The Dark Side of Platinum Based Cytostatic Drugs: From Detection to Removal
Abstract
1. Introduction
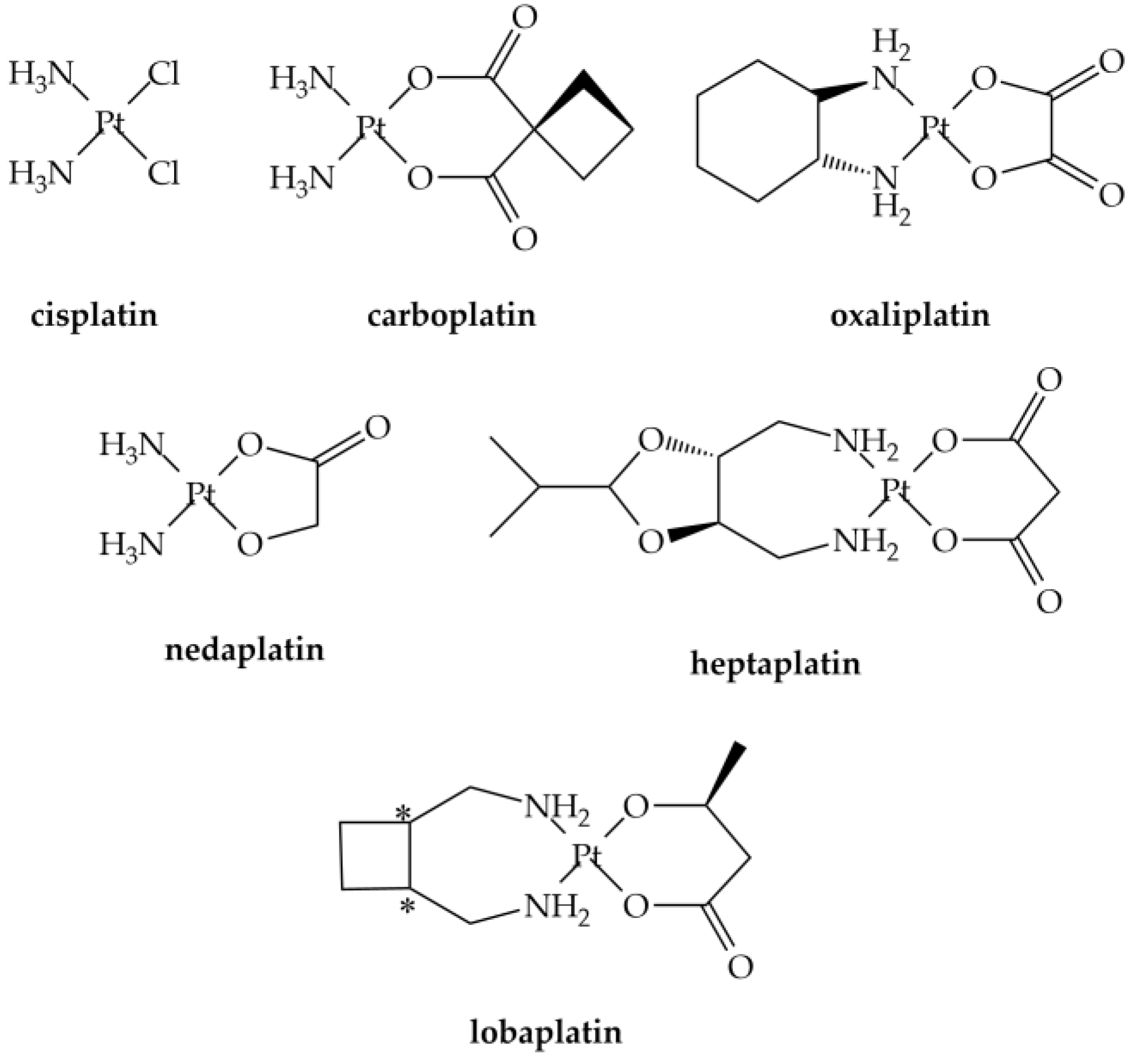
2. Action Mechanism, Speciation and Determinations Analysis of Pt-Based CDs
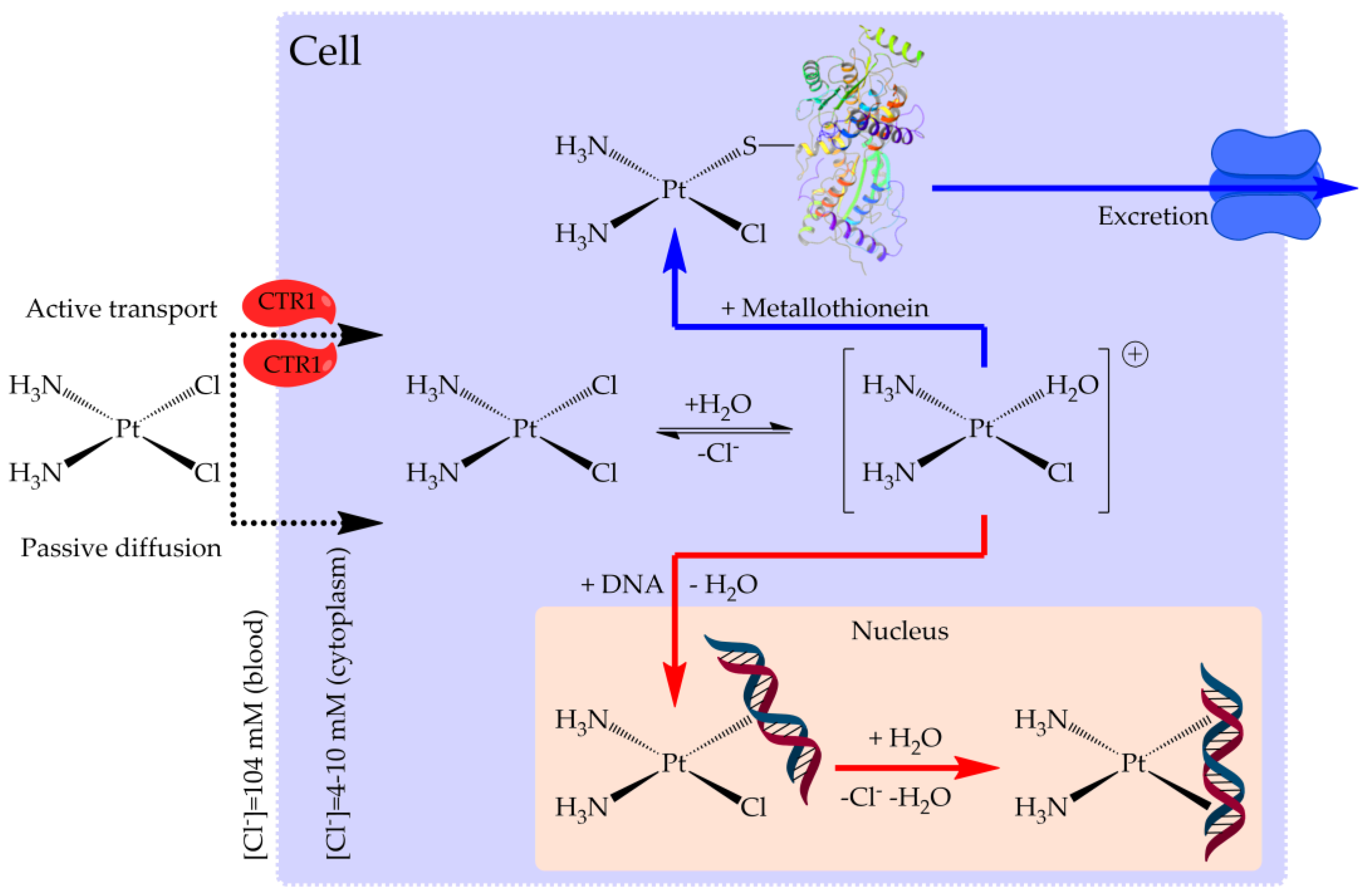
2.1. Reactivity
2.2. Prediction of Platinum-Based CDs Release into the Environment
2.3. Analytical Techniques
2.3.1. Biological Samples
2.3.2. Environmental Samples
2.3.3. Working Environments
3. Predicted Behavior in the Aquatic and Related Environments
4. Ecotoxicological Potency
5. Impact of Water Treatment Technologies
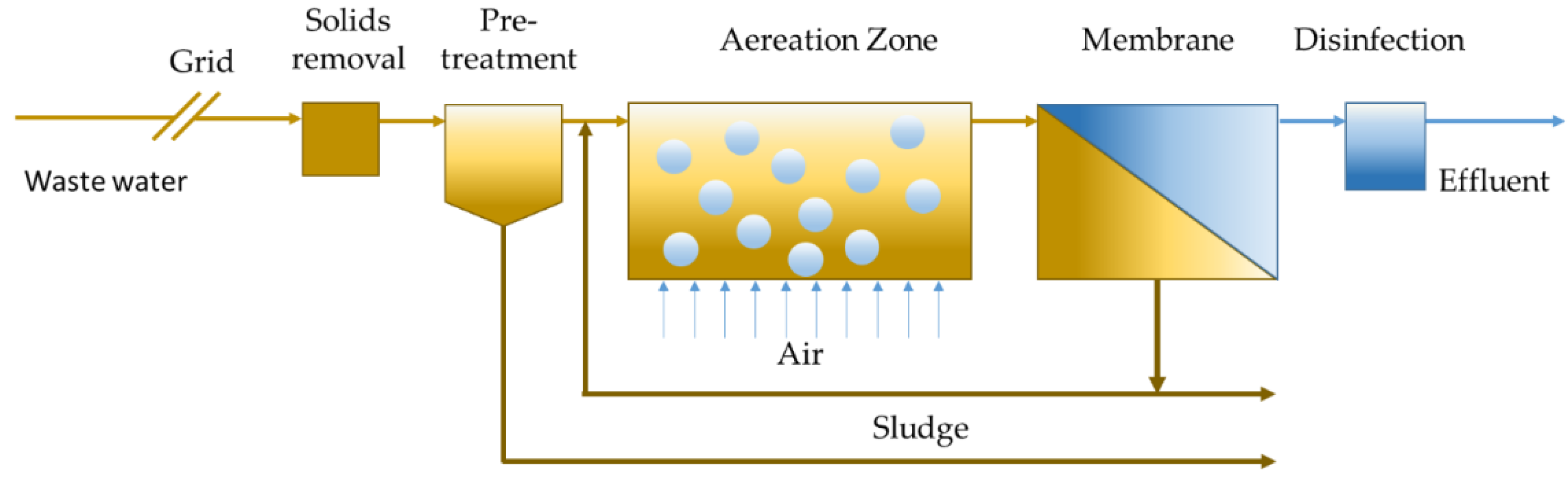
5.1. Membrane Bioreactors
5.2. Advanced Oxidation Processes
5.3. Adsorption
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- WHO. IARC Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/en (accessed on 18 October 2021).
- Gouveia, T.I.A.; Alves, A.; Santos, M.S.F. New insights on cytostatic drug risk assessment in aquatic environments based on measured concentrations in surface waters. Environ. Int. 2019, 133, 105236. [Google Scholar] [CrossRef]
- Lancharro, P.M.; De Castro-Acuña Iglesias, N.; González-Barcala, F.J.; González, J.D.M. Evidence of exposure to cytostatic drugs in healthcare staff: A review of recent literature. Farm. Hosp. 2016, 40, 604–621. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Fatta-Kassinos, D. Cytostatic Drug Residues in Wastewater Treatment Plants: Sources, Removal Efficiencies and Current Challenges. In Fate and Effects of Anticancer Drugs in the Environment; Heath, E., Isidori, M., Kosjek, T., Filipič, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 103–138. [Google Scholar]
- Zhang, J.; Chang, V.W.C.; Giannis, A.; Wang, J.-Y. Removal of cytostatic drugs from aquatic environment: A review. Sci. Total Environ. 2013, 445–446, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Toolaram, A.P.; Kümmerer, K.; Schneider, M. Environmental risk assessment of anti-cancer drugs and their transformation products: A focus on their genotoxicity characterization-state of knowledge and short comings. Mutat. Res. Mutat. Res. 2014, 760, 18–35. [Google Scholar] [CrossRef]
- Kosjek, T.; Negreira, N.; Heath, E.; López de Alda, M.; Barceló, D. Aerobic activated sludge transformation of vincristine and identification of the transformation products. Sci. Total Environ. 2018, 610–611, 892–904. [Google Scholar] [CrossRef]
- Booker, V.; Halsall, C.; Llewellyn, N.; Johnson, A.; Williams, R. Prioritising anticancer drugs for environmental monitoring and risk assessment purposes. Sci. Total Environ. 2014, 473–474, 159–170. [Google Scholar] [CrossRef]
- Jureczko, M.; Kalka, J. Cytostatic pharmaceuticals as water contaminants. Eur. J. Pharmacol. 2020, 866, 172816. [Google Scholar] [CrossRef]
- Mitra, R.; Goddard, R.; Pörschke, K.R. 9,9-Difluorobispidine Analogues of Cisplatin, Carboplatin, and Oxaliplatin. Inorg. Chem. 2017, 56, 6712–6724. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, B.P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Wheate, N.J.; Walker, S.; Craig, G.E.; Oun, R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalt. Trans. 2010, 39, 8113–8127. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt (II) Agents, Nanoparticle Delivery, and Pt (IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. Third row transition metals for the treatment of cancer. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373. [Google Scholar] [CrossRef] [PubMed]
- Franquet-Griell, H.; Gómez-Canela, C.; Ventura, F.; Lacorte, S. Predicting concentrations of cytostatic drugs in sewage effluents and surface waters of Catalonia (NE Spain). Environ. Res. 2015, 138, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.C.; Oldenkamp, R.; Dumont, E.; Sumpter, J.P. Predicting concentrations of the cytostatic drugs cyclophosphamide, carboplatin, 5-fluorouracil, and capecitabine throughout the sewage effluents and surface waters of europe. Environ. Toxicol. Chem. 2013, 32, 1954–1961. [Google Scholar] [CrossRef]
- Valcárcel, Y.; González Alonso, S.; Rodríguez-Gil, J.L.; Gil, A.; Catalá, M. Detection of pharmaceutically active compounds in the rivers and tap water of the Madrid Region (Spain) and potential ecotoxicological risk. Chemosphere 2011, 84, 1336–1348. [Google Scholar] [CrossRef]
- Queirós, V.; Azeiteiro, U.M.; Soares, A.M.V.M.; Freitas, R. The antineoplastic drugs cyclophosphamide and cisplatin in the aquatic environment—Review. J. Hazard. Mater. 2021, 412, 125028. [Google Scholar] [CrossRef]
- Tripathi, A.K.; David, A.; Govil, T.; Rauniyar, S.; Rathinam, N.K.; Goh, K.M.; Sani, R.K. Environmental Remediation of Antineoplastic Drugs: Present Status, Challenges, and Future Directions. Processes 2020, 8, 747. [Google Scholar] [CrossRef]
- Franquet-Griell, H.; Cornadó, D.; Caixach, J.; Ventura, F.; Lacorte, S. Determination of cytostatic drugs in Besòs River (NE Spain) and comparison with predicted environmental concentrations. Environ. Sci. Pollut. Res. 2017, 24, 6492–6503. [Google Scholar] [CrossRef]
- Santos, M.S.F.; Franquet-Griell, H.; Lacorte, S.; Madeira, L.M.; Alves, A. Anticancer drugs in Portuguese surface waters—Estimation of concentrations and identification of potentially priority drugs. Chemosphere 2017, 184, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and ecotoxicological risk assessment of 14 cytostatic drugs in wastewater. Water. Air. Soil Pollut. 2014, 225, 1896. [Google Scholar] [CrossRef]
- Franquet-Griell, H.; Pueyo, V.; Silva, J.; Orera, V.M.; Lacorte, S. Development of a macroporous ceramic passive sampler for the monitoring of cytostatic drugs in water. Chemosphere 2017, 182, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Quadra, G.R.; Oliveira de Souza, H.; Costa, R.d.S.; Fernandez, M.A.d.S. Do pharmaceuticals reach and affect the aquatic ecosystems in Brazil? A critical review of current studies in a developing country. Environ. Sci. Pollut. Res. 2017, 24, 1200–1218. [Google Scholar] [CrossRef]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platimun Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Van Camp, L.; Trosko, J.E.; Mansout, V.H. Platinum Compounds: A New Class of Potente Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef]
- Berners-Price, S.J.; Appleton, T.G. The Chemistry of Cisplatin in Aqueous Solution. In Platinum-Based Drugs in Cancer Therapy; Kelland, L.R., Farrell, N.P., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 3–35. [Google Scholar]
- Melchior, A.; Sánchez Marcos, E.; Pappalardo, R.R.; Martínez, J.M. Comparative study of the hydrolysis of a third- and a first-generation platinum anticancer complexes. Theor. Chem. Acc. 2011, 128, 627–638. [Google Scholar] [CrossRef]
- Veclani, D.; Tolazzi, M.; Melchior, A. Molecular interpretation of pharmaceuticals’ adsorption on carbon nanomaterials: Theory meets experiments. Processes 2020, 8, 642. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Miller, S.E.; House, D.A. The hydrolysis products of cis-diamminedichloroplatinum(II). I. The kinetics of formation and anation of the cis-diammine(aqua)chloroplatinum(II) cation in acidic aqueous solution. Inorg. Chim. Acta 1989, 161, 131–137. [Google Scholar] [CrossRef]
- Miller, S.E.; House, D.A. The hydrolysis products of cis-dichlorodiammineplatinum(II) 2. The kinetics of formation and anation of the cis-diamminedi(aqua)platinum(II) cation. Inorg. Chim. Acta 1989, 166, 189–197. [Google Scholar] [CrossRef]
- Miller, S.E.; House, D.A. The hydrolysis products of cis-diamminedichloroplatinum(II) 5. The anation kinetics of cis-Pt(X)(NH3)2(OH2)+ (X=Cl, OH) with glycine, monohydrogen malonate and chloride. Inorg. Chim. Acta 1991, 187, 125–132. [Google Scholar] [CrossRef]
- Ahmad, S. Kinetic aspects of platinum anticancer agents. Polyhedron 2017, 138, 109–124. [Google Scholar] [CrossRef]
- Credendino, R.; Minenkov, Y.; Liguori, D.; Piemontesi, F.; Melchior, A.; Morini, G.; Tolazzi, M.; Cavallo, L. Accurate experimental and theoretical enthalpies of association of TiCl4 with typical Lewis bases used in heterogeneous Ziegler–Natta catalysis. Phys. Chem. Chem. Phys. 2017, 19, 26996–27006. [Google Scholar] [CrossRef]
- Veclani, D.; Tolazzi, M.; Cerón-Carrasco, J.P.; Melchior, A. Intercalation Ability of Novel Monofunctional Platinum Anticancer Drugs: A Key Step in Their Biological Action. J. Chem. Inf. Model. 2021, 61, 4391–4399. [Google Scholar] [CrossRef]
- Dell’Anna, M.M.; Censi, V.; Carrozzini, B.; Caliandro, R.; Denora, N.; Franco, M.; Veclani, D.; Melchior, A.; Tolazzi, M.; Mastrorilli, P. Triphenylphosphane Pt(II) complexes containing biologically active natural polyphenols: Synthesis, crystal structure, molecular modeling and cytotoxic studies. J. Inorg. Biochem. 2016, 163, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Hann, S.; Koellensperger, G.; Stefánka, Z.; Stingeder, G.; Fürhacker, M.; Buchberger, W.; Mader, R.M. Application of HPLC-ICP-MS to speciation of cisplatin and its degradation products in water containing different chloride concentrations and in human urine. J. Anal. At. Spectrom. 2003, 18, 1391–1395. [Google Scholar] [CrossRef]
- Boyd, L.; Muggia, F. Carboplatin/Paclitaxel Induction in Ovarian Cancer: The Finer Points. Oncology 2018, 32, 422–424. [Google Scholar]
- Gridelli, C.; Chen, T.; Ko, A.; O’Brien, M.E.; Ong, T.J.; Socinski, M.A.; Postmus, P.E. Nab-paclitaxel/carboplatin in elderly patients with advanced squamous non-small cell lung cancer: A retrospective analysis of a phase iii trial. Drug Des. Devel. Ther. 2018, 12, 1445–1451. [Google Scholar] [CrossRef]
- Narveson, L.; Kathol, E.; Rockey, M.; Henry, D.; Grauer, D.; Neupane, P. Evaluation of Weekly Paclitaxel, Carboplatin, and Cetuximab in Head and Neck Cancer Patients With Incurable Disease. Int. J. Radiat. Oncol. 2016, 94, 933–934. [Google Scholar] [CrossRef][Green Version]
- Alexander, C.; Nithyakumar, A.; Paul, M.W.B.; Arockia Samy, N. Platinum(II) complexes of imidazophenanthroline-based polypyridine ligands as potential anticancer agents: Synthesis, characterization, in vitro cytotoxicity studies and a comparative ab initio, and DFT studies with cisplatin, carboplatin, and oxaliplatin. J. Biol. Inorg. Chem. 2018, 23, 833–848. [Google Scholar] [CrossRef]
- Riddell, I.A. Cisplatin and Oxaliplatin: Our Current Understanding of Their Actions. Met. Ions Life Sci. 2018, 18. [Google Scholar] [CrossRef]
- Rogers, B.B.; Cuddahy, T.; Briscella, C.; Ross, N.; Olszanski, A.J.; Denlinger, C.S. Oxaliplatin: Detection and management of hypersensitivity reactions. Clin. J. Oncol. Nurs. 2019, 23, 68–75. [Google Scholar] [CrossRef]
- Lenz, K.; Hann, S.; Koellensperger, G.; Stefanka, Z.; Stingeder, G.; Weissenbacher, N.; Mahnik, S.N.; Fuerhacker, M. Presence of cancerostatic platinum compounds in hospital wastewater and possible elimination by adsorption to activated sludge. Sci. Total Environ. 2005, 345, 141–152. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Yin, J.; Duan, H.; Wu, Y.; Shao, B. Analysis of hormone antagonists in clinical and municipal wastewater by isotopic dilution liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2010, 396, 2977–2985. [Google Scholar] [CrossRef]
- Kümmerer, K.; Helmers, E.; Hubner, P.; Mascart, G.; Milandri, M.; Reinthaler, F.; Zwakenberg, M. European hospitals as a source for platinum in the environment in comparison with other sources. Sci. Total Environ. 1999, 225, 155–165. [Google Scholar] [CrossRef]
- Kümmerer, K. Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—A review. Chemosphere 2001, 45, 957–969. [Google Scholar] [CrossRef]
- Johnson, A.C.; Jürgens, M.D.; Williams, R.J.; Kümmerer, K.; Kortenkamp, A.; Sumpter, J.P. Do cytotoxic chemotherapy drugs discharged into rivers pose a risk to the environment and human health? An overview and UK case study. J. Hydrol. 2008, 348, 167–175. [Google Scholar] [CrossRef]
- Lenz, K.; Koellensperger, G.; Hann, S.; Weissenbacher, N.; Mahnik, S.N.; Fuerhacker, M. Fate of cancerostatic platinum compounds in biological wastewater treatment of hospital effluents. Chemosphere 2007, 69, 1765–1774. [Google Scholar] [CrossRef]
- Vyas, N.; Turner, A.; Sewell, G. Platinum-based anticancer drugs in waste waters of a major UK hospital and predicted concentrations in recipient surface waters. Sci. Total Environ. 2014, 493, 324–329. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Russo, C.; Kundi, M.; Žegura, B.; Novak, M.; Filipič, M.; Mišík, M.; Knasmueller, S.; de Alda, M.L.; et al. Chemical and toxicological characterisation of anticancer drugs in hospital and municipal wastewaters from Slovenia and Spain. Environ. Pollut. 2016, 219, 275–287. [Google Scholar] [CrossRef]
- Besse, J.-P.; Latour, J.-F.; Garric, J. Anticancer drugs in surface waters: What can we say about the occurrence and environmental significance of cytotoxic, cytostatic and endocrine therapy drugs? Environ. Int. 2012, 39, 73–86. [Google Scholar] [CrossRef]
- Ghafuria, Y.; Yunesian, M.; Nabizadeh, R.; Mesdaghinia, A.; Dehghani, M.H.; Alimohammadi, M. Environmental risk assessment of platinum cytotoxic drugs: A focus on toxicity characterization of hospital effluents. Int. J. Environ. Sci. Technol. 2018, 15, 1983–1990. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mehta, D.; Dhangar, K.; Kumar, M. Environmental fate, distribution and state-of-the-art removal of antineoplastic drugs: A comprehensive insight. Chem. Eng. J. 2021, 407, 127184. [Google Scholar] [CrossRef]
- da Silva, R.F.; de Lima Moura, L.; Gavião, L.O.; Brito Alves Lima, G.; Dausacker Bidone, E. Local environmental risk assessment of anticancer drugs in a developing country. Hum. Ecol. Risk Assess. Int. J. 2020, 26, 2142–2161. [Google Scholar] [CrossRef]
- Santana-Viera, S.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Analytical Methodologies for the Determination of Cytostatic Compounds in Environmental Matrices. In Fate and Effects of Anticancer Drugs in the Environment; Heath, E., Isidori, M., Kosjek, T., Filipič, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 165–195. ISBN 978-3-030-21048-9. [Google Scholar]
- Kizu, R.; Yamamoto, T.; Yokoyama, T.; Tanaka, M.; Miyazaki, M. A Sensitive Postcolumn Derivatization/UV Detection System for HPLC Determination of Antitumor Divalent and Quadrivalent Platinum Complexes. Chem. Pharm. Bull. 1995, 43, 108–114. [Google Scholar] [CrossRef]
- Burns, R.B.; Embree, L. Validation of high-performance liquid chromatographic assay methods for the analysis of carboplatin in plasma ultrafiltrate. J. Chromatogr. B Biomed. Sci. Appl. 2000, 744, 367–376. [Google Scholar] [CrossRef]
- Koellensperger, G.; Hann, S. Ultra-fast HPLC-ICP-MS analysis of oxaliplatin in patient urine. Anal. Bioanal. Chem. 2010, 397, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Koellensperger, G.; Stefanka, Z.; Meelich, K.; Galanski, M.; Keppler, B.K.; Stingeder, G.; Hann, S. Species specific IDMS for accurate quantification of carboplatin in urine by LC-ESI-TOFMS and LC-ICP-QMS. J. Anal. At. Spectrom. 2008, 23, 29–36. [Google Scholar] [CrossRef]
- Shaik, A.N.; Altomare, D.A.; Lesko, L.J.; Trame, M.N. Development and validation of a LC–MS/MS assay for quantification of cisplatin in rat plasma and urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1046, 243–249. [Google Scholar] [CrossRef]
- da Costa, A.C.; Vieira, M.A.; Luna, A.S.; de Campos, R.C. Determination of platinum originated from antitumoral drugs in human urine by atomic absorption spectrometric methods. Talanta 2010, 82, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Martinčič, A.; Cemazar, M.; Sersa, G.; Kovač, V.; Milačič, R.; Ščančar, J. A novel method for speciation of Pt in human serum incubated with cisplatin, oxaliplatin and carboplatin by conjoint liquid chromatography on monolithic disks with UV and ICP-MS detection. Talanta 2013, 116, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Cai, S.; Forrest, W.C.; Mohr, E.; Yang, Q.; Forrest, M.L. Development and Validation of an Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Method for Quantitative Analysis of Platinum in Plasma, Urine, and Tissues. Appl. Spectrosc. 2016, 70, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Zachariadis, G.A.; Misopoulou, O.E. Determination of Cisplatin and Carboplatin Anticancer Drugs by Non-suppressed Ion Chromatography with an Inductively Coupled Plasma Atomic Emission Detector. Anal. Lett. 2018, 51, 1060–1070. [Google Scholar] [CrossRef]
- Lemoine, L.; Thijssen, E.; Noben, J.-P.; Adriaensens, P.; Carleer, R.; Speeten, K. Van der A validated inductively coupled plasma mass spectrometry (ICP-MS) method for the quantification of total platinum content in plasma, plasma ultrafiltrate, urine and peritoneal fluid. J. Pharm. Biomed. Anal. 2018, 152, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Folens, K.; Mortier, S.T.F.C.; Baeten, J.; Couvreur, K.; Michelet, R.; Gernaey, K.V.; De Beer, T.; Du Laing, G.; Nopens, I. Modelling and sensitivity analysis of urinary platinum excretion in anticancer chemotherapy for the recovery of platinum. Sustain. Chem. Pharm. 2016, 4, 46–56. [Google Scholar] [CrossRef]
- Wu, Y.; Lai, R.Y. Tunable Signal-Off and Signal-On Electrochemical Cisplatin Sensor. Anal. Chem. 2017, 89, 9984–9989. [Google Scholar] [CrossRef]
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin electrochemical biosensor. Electrochim. Acta 2006, 51, 5169–5173. [Google Scholar] [CrossRef]
- Volder, M.F.L.D.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-Based Materials for Biosensors: A Review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Gholivand, M.B.; Ahmadi, E.; Mavaei, M. A novel voltammetric sensor based on graphene quantum dots-thionine/nano-porous glassy carbon electrode for detection of cisplatin as an anti-cancer drug. Sens. Actuators B Chem. 2019, 299, 126975. [Google Scholar] [CrossRef]
- Materon, E.M.; Wong, A.; Klein, S.I.; Liu, J.; Sotomayor, M.D.P.T. Multi-walled carbon nanotubes modified screen-printed electrodes for cisplatin detection. Electrochim. Acta 2015, 158, 271–276. [Google Scholar] [CrossRef]
- Mitchell, L.; Shen, C.; Timmins, H.C.; Park, S.B.; New, E.J. A Versatile Fluorescent Sensor Array for Platinum Anticancer Drug Detection in Biological Fluids. ACS Sens. 2021, 6, 1261–1269. [Google Scholar] [CrossRef]
- Lenz, K.; Mahnik, S.N.; Weissenbacher, N.; Mader, R.M.; Krenn, P.; Hann, S.; Koellensperger, G.; Uhl, M.; Knasmüller, S.; Ferk, F.; et al. Monitoring, removal and risk assessment of cytostatic drugs in hospital wastewater. Water Sci. Technol. 2007, 56, 141–149. [Google Scholar] [CrossRef]
- Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D. Development of a UPLC-MS/MS method for the determination of ten anticancer drugs in hospital and urban wastewaters, and its application for the screening of human metabolites assisted by information-dependent acquisition tool (IDA) in sewage samples. Anal. Bioanal. Chem. 2013, 405, 5937–5952. [Google Scholar] [CrossRef] [PubMed]
- Santana-Viera, S.; Padrón, M.E.T.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Quantification of cytostatic platinum compounds in wastewater by inductively coupled plasma mass spectrometry after ion exchange extraction. Microchem. J. 2020, 157, 104862. [Google Scholar] [CrossRef]
- Jadon, N.; Jain, R.; Sharma, S.; Singh, K. Recent trends in electrochemical sensors for multianalyte detection—A review. Talanta 2016, 161, 894–916. [Google Scholar] [CrossRef]
- Kominkova, M.; Heger, Z.; Zitka, O.; Kynicky, J.; Pohanka, M.; Beklova, M.; Adam, V.; Kizek, R. Flow injection analysis with electrochemical detection for rapid identification of platinum-based cytostatics and platinum chlorides in water. Int. J. Environ. Res. Public Health 2014, 11, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Ghafuria, Y.; Yunesian, M.; Nabizadeh, R.; Mesdaghinia, A.; Dehghani, M.H.; Alimohammadi, M. Platinum cytotoxic drugs in the municipal wastewater and drinking water, a validation method and health risk assessment. Hum. Ecol. Risk Assess. 2018, 24, 784–796. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Asadi-Ghalhari, M.; Ghafuri, Y. Development of an analytical method for determination of carboplatin and oxaliplatin in resource water, prediction and environmental risk assessment. J. Environ. Treat. Tech. 2020, 8, 1168–1175. [Google Scholar]
- Hann, S.; Stefánka, Z.; Lenz, K.; Stingeder, G. Novel separation method for highly sensitive speciation of cancerostatic platinum compounds by HPLC-ICP-MS. Anal. Bioanal. Chem. 2005, 381, 405–412. [Google Scholar] [CrossRef]
- Kato, R.; Sato, T.; Kanamori, M.; Miyake, M.; Fujimoto, A.; Ogawa, K.; Kobata, D.; Fujikawa, T.; Wada, Y.; Mitsuishi, R.; et al. A novel analytical method of cisplatin using the HPLC with a naphthylethyl group bonded with silica gel (πNAP) column. Biol. Pharm. Bull. 2017, 40, 290–296. [Google Scholar] [CrossRef]
- Nygren, O.; Lundgren, C. Determination of platinum in workroom air and in blood and urine from nursing staff attending patients receiving cisplatin chemotherapy. Int. Arch. Occup. Environ. Health 1997, 70, 209–214. [Google Scholar] [CrossRef]
- Schreiber, C.; Radon, K.; Pethran, A.; Schierl, R.; Hauff, K.; Grimm, C.-H.; Boos, K.-S.; Nowak, D. Uptake of antineoplastic agents in pharmacy personnel. Part II: Study of work-related risk factors. Int. Arch. Occup. Environ. Health 2003, 76, 11–16. [Google Scholar] [CrossRef]
- Raghavan, R.; Burchett, M.; Loffredo, D.; Mulligan, J.A. Low-Level (PPB)Determination of Cisplatin in Cleaning Validation (Rinse Water) Samples. II. A High-Performance Liquid Chromatogrphic Method. Drug Dev. Ind. Pharm. 2000, 26, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Hori, A.; Shimura, M.; Iida, Y.; Yamada, K.; Nohara, K.; Ichinose, T.; Yamashita, A.; Shirataki, J.; Hagiwara, S. Occupational exposure of platinum-based anti-cancer drugs: Five-year monitoring of hair and environmental samples in a single hospital. J. Occup. Med. Toxicol. 2020, 15, 29. [Google Scholar] [CrossRef]
- Jeronimo, M.; Colombo, M.; Astrakianakis, G.; Hon, C.-Y. A surface wipe sampling and LC–MS/MS method for the simultaneous detection of six antineoplastic drugs commonly handled by healthcare workers. Anal. Bioanal. Chem. 2015, 407, 7083–7092. [Google Scholar] [CrossRef] [PubMed]
- Böhlandt, A.; Sverdel, Y.; Schierl, R. Antineoplastic drug residues inside homes of chemotherapy patients. Int. J. Hyg. Environ. Health 2017, 220, 757–765. [Google Scholar] [CrossRef]
- Goykhman, N.; Dror, I.; Berkowitz, B. Transport of platinum-based pharmaceuticals in water-saturated sand and natural soil: Carboplatin and cisplatin species. Chemosphere 2019, 219, 390–399. [Google Scholar] [CrossRef]
- Curis, E.; Provost, K.; Nicolis, I.; Bouvet, D.; Bénazeth, S.; Crauste-Manciet, S.; Brion, F.; Brossard, D. Carboplatin decomposition in aqueous solution with chloride ions monitored by X-ray absorption spectroscopy. New J. Chem. 2000, 24, 1003–1008. [Google Scholar] [CrossRef]
- Myers, A.L.; Zhang, Y.P.; Kawedia, J.D.; Trinh, V.A.; Tran, H.; Smith, J.A.; Kramer, M.A. Stability study of carboplatin infusion solutions in 0.9% sodium chloride in polyvinyl chloride bags. J. Oncol. Pharm. Pract. 2016, 22, 31–36. [Google Scholar] [CrossRef]
- Di Pasqua, A.J.; Goodisman, J.; Dabrowiak, J.C. Understanding how the platinum anticancer drug carboplatin works: From the bottle to the cell. Inorg. Chim. Acta 2012, 389, 29–35. [Google Scholar] [CrossRef]
- Goykhman, N.; Dror, I.; Berkowitz, B. Transport of oxaliplatin species in water-saturated natural soil. Chemosphere 2018, 208, 829–837. [Google Scholar] [CrossRef]
- Wormington, A.M.; De María, M.; Kurita, H.G.; Bisesi, J.H.; Denslow, N.D.; Martyniuk, C.J. Antineoplastic Agents: Environmental Prevalence and Adverse Outcomes in Aquatic Organisms. Environ. Toxicol. Chem. 2020, 39, 967–985. [Google Scholar] [CrossRef]
- Nassour, C.; Nabhani-Gebara, S.; Barton, S.J.; Barker, J. Aquatic ecotoxicology of anticancer drugs: A systematic review. Sci. Total Environ. 2021, 800, 149598. [Google Scholar] [CrossRef]
- Brezovšek, P.; Eleršek, T.; Filipič, M. Toxicities of four anti-neoplastic drugs and their binary mixtures tested on the green alga Pseudokirchneriella subcapitata and the cyanobacterium Synechococcus leopoliensis. Water Res. 2014, 52, 168–177. [Google Scholar] [CrossRef]
- Supalkova, V.; Beklova, M.; Baloun, J.; Singer, C.; Sures, B.; Adam, V.; Huska, D.; Pikula, J.; Rauscherova, L.; Havel, L.; et al. Affecting of aquatic vascular plant Lemna minor by cisplatin revealed by voltammetry. Bioelectrochemistry 2008, 72, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kajander, V.; Sewell, G.; Turner, A. Bioaccumulation and toxicity of oxaliplatin in fresh water: A study with Lemna minor. Environ. Adv. 2021, 3, 100030. [Google Scholar] [CrossRef]
- Parrella, A.; Lavorgna, M.; Criscuolo, E.; Russo, C.; Fiumano, V.; Isidori, M. Acute and chronic toxicity of six anticancer drugs on rotifers and crustaceans. Chemosphere 2014, 115, 59–66. [Google Scholar] [CrossRef]
- Hung, G.-Y.; Wu, C.-L.; Chou, Y.-L.; Chien, C.-T.; Horng, J.-L.; Lin, L.-Y. Cisplatin exposure impairs ionocytes and hair cells in the skin of zebrafish embryos. Aquat. Toxicol. 2019, 209, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Easton, C.; Turner, A.; Sewell, G. An evaluation of the toxicity and bioaccumulation of cisplatin in the marine environment using the macroalga, Ulva lactuca. Environ. Pollut. 2011, 159, 3504–3508. [Google Scholar] [CrossRef]
- Mišík, M.; Filipic, M.; Nersesyan, A.; Kundi, M.; Isidori, M.; Knasmueller, S. Environmental risk assessment of widely used anticancer drugs (5-fluorouracil, cisplatin, etoposide, imatinib mesylate). Water Res. 2019, 164, 114953. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Graziani, V.; Lavorgna, M.; D’Abrosca, B.; Piscitelli, C.; Fiorentino, A.; Scognamiglio, M.; Isidori, M. Lymphocytes exposed to vegetables grown in waters contaminated by anticancer drugs: Metabolome alterations and genotoxic risks for human health. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2019, 842, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Dehghanpour, S.; Pourzamani, H.R.; Amin, M.M.; Ebrahimpour, K. Evaluation of toxic effects of platinum-based antineoplastic drugs (cisplatin, carboplatin and oxaliplatin) on green alga Chlorella vulgaris. Aquat. Toxicol. 2020, 223, 105495. [Google Scholar] [CrossRef]
- Bownik, A.; Ślaska, B.; Dudka, J. Cisplatin affects locomotor activity and physiological endpoints of Daphnia magna. J. Hazard. Mater. 2020, 384, 1–8. [Google Scholar] [CrossRef]
- Fonseca, T.G.; Morais, M.B.; Rocha, T.; Abessa, D.M.S.; Aureliano, M.; Bebianno, M.J. Ecotoxicological assessment of the anticancer drug cisplatin in the polychaete Nereis diversicolor. Sci. Total Environ. 2017, 575, 162–172. [Google Scholar] [CrossRef]
- Kolarević, S.; Gačić, Z.; Kostić, J.; Sunjog, K.; Kračun-Kolarević, M.; Paunović, M.; Knežević-Vukčević, J.; Vuković-Gačić, B. Impact of Common Cytostatics on DNA Damage in Freshwater Mussels Unio pictorum and Unio tumidus. CLEAN Soil Air Water 2016, 44, 1471–1476. [Google Scholar] [CrossRef]
- Trombini, C.; Garcia da Fonseca, T.; Morais, M.; Rocha, T.L.; Blasco, J.; Bebianno, M.J. Toxic effects of cisplatin cytostatic drug in mussel Mytilus galloprovincialis. Mar. Environ. Res. 2016, 119, 12–21. [Google Scholar] [CrossRef]
- Mello, L.C.; da Fonseca, T.G.; Denis Moledode de Souza, A. Ecotoxicological assessment of chemotherapeutic agents using toxicity tests with embryos of Mellita quinquiesperforata. Mar. Pollut. Bull. 2020, 159, 111493. [Google Scholar] [CrossRef]
- Grzesiuk, M.; Bednarska, A.; Mielecki, D.; Garbicz, D.; Marcinkowski, M.; Pilžys, T.; Malinowska, A.; Świderska, B.; Grzesiuk, E. Anticancer agents found in environment affect Daphnia at population, individual and molecular levels. Aquat. Toxicol. 2019, 215, 105288. [Google Scholar] [CrossRef]
- Nath, J.; Dror, I.; Berkowitz, B. Effect of nanoplastics on the transport of platinum-based pharmaceuticals in water-saturated natural soil and their effect on a soil microbial community. Environ. Sci. Nano 2020, 7, 3178–3188. [Google Scholar] [CrossRef]
- Siedlecka, E.M. Removal of Cytostatic Drugs from Water and Wastewater: Progress in the Development of Advanced Treatment Methods. In Fate and Effects of Anticancer Drugs in the Environment; Healt, E., Isidor, M., Kosjek, T., Filipič, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Delgado, L.F.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Dorandeu, C.; Marion, B.; Schetrite, S.; Albasi, C. Cytotoxicity micropollutant removal in a crossflow membrane bioreactor. Bioresour. Technol. 2011, 102, 4395–4401. [Google Scholar] [CrossRef]
- Köhler, C.; Venditti, S.; Igos, E.; Klepiszewski, K.; Benetto, E.; Cornelissen, A. Elimination of pharmaceutical residues in biologically pre-treated hospital wastewater using advanced UV irradiation technology: A comparative assessment. J. Hazard. Mater. 2012, 239–240, 70–77. [Google Scholar] [CrossRef]
- Kazner, C.; Lehnberg, K.; Kovalova, L.; Wintgens, T.; Melin, T.; Hollender, J.; Dott, W. Removal of endocrine disruptors and cytostatics from effluent by nanofiltration in combination with adsorption on powdered activated carbon. Water Sci. Technol. 2008, 58, 1699–1706. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Tajahmadi, S.; Rezakazemi, M.; Sehat, A.A.; Molavi, H.; Aminabhavi, T.M.; Arjmand, M. Aluminum-based metal-organic frameworks for adsorptive removal of anti-cancer (methotrexate) drug from aqueous solutions. J. Environ. Manag. 2021, 277, 111448. [Google Scholar] [CrossRef]
- Hirose, J.; Kondo, F.; Nakano, T.; Kobayashi, T.; Hiro, N.; Ando, Y.; Takenaka, H.; Sano, K. Inactivation of antineoplastics in clinical wastewater by electrolysis. Chemosphere 2005, 60, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Ramos, Y.; Fernández, L.A.; Ledea, O.; Bataller, M.; Véliz, E.; Besada, V.; Rosado, A. Ozonation of cisplatin in aqueous solution at pH 9. Ozone Sci. Eng. 2008, 30, 189–196. [Google Scholar] [CrossRef]
- Janssens, R.; Mandal, M.K.; Dubey, K.K.; Luis, P. Slurry photocatalytic membrane reactor technology for removal of pharmaceutical compounds from wastewater: Towards cytostatic drug elimination. Sci. Total Environ. 2017, 599–600, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Farías, T.; Hajizadeh, S.; Ye, L. Cryogels with high cisplatin adsorption capacity: Towards removal of cytotoxic drugs from wastewater. Sep. Purif. Technol. 2020, 235, 116203. [Google Scholar] [CrossRef]
- Judd, S. The status of membrane bioreactor technology. Trends Biotechnol. 2008, 26, 109–116. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane bioreactor (Mbr) technology for wastewater treatment and reclamation: Membrane fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Gao, W.; Meng, F.; Liao, B.Q.; Leung, K.T.; Zhao, L.; Chen, J.; Hong, H. Membrane bioreactors for industrial wastewater treatment: A critical review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 677–740. [Google Scholar] [CrossRef]
- Mutamim, N.S.A.; Noor, Z.Z.; Hassan, M.A.A.; Yuniarto, A.; Olsson, G. Membrane bioreactor: Applications and limitations in treating high strength industrial wastewater. Chem. Eng. J. 2013, 225, 109–119. [Google Scholar] [CrossRef]
- Clara, M.; Kreuzinger, N.; Strenn, B.; Gans, O.; Kroiss, H. The solids retention time—A suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res. 2005, 39, 97–106. [Google Scholar] [CrossRef]
- De Wever, H.; Weiss, S.; Reemtsma, T.; Vereecken, J.; Müller, J.; Knepper, T.; Rörden, O.; Gonzalez, S.; Barcelo, D.; Dolores Hernando, M. Comparison of sulfonated and other micropollutants removal in membrane bioreactor and conventional wastewater treatment. Water Res. 2007, 41, 935–945. [Google Scholar] [CrossRef]
- García, L.A.F.; Lozano, O.L.; Lorenzo, E.V.; Venta, M.B.; Rodríguez, Y.R.; Castro, C.H.; Trujillo, C.G.; Torres, I.F. Review of cytostatic wastewater degradation by ozone and advanced oxidation processes: Results from cuban studies. Environ. Rev. 2020, 28, 21–31. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review. Chemosphere 2017, 174, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals and endocrine disrupting compounds from water by zinc oxide-based photocatalytic degradation: A review. Sustain. Cities Soc. 2016, 27, 407–418. [Google Scholar] [CrossRef]
- Giri, R.R.; Ozaki, H.; Takayanagi, Y.; Taniguchi, S.; Takanami, R. Efficacy of ultraviolet radiation and hydrogen peroxide oxidation to eliminate large number of pharmaceutical compounds in mixed solution. Int. J. Environ. Sci. Technol. 2011, 8, 19–30. [Google Scholar] [CrossRef]
- Prado, M.; Borea, L.; Cesaro, A.; Liu, H.; Naddeo, V.; Belgiorno, V.; Ballesteros, F. Removal of emerging contaminant and fouling control in membrane bioreactors by combined ozonation and sonolysis. Int. Biodeterior. Biodegrad. 2017, 119, 577–586. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hirose, J.; Sano, K.; Hiro, N.; Ijiri, Y.; Takiuchi, H.; Tamai, H.; Takenaka, H.; Tanaka, K.; Nakano, T. Evaluation of an electrolysis apparatus for inactivating antineoplastics in clinical wastewater. Chemosphere 2008, 72, 659–665. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Lanas, S.G.; Valiente, M.; Aneggi, E.; Trovarelli, A.; Tolazzi, M.; Melchior, A. Efficient fluoride adsorption by mesoporous hierarchical alumina microspheres. RSC Adv. 2016, 6, 42288–42296. [Google Scholar] [CrossRef]
- Tao, Z.; Toms, B.; Goodisman, J.; Asefa, T. Mesoporous Silica Microparticles Enhance the Cytotoxicity of Anticancer Platinum Drugs. ACS Nano 2010, 4, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Xiao, H.; Li, C.; Dai, Y.; Cheng, Z.; Hou, Z.; Lin, J. Inorganic nanocarriers for platinum drug delivery. Mater. Today 2015, 18, 554–564. [Google Scholar] [CrossRef]
- Hilder, T.A.; Hill, J.M. Modelling the encapsulation of the anticancer drug cisplatin into carbon nanotubes. Nanotechnology 2007, 18, 275704. [Google Scholar] [CrossRef]
- Mejri, A.; Vardanega, D.; Tangour, B.; Gharbi, T.; Picaud, F. Encapsulation into carbon nanotubes and release of anticancer cisplatin drug molecule. J. Phys. Chem. B 2015, 119, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Veclani, D.; Melchior, A. Adsorption of ciprofloxacin on carbon nanotubes: Insights from molecular dynamics simulations. J. Mol. Liq. 2020, 298. [Google Scholar] [CrossRef]
- Melchior, A.; Lanas, S.G.; Valiente, M.; Tolazzi, M. Thermodynamics of sorption of platinum on superparamagnetic nanoparticles functionalized with mercapto groups. J. Therm. Anal. Calorim. 2018, 134, 1261–1266. [Google Scholar] [CrossRef]
- Callari, M.; Aldrich-Wright, J.R.; de Souza, P.L.; Stenzel, M.H. Polymers with platinum drugs and other macromolecular metal complexes for cancer treatment. Prog. Polym. Sci. 2014, 39, 1614–1643. [Google Scholar] [CrossRef]
- Solomevich, S.O.; Dmitruk, E.I.; Bychkovsky, P.M.; Nebytov, A.E.; Yurkshtovich, T.L.; Golub, N.V. Fabrication of oxidized bacterial cellulose by nitrogen dioxide in chloroform/cyclohexane as a highly loaded drug carrier for sustained release of cisplatin. Carbohydr. Polym. 2020, 248, 116745. [Google Scholar] [CrossRef] [PubMed]
- Folens, K.; Abebe, A.; Tang, J.; Ronsse, F.; Du Laing, G. Biosorption of residual cisplatin, carboplatin and oxaliplatin antineoplastic drugs in urine after chemotherapy treatment. Environ. Chem. 2018, 15, 506–512. [Google Scholar] [CrossRef]
- Ogata, F.; Inoue, K.; Tominaga, H.; Iwata, Y.; Ueda, A.; Tanaka, Y.; Kawasaki, N. Use of Calcined Gibbsite to Remove Cisplatin from Aqueous Solutions. J. Water Environ. Technol. 2014, 12, 13–23. [Google Scholar] [CrossRef]
- Ertürk, G.; Mattiasson, B. Cryogels-versatile tools in bioseparation. J. Chromatogr. A 2014, 1357, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, J.; Dąbrowska, M.; Olchowski, R.; Zięba, E.; Dobrowolski, R. Development of a method for removal of platinum from hospital wastewater by novel ion-imprinted mesoporous organosilica. J. Environ. Chem. Eng. 2021, 9, 105302. [Google Scholar] [CrossRef]



| CD | 2004 | 2008 |
|---|---|---|
| carboplatin | 1.47 | 1.91 |
| oxaliplatin | 0.46 | 0.76 |
| cisplatin | 0.40 | 0.52 |
| Biological Samples | |||||
|---|---|---|---|---|---|
| Compound | Method | Experimental Remarks | Sample | LOD | Ref. |
| cisplatin carboplatin oxaliplatin | HPLC/UV-Vis | post-column derivatization with sodium bisulfite | plasma, urine | 20–60 nM | [61] |
| cisplatin carboplatin oxaliplatin | HPLC UV-Vis | Reaction with DDTC | plasma and urine | 20–60 nM | [61] |
| cisplatin | HPLC UV-Vis | Reaction with DDTC | ultrafiltrate plasma | 0.025 μg/mL | [62] |
| oxaliplatin | HPLC-ICP-MS | Samples collected and stored at different temperatures | urine | 0.05 µg L−1 | [63] |
| carboplatin | LC-ICP-QMS LC-ESI-TOFMS | 194Pt enriched carboplatin for species specific isotope dilution analysis | urine | 1 ng g−1 15 ng g−1 | [64] |
| cisplatin carboplatin | AAS | Samples collected and stored at −4 °C | urine | 0.004 mg L−1 | [66] |
| cisplatin carboplatin oxaliplatin | CLC/ICP-MS | Rapid two-dimensional separation | human serum | <2.4 ng mL−1 | [67]. |
| total Pt | ICP-MS | Microwave digestion of the samples | rat urine, plasma and tissues | 5 ppb | [68] |
| cisplatin carboplatin | ICP-AES | Laboratory-prepared samples | urine | 0.1 mg L−1 | [69] |
| oxaliplatin | ICP-MS | Possible short-term storage of the samples, for 14 days at −4 °C, −24 °C, and −80 °C. Storage at −80 °C allows stability up to 5 months | ultrafiltrate plasma urine peritoneal fluid | 1.76 ng mL−1 0.39 ng mL−1 0.20 ng mL−1 | [70] |
| cisplatin | CV | nano-porous glassy carbon electrode (npGCE) modified with graphene quantum dots (GQDs) functionalized with thionine groups | blood serum and urine samples | 90 nM | [76] |
| cisplatin | adsorptive transfer stripping and pulse voltammetry | drop electrode with metallothionine protein | human serum | 0.5 μM | [73] |
| cisplatin | LC–MS/MS | - | rat plasma and urine | 1 ng mL−1 | [65] |
| cisplatin | differential pulse voltammetry | functionalization of screen printed electrodes with carbon nanotubes | human serum | 4.6 μmol L−1 | [77] |
| cisplatin | ICP-MS | determination of total platinum in urine samples taken at −20 °C after collection | urine | 0.005µg L−1 | [71] |
| Environmental Samples | |||||
| Compound | Method | Experimental Remarks | Sample | LOD | Ref. |
| cisplatin carboplatin oxaliplatin | ICP-MS | Evaluated the total platinum and the fraction in solution and adsorbed on activated sludge | HWW | 10 ng L−1 | [48] |
| total Pt | ICP-MS | Sampling for several days from two different hospital drains one after the oncological ward | HWW | 15 ng L−1 | [54] |
| total Pt | ICP-MS | Determination of total platinum from WWTP and HWW effluents | WWTP HWW | 1.0 ng L−1 | [55] |
| total Pt | HPLC-ICP-MS | Determination for the influent and effluent of a pilot MBR for several months | HWW | 10.0 ng L−1 | [79] |
| total Pt | ICP-MS | Samples taken from the influent and effluent of a WWTP and from the effluent of a hospital, both located in Gran Canaria island. Samples stored at −4 °C and then extracted. | WWTP HWW | 0.74 ng L−1 a | [81] |
| cisplatin, carboplatin, oxaliplatin, PtCl42− PtCl2 | FIA-ED | electrochemical detector consisting in a glassy carbon electrode as a working electrode | Ponavka river | - | [83] |
| total Pt | ICP-OES | Samples taken from several hospitals of the city of Qom | HWW | 1 μg L−1 | [84] |
| cisplatin | HPLC-ICP-MS | pentafluorophenylpropyl-functionalized silica gel column | HWW | 0.09 μg L−1 | [86] |
| carboplatin | 0.10 μg L−1 | ||||
| oxaliplatin | 0.15 μg L−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roque-Diaz, Y.; Sanadar, M.; Han, D.; López-Mesas, M.; Valiente, M.; Tolazzi, M.; Melchior, A.; Veclani, D. The Dark Side of Platinum Based Cytostatic Drugs: From Detection to Removal. Processes 2021, 9, 1873. https://doi.org/10.3390/pr9111873
Roque-Diaz Y, Sanadar M, Han D, López-Mesas M, Valiente M, Tolazzi M, Melchior A, Veclani D. The Dark Side of Platinum Based Cytostatic Drugs: From Detection to Removal. Processes. 2021; 9(11):1873. https://doi.org/10.3390/pr9111873
Chicago/Turabian StyleRoque-Diaz, Yessica, Martina Sanadar, Dong Han, Montserrat López-Mesas, Manuel Valiente, Marilena Tolazzi, Andrea Melchior, and Daniele Veclani. 2021. "The Dark Side of Platinum Based Cytostatic Drugs: From Detection to Removal" Processes 9, no. 11: 1873. https://doi.org/10.3390/pr9111873
APA StyleRoque-Diaz, Y., Sanadar, M., Han, D., López-Mesas, M., Valiente, M., Tolazzi, M., Melchior, A., & Veclani, D. (2021). The Dark Side of Platinum Based Cytostatic Drugs: From Detection to Removal. Processes, 9(11), 1873. https://doi.org/10.3390/pr9111873










