 26, 33–46. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Mendrick, D.L.; Diehl, A.M.; Topor, L.S.; Dietert, R.R.; Will, Y.; La Merrill, M.A.; Bouret, S.; Varma, V.; Hastings, K.L.; Schug, T.T.; et al. Metabolic syndrome and associated diseases: From the bench to the clinic. Toxicol. Sci. 2018, 162, 36–42. [Google Scholar]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Deen, D. Metabolic syndrome: Time for action. Am. Fam. Physician 2004, 69, 2875–2882. [Google Scholar]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Wagh, A.; Stone, N.J. Treatment of metabolic syndrome. Expert Rev. Cardiovasc. Ther. 2004, 2, 213–228. [Google Scholar] [CrossRef]
- Lim, S.; Eckel, R.H. Pharmacological treatment and therapeutic perspectives of metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 329–341. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kim, Y.K.; Ryoo, K.K.; Lee, Y.B.; Park, E.J. Electrolyzed-reduced water protects against oxidative damage to DNA, RNA, and protein. Appl. Biochem. Biotechnol. 2006, 135, 133–144. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Joo, K.-B.; Lee, K.-J. Clinical effect and mechanism of alkaline reduced water. J. Food Drug Anal. 2020, 20. [Google Scholar] [CrossRef]
- Magro, M.; Corain, L.; Ferro, S.; Baratella, D.; Bonaiuto, E.; Terzo, M.; Corraducci, V.; Salmaso, L.; Vianello, F. Alkaline water and longevity: A murine study. Evid. -Based Complementary Altern. Med. 2016, 2016, e3084126. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Aster, J.C. Robbins and Cotran Pathologic Basis of Disease; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014; ISBN 9780323609937. [Google Scholar]
- Maurizi, G.; Della Guardia, L.; Maurizi, A.; Poloni, A. Adipocytes properties and crosstalk with immune system in obesity-related inflammation. J. Cell. Physiol. 2018, 233, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Sikaris, K.A. The clinical biochemistry of obesity. Clin. Biochem. Rev. 2004, 25, 165–181. [Google Scholar] [PubMed]
- Schipper, H.S.; Rakhshandehroo, M.; van de Graaf, S.F.J.; Venken, K.; Koppen, A.; Stienstra, R.; Prop, S.; Meerding, J.; Hamers, N.; Besra, G.; et al. Natural killer t cells in adipose tissue prevent insulin resistance. J. Clin. Investig. 2012, 122, 3343–3354. [Google Scholar] [PubMed] [Green Version]
- Singh, P.; Rai, S.N. Factors affecting obesity and its treatment. Obes. Med. 2019, 16, 100140. [Google Scholar] [CrossRef]
- Kamimura, N.; Nishimaki, K.; Ohsawa, I.; Ohta, S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in Db/Db mice. Obesity 2011, 19, 1396–1403. [Google Scholar] [CrossRef]
- Ignacio, R.M.C.; Kang, T.-Y.; Kim, C.-S.; Kim, S.-K.; Yang, Y.-C.; Sohn, J.-H.; Lee, K.-J. Anti-obesity effect of alkaline reduced water in high fat-fed obese mice. Biol. Pharm. Bull. 2013, 36, 1052–1059. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.; Park, S.K.; Lee, Y.M.; Yoon, Y.S.; Kim, D.H.; Deung, Y.K.; Lee, K.J. Effect of mineral-induced alkaline reduced water on sprague-dawley rats fed on high-fat diet. J. Exp. Biomed. Sci. 2006, 12, 1–7. [Google Scholar]
- Park, J.; Jang, H.-J. Anti-diabetic effects of natural products an overview of therapeutic strategies. Mol. Cell. Toxicol. 2017, 13, 1–20. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins Basic Pathology; Elsevier Health Science: Amsterdam, The Netherlands, 2017; ISBN 9780323394130. [Google Scholar]
- Jin, D.; Ryu, S.H.; Kim, H.W.; Yang, E.J.; Lim, S.J.; Ryang, Y.S.; Chung, C.H.; Park, S.K.; Lee, K.J. Anti-diabetic effect of alkaline-reduced water on OLETF rats. Biosci. Biotechnol. Biochem. 2006, 70, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Siswantoro, E.; Purwanto, N.H. Effectiveness of alkali water consumption to reduce blood sugar levels in diabetes mellitus type 2. J. Diabetes Mellit. 2017, 7, 249–264. [Google Scholar] [CrossRef] [Green Version]
- Rias, Y.A.; Kurniawan, A.L.; Chang, C.W.; Gordon, C.J.; Tsai, H.T. Synergistic effects of regular walking and alkaline electrolyzed water on decreasing inflammation and oxidative stress and increasing quality of life in individuals with type 2 diabetes: A community based randomized controlled trial. Antioxidants 2020, 9, 946. [Google Scholar]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.; Dressler, N.; Ben-Shushan, R.S.; Meerson, A.; LeBaron, T.W.; Tamir, S. Effects of alkaline-electrolyzed and hydrogen-rich water, in a high-fat-diet non-alcoholic fatty liver disease mouse model. World J. Gastroenterol. 2018, 24, 5095–5108. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, X.; Lu, J.; Zhang, Y.; Sun, X.; Huang, Q.; Wang, Q. Hydrogen-rich saline improves nonalcoholic fatty liver disease by alleviating oxidative stress and activating hepatic PPARα and PPARγ. Mol. Med. Rep. 2017, 15, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Kawai, D.; Takaki, A.; Nakatsuka, A.; Wada, J.; Tamaki, N.; Yasunaka, T.; Koike, K.; Tsuzaki, R.; Matsumoto, K.; Miyake, Y.; et al. Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice. Hepatology 2012, 56, 912–921. [Google Scholar] [CrossRef]
- Lee, K.-J.; Park, S.-K.; Kim, J.-W.; Kim, G.-Y.; Ryang, Y.-S.; Kim, G.-H.; Cho, H.-C.; Kim, S.-K.; Kim, H.-W. Anticancer effect of alkaline reduced water(International conference on mind body science: Physical and physiological approach joint with the eighteenth symposium on life information science). J. Int. Soc. Life Inf. Sci. 2004, 22, 302–305. [Google Scholar]
- Ye, J.; Li, Y.; Hamasaki, T.; Nakamichi, N.; Komatsu, T.; Kashiwagi, T.; Teruya, K.; Nishikawa, R.; Kawahara, T.; Osada, K.; et al. Inhibitory effect of electrolyzed reduced water on tumor angiogenesis. Biol. Pharm. Bull. 2008, 31, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Kinjo, T.; Ye, J.; Yan, H.; Hamasaki, T.; Nakanishi, H.; Toh, K.; Nakamichi, N.; Kabayama, S.; Teruya, K.; Shirahata, S. Suppressive effects of electrochemically reduced water on matrix metalloproteinase-2 activities and in vitro invasion of human fibrosarcoma HT1080 cells. Cytotechnology 2012, 64, 357–371. [Google Scholar]
- Lindenmeyer, C.C.; McCullough, A.J. The natural history of nonalcoholic fatty liver disease—An evolving view. Clin. Liver Dis. 2018, 22, 11–21. [Google Scholar] [CrossRef]
- Groop, L.C.; Bonadonna, R.C.; DelPrato, S.; Ratheiser, K.; Zyck, K.; Ferrannini, E.; DeFronzo, R.A. Glucose and Free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Investig. 1989, 84, 205–213. [Google Scholar]
- Yu, J.; Marsh, S.; Hu, J.; Feng, W.; Wu, C. The pathogenesis of nonalcoholic fatty liver disease: Interplay between diet, gut microbiota, and genetic background. Gastroenterol. Res. Pract. 2016, 2016, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petta, S.; Gastaldelli, A.; Rebelos, E.; Bugianesi, E.; Messa, P.; Miele, L.; Svegliati-Baroni, G.; Valenti, L.; Bonino, F. Pathophysiology of non alcoholic fatty liver disease. Int. J. Mol. Sci. 2016, 17, 2082. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, K.; Abrams, G.A. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroentero 2007, 13, 3540–3553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Iio, A.; Ito, M.; Itoh, T.; Terazawa, R.; Fujita, Y.; Nozawa, Y.; Ohsawa, I.; Ohno, K.; Ito, M. Molecular hydrogen attenuates fatty acid uptake and lipid accumulation through downregulating CD36 expression in HepG2 cells. Med. Gas Res. 2013, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Connolly, J.L.; Schnitt, S.J.; Wang, H.H.; Longtine, J.A.; Dvorak, A.; Dvorak, H.F. Principles of cancer pathology. In Holland-Frei Cancer Medicine, 5th ed.; BC Decker Inc.: Hamilton, ON, Canada, 2003. [Google Scholar]
- Tomasetti, C.; Li, L.; Vogelstein, B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 2017, 355, 1330–1334. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.Y.H.P.; Muller, W.J. Oncogenes and tumor suppressor genes. Cold Spring Harb. Perspect. Biol. 2010, 2, a003236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Powers, S.; Zhu, W.; Hannun, Y.A. Substantial contribution of extrinsic risk factors to cancer development. Nature 2016, 529, 43–47. [Google Scholar] [CrossRef]
- El-Zimaity, H.; Di Pilato, V.; Novella Ringressi, M.; Brcic, I.; Rajendra, S.; Langer, R.; Dislich, B.; Tripathi, M.; Guindi, M.; Riddell, R. Risk factors for esophageal cancer: Emphasis on infectious agents. Ann. N. Y. Acad. Sci. 2018, 1434, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Inoue-Choi, M.; Hartge, P.; Liao, L.M.; Caporaso, N.; Freedman, N.D. Association between long-term low-intensity cigarette smoking and incidence of smoking-related cancer in the national institutes of health-AARP cohort: Long-term low-intensity cigarette smoking and incidence of smoking-related cancer. Int. J. Cancer 2018, 142, 271–280. [Google Scholar] [CrossRef]
- Scoccianti, C.; Cecchini, M.; Anderson, A.S.; Berrino, F.; Boutron-Ruault, M.-C.; Espina, C.; Key, T.J.; Leitzmann, M.; Norat, T.; Powers, H.; et al. European code against cancer 4th edition: Alcohol drinking and cancer. Cancer Epidemiol. 2015, 39, S67–S74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Allen, A.E.; Locasale, J.W. The molecular link from diet to cancer cell metabolism. Mol. Cell 2020, 80, 554. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar] [PubMed]
- Argilés, J.M.; Stemmler, B.; López-Soriano, F.J.; Busquets, S. Inter-tissue communication in cancer cachexia. Nat. Rev. Endocrinol. 2019, 15, 9–20. [Google Scholar] [CrossRef]
- Sakurai, T.; Kudo, M. Signaling pathways governing tumor angiogenesis. Oncology 2011, 81, 24–29. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef]
- Sahai, E. Mechanisms of cancer cell invasion. Curr. Opin. Genet. Dev. 2005, 15, 87–96. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Al-Hilu, S.A.; Al-Shujairi, W.H. Dual role of bacteria in carcinoma: Stimulation and inhibition. Int. J. Microbiol. 2020, 2020, 1–15. [Google Scholar]
- Jia, B.; Jeon, C.O. Promotion and induction of liver cancer by gut microbiome-mediated modulation of bile acids. PLoS Pathog. 2019, 15, e1007954. [Google Scholar]
- Ma, C.; Han, M.; Heinrich, B.; Fu, Q.; Zhang, Q.; Sandhu, M.; Agdashian, D.; Terabe, M.; Berzofsky, J.A.; Fako, V.; et al. Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science 2018, 360, 6931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.H.; Park, J.P.; Jeon, S.H.; Lee, Y.J.; Choi, H.J.; Jeong, K.M.; Lee, J.G.; Choi, S.P.; Lim, J.H.; Kim, Y.H.; et al. Purulent pericarditis caused by group g streptococcus as an initial presentation of colon cancer. J. Korean Med. Sci. 2002, 17, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegert, C.E.; Overbosch, D. Carcinoma of the colon presenting as streptococcus sanguis bacteremia. Am. J. Gastroenterol. 1995, 90, 1528–1529. [Google Scholar] [PubMed]
- Wu, S.; Rhee, K.-J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.-R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A human colonic commensal promotes colon tumorigenesis via activation of t helper type 17 T cell responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Karpiński, T. Role of oral microbiota in cancer development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Dominguez-Recio, M.E.; Jimenez-Rodriguez, B.; Lavado, R.; Comino-Méndez, I.; Alba, E.; Queipo-Ortuño, M.I. Breast and gut microbiota action mechanisms in breast cancer pathogenesis and treatment. Cancers 2020, 12, E2465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xia, Y.; Sun, J. Breast and gut microbiome in health and cancer. Genes Dis. 2021, 8, 581–589. [Google Scholar] [PubMed]
- Sharma, V.R.; Singh, M.; Kumar, V.; Yadav, M.; Sehrawat, N.; Sharma, D.K.; Sharma, A.K. Microbiome dysbiosis in cancer: Exploring therapeutic strategies to counter the disease. Semin. Cancer Biol. 2021, 70, 61–70. [Google Scholar] [CrossRef]
- Vergara, D.; Simeone, P.; Damato, M.; Maffia, M.; Lanuti, P.; Trerotola, M. The cancer microbiota: EMT and inflammation as shared molecular mechanisms associated with plasticity and progression. J. Oncol. 2019, 2019, 1–16. [Google Scholar] [CrossRef]
- Gevers, D.; Knight, R.; Petrosino, J.F.; Huang, K.; McGuire, A.L.; Birren, B.W.; Nelson, K.E.; White, O.; Methé, B.A.; Huttenhower, C. The human microbiome project: A community resource for the healthy human microbiome. PLoS Biol. 2012, 10, e1001377. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Al-Asmakh, M.; Zadjali, F. Use of germ-free animal models in microbiota-related research. J. Microbiol. Biotechnol. 2015, 25, 1583–1588. [Google Scholar] [CrossRef] [Green Version]
- Kostic, A.D.; Howitt, M.R.; Garrett, W.S. Exploring host-microbiota interactions in animal models and humans. Genes Dev. 2013, 27, 701–718. [Google Scholar] [CrossRef] [Green Version]
- Wiley, N.C.; Dinan, T.G.; Ross, R.P.; Stanton, C.; Clarke, G.; Cryan, J.F. The microbiota-gut-brain axis as a key regulator of neural function and the stress response: Implications for human and animal health1,2. J. Anim. Sci. 2017, 95, 3225–3246. [Google Scholar]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- de La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clément, K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat. Rev. Nephrol. 2016, 12, 169–181. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef]
- Higashimura, Y.; Baba, Y.; Inoue, R.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Hirai, Y.; Ushiroda, C.; Tanaka, Y.; Naito, Y. Effects of molecular hydrogen-dissolved alkaline electrolyzed water on intestinal environment in mice. Med. Gas Res. 2018, 8, 6. [Google Scholar] [PubMed] [Green Version]
- Nagpal, R.; Wang, S.; Solberg Woods, L.C.; Seshie, O.; Chung, S.T.; Shively, C.A.; Register, T.C.; Craft, S.; McClain, D.A.; Yadav, H. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front. Microbiol. 2018, 9, 2897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimann, E.; Nyman, M.; Degerman, E. Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. Adipocyte 2015, 4, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Chaves, V.E.; Frasson, D.; Kawashita, N.H. Several agents and pathways regulate lipolysis in adipocytes. Biochimie 2011, 93, 1631–1640. [Google Scholar] [CrossRef]
- Hansen, T.H.; Thomassen, M.T.; Madsen, M.L.; Kern, T.; Bak, E.G.; Kashani, A.; Allin, K.H.; Hansen, T.; Pedersen, O. The effect of drinking water PH on the human gut microbiota and glucose regulation: Results of a randomized controlled cross-over intervention. Sci. Rep. 2018, 8, 16626. [Google Scholar] [CrossRef]
- Shin, D.W.; Yoon, H.; Kim, H.S.; Choi, Y.J.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H. Effects of alkaline-reduced drinking water on irritable bowel syndrome with diarrhea: A randomized double-blind, placebo-controlled pilot study. Evid. Based Complement. Alternat. Med. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Shang, G.; Tanaka, Y.; Saihara, Y.; Hou, L.; Velasquez, N.; Liu, W.; Lu, Y. Dose-dependent inhibition of gastric injury by hydrogen in alkaline electrolyzed drinking water. BMC Complement. Altern. Med. 2014, 14, 81. [Google Scholar] [CrossRef]
- Tanaka, Y.; Saihara, Y.; Izumotani, K.; Nakamura, H. Daily ingestion of alkaline electrolyzed water containing hydrogen influences human health, including gastrointestinal symptoms. Med. Gas Res. 2018, 8, 160–166. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kiuchi, M.; Higashimura, Y.; Naito, Y.; Koyama, K. The effects of ingestion of hydrogen-dissolved alkaline electrolyzed water on stool consistency and gut microbiota: A double-blind randomized trial. Med. Gas Res. 2021, 11, 138–144. [Google Scholar]
- O’Toole, P.W.; Shiels, P.G. The role of the microbiota in sedentary lifestyle disorders and ageing: Lessons from the animal kingdom. J. Intern. Med. 2020, 287, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Ramadhan, A.; Wicaksono, S.A.; Nugroho, T.E.; Utami, S.B. The effects of alkaline ionized water administration to the total cholesterol levels in patients with type 2 diabetes mellitus accompanied by dyslipidemia. Pak. J. Med. Health Sci. 2021, 15, 1449–1455. [Google Scholar]
- Cian, C.; Barraud, P.A.; Melin, B.; Raphel, C. Effects of fluid ingestion on cognitive function after heat stress or exercise-induced dehydration. Int. J. Psychophysiol. 2001, 42, 243–251. [Google Scholar] [CrossRef]
- Montain, S.J. Hydration recommendations for sport 2008. Curr. Sports Med. Rep. 2008, 7, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Chycki, J.; Zając, T.; Maszczyk, A.; Kurylas, A. The effect of mineral-based alkaline water on hydration status and the metabolic response to short-term anaerobic exercise. Biol. Sport 2017, 34, 255–261. [Google Scholar] [CrossRef] [Green Version]
- Coyle, E.F. Fluid and fuel intake during exercise. J. Sports Sci. 2004, 22, 39–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurylas, A.; Zajac, T.; Zydek, G.; Zajac, A. The effectiveness of alkaline water in hydrating athletes. J. Nutr. Health Food Sci. 2017, 5. [Google Scholar] [CrossRef]
- Chycki, J.; Kurylas, A.; Maszczyk, A.; Golas, A.; Zajac, A. Alkaline water improves exercise-induced metabolic acidosis and enhances anaerobic exercise performance in combat sport athletes. PLoS ONE 2018, 13, e0205708. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Stojanovic, M.D. Hydrogen-rich water affected blood alkalinity in physically active men. Res. Sports Med. 2014, 22, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Remig, V.; Franklin, B.; Margolis, S.; Kostas, G.; Nece, T.; Street, J.C. Trans fats in America: A review of their use, consumption, health implications, and regulation. J. Am. Diet. Assoc. 2010, 110, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Henry, M.; Chambron, J. Physico-chemical, biological and therapeutic characteristics of electrolyzed reduced alkaline water (ERAW). Water 2013, 5, 2094–2115. [Google Scholar] [CrossRef]
- Shirahata, S.; Hamasaki, T.; Teruya, K. Advanced research on the health benefit of reduced water. Trends Food Sci. Technol. 2012, 23, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.-C.; Yang, C.-C.; Hsu, S.-P.; Lee, K.-T.; Liu, H.-W.; Morisawa, S.; Otsubo, K.; Chien, C.-T. Electrolyzed-reduced water reduced hemodialysis-induced erythrocyte impairment in end-stage renal disease patients. Kidney Int. 2006, 70, 391–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauns, J.; Turek, T. Alkaline water electrolysis powered by renewable energy: A review. Processes 2020, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-R.; Hung, Y.-C.; Hsu, S.-Y.; Huang, Y.-W.; Hwang, D.-F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Yan, H.; Kinjo, T.; Tian, H.; Hamasaki, T.; Teruya, K.; Kabayama, S.; Shirahata, S. Mechanism of the lifespan extension of Caenorhabditis Elegans by electrolyzed reduced water—Participation of Pt nanoparticles. Biosci. Biotechnol. Biochem. 2011, 75, 1295–1299. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.-C.; Yang, C.-C.; Lee, K.-T.; Chien, C.-T. Reduced hemodialysis-induced oxidative stress in end-stage renal disease patients by electrolyzed reduced water. Kidney Int. 2003, 64, 704–714. [Google Scholar] [CrossRef] [Green Version]
- Koseki, M.; Tanaka, Y.; Noguchi, H.; Nishikawa, T. Effect of PH on the taste of alkaline electrolyzed water. J. Food Sci. 2007, 72, S298–S302. [Google Scholar] [CrossRef]
- Lee, K.J.; Jin, D.; Chang, B.S.; Teng, Y.C.; Kim, D.H. The immunological effects of electrolyzed reduced water on the echinostoma hortense infection in C57BL/6 mice. Biol. Pharm. Bull. 2009, 32, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.-J.; Kim, J.-R.; Ryang, Y.-S.; Kim, D.-H.; Deung, Y.-K.; Park, S.-K.; Lee, K.-J. A clinical trial of orally administered alkaline reduced water. Biomed. Sci. Lett. 2007, 13, 83–89. [Google Scholar]
- Vorobjeva, N.V. Selective stimulation of the growth of anaerobic microflora in the human intestinal tract by electrolyzed reducing water. Med. Hypotheses 2005, 64, 543–546. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Kim, D.H.; Kim, S.K.; Song, S.B.; Uh, Y.; Jin, D.; Qi, X.F.; Teng, Y.C.; Lee, K.J. The melamine excretion effect of the electrolyzed reduced water in melamine-fed mice. Food Chem. Toxicol. 2011, 49, 1814–1819. [Google Scholar] [CrossRef]
- Kim, J.M.; Kazuhito, Y. Effects of alkaline ionized water on spontaneously diabetic GK-rats fed sucrose. Korean J. Lab. Anim. Sci. Korea Repub. 1997, 13, 187–190. [Google Scholar]
- Watanabe, T.; Kishikawa, Y.; Shirai, W. Influence of alkaline ionized water on rat erythrocyte hexokinase activity and myocardium. J. Toxicol. Sci. 1997, 22, 141–152. [Google Scholar] [CrossRef]
- Li, Y.; Hamasaki, T.; Nakamichi, N.; Kashiwagi, T.; Komatsu, T.; Ye, J.; Teruya, K.; Abe, M.; Yan, H.; Kinjo, T.; et al. Suppressive effects of electrolyzed reduced water on alloxan-induced apoptosis and type 1 diabetes mellitus. Cytotechnology 2011, 63, 119–131. [Google Scholar] [CrossRef] [Green Version]
- Ignacio, R.M.C.; Kim, C.S.; Kim, S.K. Immunological profiling of obesity. J. Lifestyle Med. 2014, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pullinger, C.R.; Eng, C.; Salen, G.; Shefer, S.; Batta, A.K.; Erickson, S.K.; Verhagen, A.; Rivera, C.R.; Mulvihill, S.J.; Malloy, M.J.; et al. Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Invest. 2002, 110, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H.K. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci. 2006, 79, 2288–2292. [Google Scholar] [CrossRef]
| Metabolic Syndrome | Model Used | Results | Possible Mechanism | Reference |
|---|---|---|---|---|
| Diabetes | db/db Obesity Model Mice |
| Upregulation of hepatic hormone, FGF21, which increases glucose and fatty acid expenditures. | [27] |
| OLETF Rats |
| ARW functions as an antioxidant against ROS generated within blood vessels by the non-phagocytic NAD(P)H oxidase protein. | [32] | |
| Clinical |
| Water with alkaline pH acts as an antioxidant against free radicals generated by hyperglycemia. Alkaline water donates electrons to these free radicals. Free radicals react with cellular unsaturated fatty acids that cause the production of harmful peroxides that damage the cells. | [33] | |
| Clinical |
| ROS scavenger (antioxidant), which reduces inflammation and protects DNA from damages. Hydrogen readily diffuses into the cell, which binds to hydroxyl radicals. Inflammation, being a product of oxidative stress, was also seen to decrease upon treatment of ARW—reduces IL-6, TNF, and IL-12, as well as IFN-gamma. | [34] | |
| Clinical |
| Hydrogen suppresses oxidation/modification of LDLs. | [35] | |
| Obesity | Sprague-Dawley rats |
| MRW downregulates lipid-metabolism in the liver, thus suppressing obesity—lowers serum TG. Hydrogen acts as an antioxidant against ROS. | [29] |
| C57BL/6 Mice |
| CYP7A1 induces higher cholesterol catabolic rate. | [28] | |
| Non-alcoholic fatty liver disease | C57BL/6 High-Fat Diet Model |
| CD36 and TNF -α expression levels in the liver are significantly reduced in HRW-treated mice compared to control. | [36] |
| High-Fat Diet and Streptozotocin-induced Lipotoxicity and Glucotoxicity in Sprague-Dawley Rats |
| PPARα and PPARγ protein expression in the liver were upregulated in the HRS group. TNF-α and IL-1β were significantly decreasing in the HRS group. | [37] | |
| Methionine-choline–deficient (MCD) diet C57BL/6 Mouse Model Stelic Animal Model (STAM) of NASH-derived HCC |
| Inflammatory markers TNF-α and IL-6 were downregulated. Lipid metabolism markers FAT and AOX were significantly reduced in the HRW group. | [38] | |
| Cancer (Skin) | C56BL/6 Mice B16-BL6 Melanoma Cells of Mice |
| Inhibition of intravenous metastasis. ARW acts as an antioxidant by reducing the amount of ROS, and functions as a strong immune modulator. Magnesium ion components of ARW might aid in its anticancer effect. | [39] |
| Cancer (Lung) | A549 Cell Line |
| Activation of hydrogen with enhanced reducing potential produced during electrolysis of ERW aid in the scavenging of reactive oxygen species. ERW blocks ERK activation in A549 cells. Inactivation of ERK that leads to ERK MAPK signal pathways by ERW is suggested to play a pivotal role in inhibiting VEGF gene expression. | [40] |
| Cancer (Human Fibrosarcoma) | Human Fibrosarcoma HT1080 Cells |
| The ROS scavenging activity of ERW can be attributed to its two active substances: hydrogen molecules that protect from free radicals by enhancing the expression of genes encoding antioxidant proteins (SOD, catalase, and HO-1 enzymes); and platinum (Pt) nanoparticles that can scavenge O2-, H2O2, and OH radicals. ERW inhibits invasion of the HT1080 cells through a high reducing potential of its hydrogen molecule component, and the ROS scavenging ability of Pt nanoparticle component. ERW has an antagonizing effect on the amplified activation of p38 due to H2O2 treatment. | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delos Reyes, F.S.L.G.; Mamaril, A.C.C.; Matias, T.J.P.; Tronco, M.K.V.; Samson, G.R.; Javier, N.D.; Fadriquela, A.; Antonio, J.M.; Sajo, M.E.J.V. The Search for the Elixir of Life: On the Therapeutic Potential of Alkaline Reduced Water in Metabolic Syndromes. Processes 2021, 9, 1876. https://doi.org/10.3390/pr9111876
Delos Reyes FSLG, Mamaril ACC, Matias TJP, Tronco MKV, Samson GR, Javier ND, Fadriquela A, Antonio JM, Sajo MEJV. The Search for the Elixir of Life: On the Therapeutic Potential of Alkaline Reduced Water in Metabolic Syndromes. Processes. 2021; 9(11):1876. https://doi.org/10.3390/pr9111876
Chicago/Turabian StyleDelos Reyes, Felippe Steven Louis G., Adrian Carlo C. Mamaril, Trisha Joy P. Matias, Mary Kathleen V. Tronco, Gabriel R. Samson, Nyczl D. Javier, Ailyn Fadriquela, Jayson M. Antonio, and Ma Easter Joy V. Sajo. 2021. "The Search for the Elixir of Life: On the Therapeutic Potential of Alkaline Reduced Water in Metabolic Syndromes" Processes 9, no. 11: 1876. https://doi.org/10.3390/pr9111876
APA StyleDelos Reyes, F. S. L. G., Mamaril, A. C. C., Matias, T. J. P., Tronco, M. K. V., Samson, G. R., Javier, N. D., Fadriquela, A., Antonio, J. M., & Sajo, M. E. J. V. (2021). The Search for the Elixir of Life: On the Therapeutic Potential of Alkaline Reduced Water in Metabolic Syndromes. Processes, 9(11), 1876. https://doi.org/10.3390/pr9111876












