Isolation and Analytical Method Validation for Phytocomponents of Aqueous Leaf Extracts from Vaccinium bracteatum Thunb. in Korea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Extract
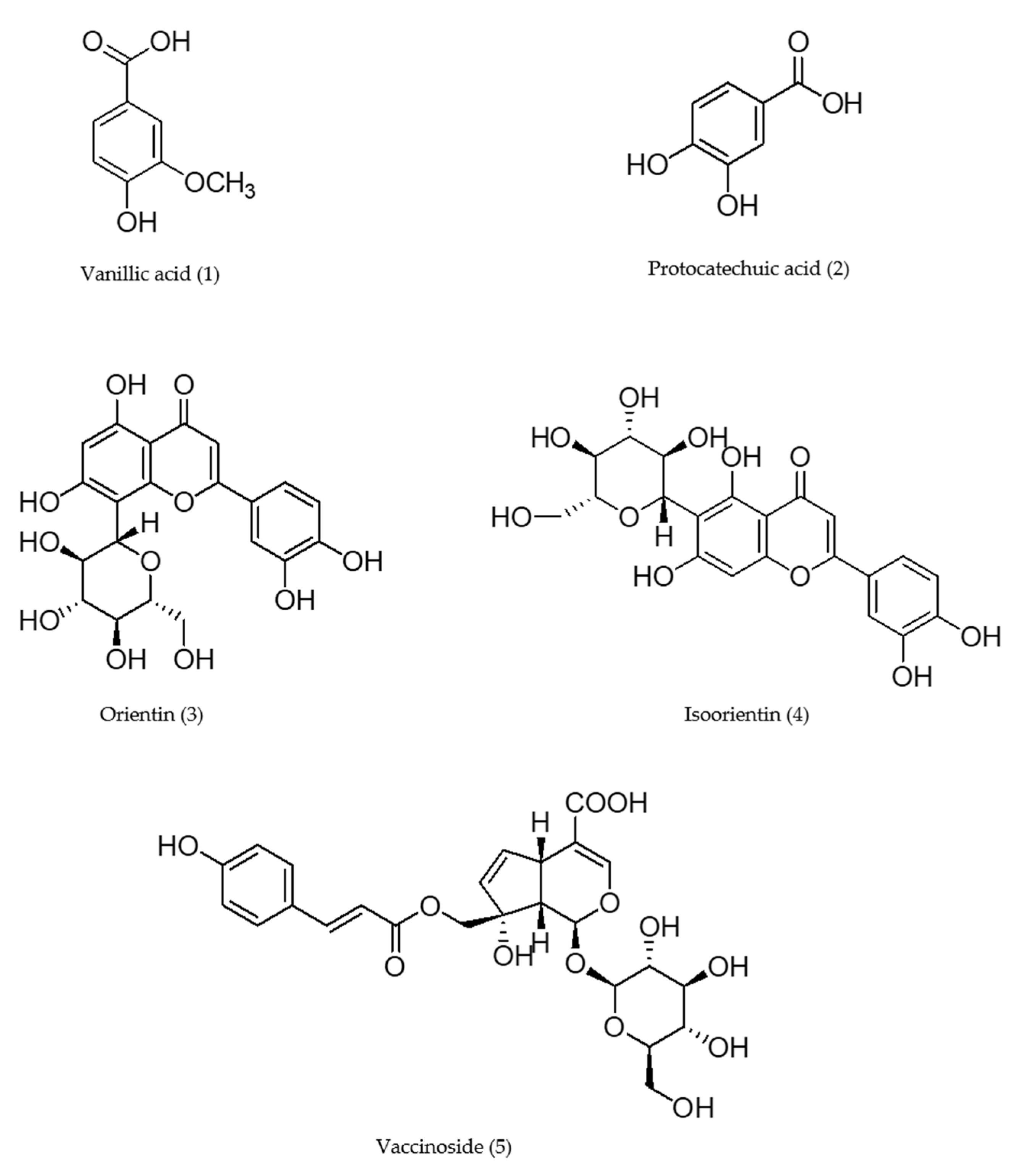
2.2. Isolation and Identification
2.3. Preparation of Extract and Standard Solution
2.4. Instrumentation and Analytical Method
2.5. Method Validation
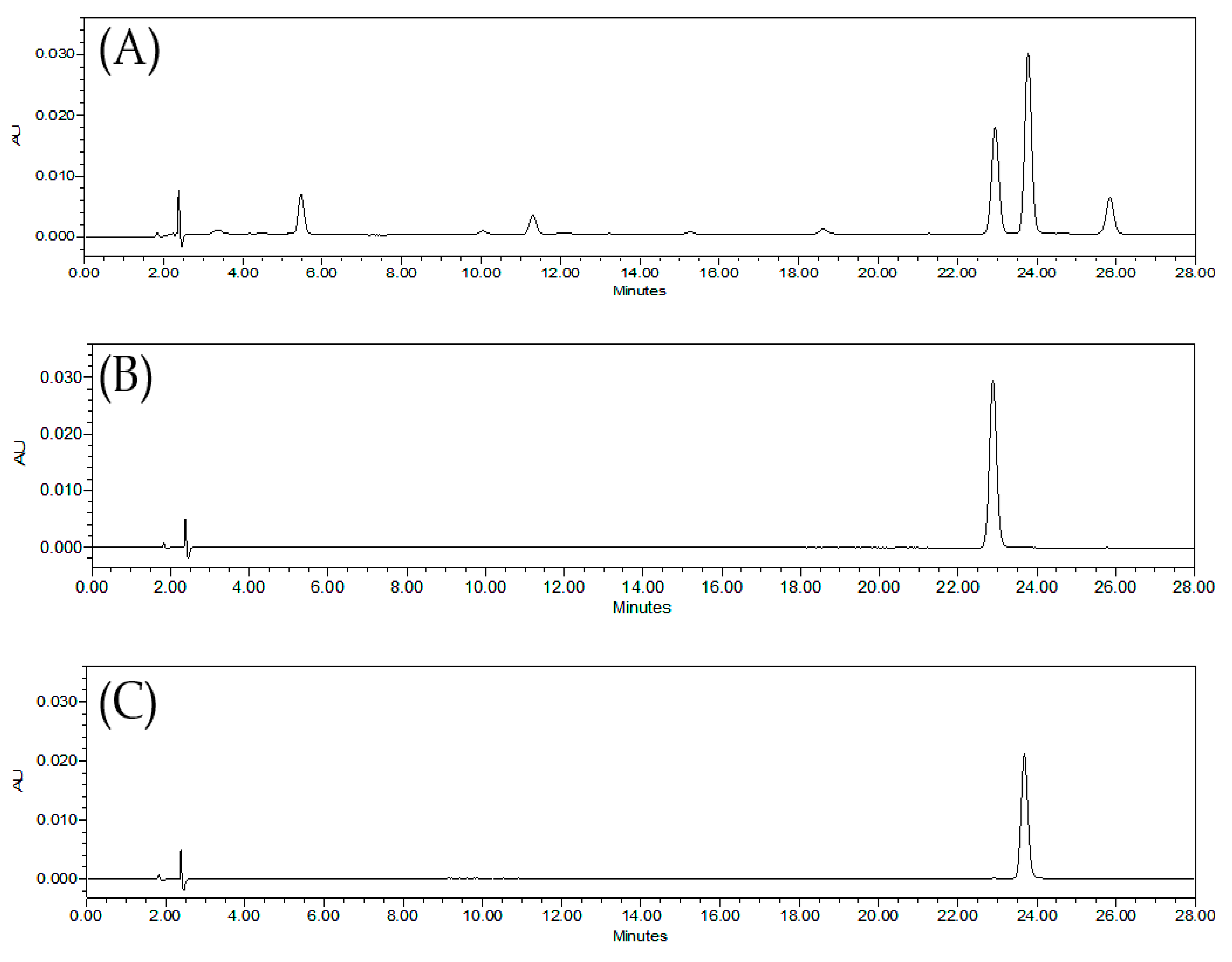
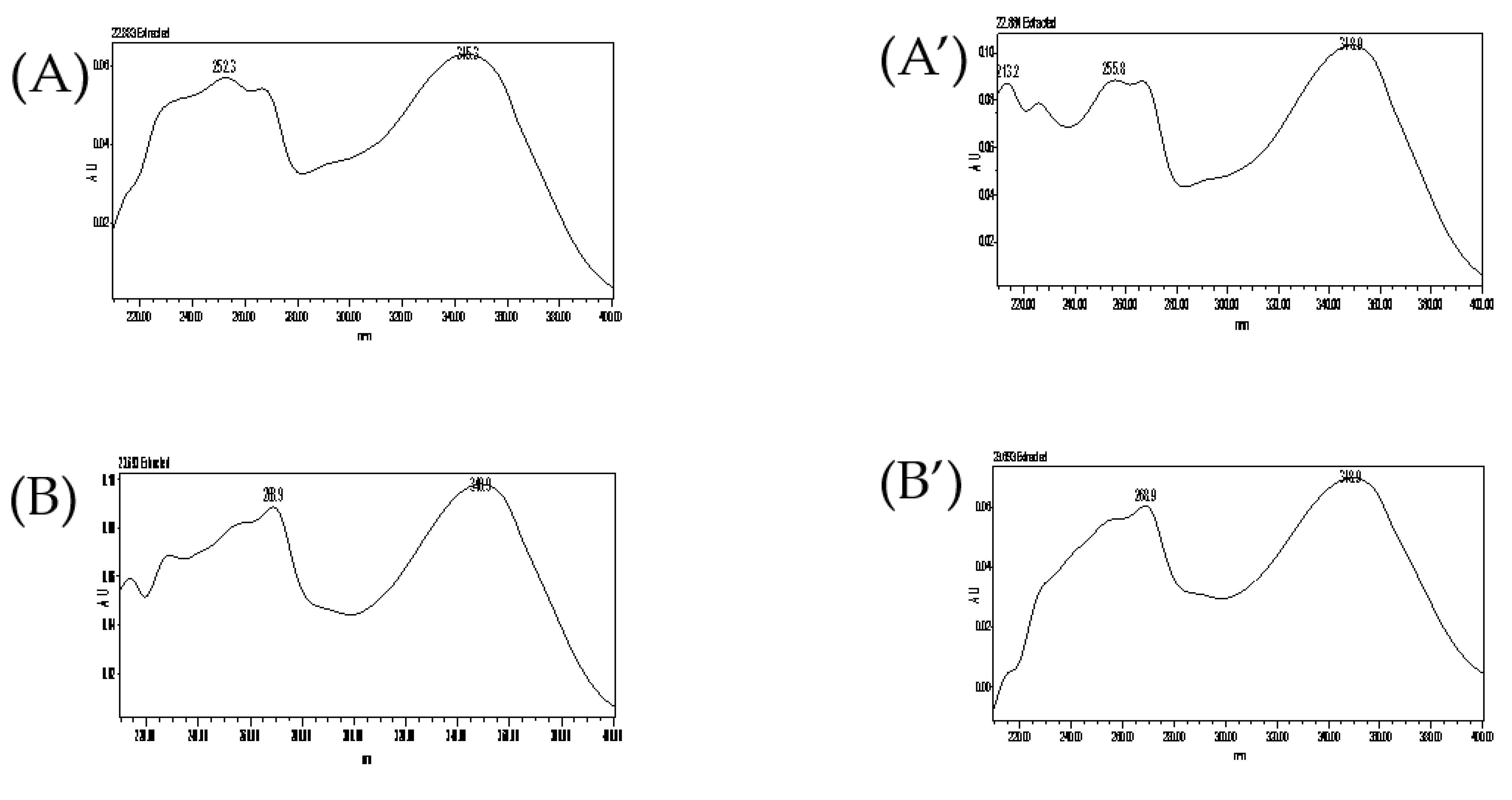
2.5.1. Specificity
2.5.2. Linearity and Range
2.5.3. LOD and LOQ Calculation
2.5.4. Precision
2.5.5. Recovery
3. Results and Discussion
3.1. Optimization of HPLC–PDA Condition
3.2. Method Validation
3.2.1. Specificity
3.2.2. Linearity, Range, LOD, and LOQ
3.2.3. Precision
3.2.4. Recovery
3.2.5. Quantitative Analysis of the Two Compounds in VB Extract
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, J.T. The Sauras of Korean Resources Plants II; II-heung: Seoul, Korea, 1989; pp. 48–49. [Google Scholar]
- Kim, T.J. Korean Resources Plants III; Seoul National University: Seoul, Korea, 1996; p. 230. [Google Scholar]
- Wang, L.; Zhang, Y.; Xu, M.; Wang, Y.; Cheng, S.; Liebrecht, A.; Qian, H.; Zhang, H.; Qi, X. Anti-diabetic activity of Vaccinium bracteatum Thunb. leaves polysaccharide in STZ-induced diabetic mice. Int. J. Biol. Macromol. 2013, 61, 317–321. [Google Scholar] [PubMed]
- Kim, M.; Park, J.; Song, K.; Kim, H.G.; Koh, J.-S.; Boo, Y.C. Screening of plant extracts for human tyrosinase inhibiting effects. Int. J. Cosmet. Sci. 2012, 34, 202–208. [Google Scholar] [CrossRef]
- Kwon, S.H.; Ma1, S.X.; Ko1, Y.H.; Seo1, J.Y.; Lee, B.R.; Lee, T.H.; Kim, S.Y.; Lee, S.Y.; Jang, C.G. Vaccinium bracteatum Thunb. Exerts Anti-Inflammatory Activity by Inhibiting NF-κB Activation in BV-2 Microglial Cells. Biomol. Ther. 2016, 24, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Landa, P.; Skalova, L.; Bousova, I.; Kutil1, Z.; Langhansova, L.; Lou, J.D.; Vanek, T. In vitro anti-proliferative and anti-inflammatory activity of leaf and fruit extracts from Vaccinium bracteatum Thunb. Pak. J. Pharm. Sci. 2014, 27, 103–106. [Google Scholar]
- Zhang, J.; Chu, C.J.; Li, X.L.; Yao, S.; Yan, B.; Ren, H.L.; Xu, N.Y.; Liang, Z.T.; Zhao, Z.Z. Isolation and identification of antioxidant compounds in Vaccinium bracteatumThunb. by UHPLC-Q-TOF LC/MS and their kidney damage protection. J. Funct. Foods 2014, 11, 62–67. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.N.; Yao, H.; Zhang, H. Phenolic Composition and Radical Scavenging Capacity of Vaccinium Bracteatum Thunb. Leaves. Int. J. Food. Proper 2011, 14, 721–725. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, L.; Liu, Y.; Shi, L.; Wan, S.; Wang, L. Evaluation of antimicrobial activity of water-soluble flavonoids extract from Vaccinium bracteatum Thunb. leaves. Food. Sci. Biotechnol. 2019, 28, 1853–1859. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.T.; Zhang, H.Y.; Yao, H.Y.; Zhang, H. Effect of Vaccinium bracteatum Thunb. leaves extract on blood glucose and plasma lipid levels in streptozotocin-induced diabetic mice. J. Ethnopharmacol. 2010, 130, 465–469. [Google Scholar] [CrossRef]
- Oh, D.R.; Kim, Y.; Jo, A.; Choi, E.J.; Oh, K.N.; Kim, J.; Kang, H.; Kim, Y.R.; Choi, C. Sedative and hypnotic effects of Vaccinium bracteatum Thunb. through the regulation of serotonegic and GABAA-ergic systems: Involvement of 5-HT1A receptor agonistic activity. Biomed. Pharmacother. 2019, 1, 109. [Google Scholar] [CrossRef]
- Oh, D.R.; Kim, Y.; Im, S.; Oh, K.N.; Shin, J.; Jeong, C.; Kim, Y.; Choi, E. Vaccinium bracteatum Improves Spatial Learning and Memory by Regulating N-methyl-D-aspartate Receptors and Tau Phosphorylation in Chronic Restraint Stress-Induced Memory Impaired Mice. Am. J. Chin. Med. 2021, 49, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.R.; Kim, M.J.; Choi, E.J.; Kim, Y.; Lee, H.S.; Bae, D.; Choi, C. Protective Effects of p-Coumaric Acid Isolated from Vaccinium bracteatum Thunb. Leaf Extract on Corticosterone-Induced Neurotoxicity in SH-SY5Y Cells and Primary Rat Cortical Neurons. Processes 2021, 9, 869. [Google Scholar] [CrossRef]
- Wang, L.; Yao, H.Y.; Chen, Z.X. Isolation, Purification and Identification of Flavonoids in Vaccinium bracteatum Thunb. Leaves. Sch. Food. Sci. Technol. 2007, 27, 121–123. [Google Scholar]
- Ren, Y.; Chang, Q.K.; Chunping, T.; Yang, Y. Divaccinosides A-D, four rare iridoid glucosidic truxillate esters from the leaves of Vaccinium bracteatum. Tetragedron. Lett. 2017, 58, 2385–2388. [Google Scholar] [CrossRef]
- Ren, Y.M.; Ke, C.Q.; Mandi, A.; Kurtan, T.; Tang, C.; Yao, S.; Ye, Y. Two new lignan-iridoid glucoside diesters from the leaves of Vaccinium bracteatum and their relative and absolute configuration determination by DFT NMR and TDDFT-ECD calculation. Tetrahedron 2017, 73, 3213–3219. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.X.; Shi, J.J.; Zhang, J.G.; Li, L.; Jiang, L.; Wei, Z.J. Thermal, emulsifying and rheological properties of polysaccharides sequentially extracted from Vaccinium bracteatum Thunb leaves. Int. J. Biol. Macromol. 2016, 93, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Amita, P.; Shalini, T. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J. Pharm. Phytochem. 2014, 2, 115–119. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Lysiuk, L.; Hudz, N. Differential Spectrophotometry: Application for Quantification of Flavonoids in Herbal Drugs and Nutraceuticals. Int. J. Trends Food. Nutr. 2017, 1, 102. [Google Scholar]
- Alzand, K.I.; Mohamed, M.A. Flavonoids: Chemistry, Biochemistry and Antioxidant activity. J. Pharm. Res 2012, 5, 4013–4020. [Google Scholar]
- Brodowska, K.M. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 2017, 7, 108–123. [Google Scholar]
- Jain, P.; Kharya, M.; Gajbhiye, A.; Sara, U.V.S.; Sharma, V.K. Flavonoids as nutraceuticals. A review. Herba Polonica 2010, 56, 105–117. [Google Scholar]
- Lee, W.H.; Bae, J.S. Antithrombotic and antiplatelet activities of orientin in vitro and in vivo. J. Func. Food 2015, 17, 388–398. [Google Scholar] [CrossRef]
- International Council for Harmonisation (ICH). Validation of Analytical Procedures: Text and Methodology Q2(R1). In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, 1 November 2005. [Google Scholar]
- Chandran, S.; Singh, R.S.P. Comparison of various international guidelines for analytical method validation. Pharmazie 2007, 62, 4–14. [Google Scholar]
- Oh, D.R.; Yoo, J.S.; Kim, Y.; Kang, H.; Lee, H.; Lim, S.J.; Choi, E.; Jung, M.A.; Bae, D.H.; Oh, G.N.; et al. Vaccinium bracteatum Leaf Extract Reverses Chronic Restraint Stress-Induced Depression-Like Behavior in Mice: Regulation of Hypothalamic-Pituitary-Adrenal Axis, Serotonin Turnover Systems, and ERK/Akt Phosphorylation. Front. Pharmacol. 2018, 9, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.W.; Kim, K.H.; Lee, I.K.; Choi, S.U.; Ryu, S.Y.; Lee, K.R. Phytochemical Constituents of Bistorta manshuriensis. Nat. Prod. Sci. 2009, 15, 234–240. [Google Scholar]
- Kikuzaki, H.; Kawai, Y.; Nakatani, N. 1,1-Diphenyl-2-picrylhydrazyl Radical-scavenging Active Compounds from Greater Cardamon (Amomum subulatum Roxb.). J. Nutr. Sci. Vitaninol. 2001, 47, 167–171. [Google Scholar] [CrossRef]
- An, L.J.; Guan, S.; Shi, G.F.; Bao, Y.M.; Duan, Y.L.; Jiang, B. Protocatechuic acid from Alpinia oxyphylla against MPP+-induced neurotoxicity in PC12 cells. Food Chem. Toxicol. 2006, 44, 436–443. [Google Scholar] [CrossRef]
- Wang, R.-F.; Yang, X.-W.; Ma, C.-M.; Liu, H.-Y.; Shang, M.-Y.; Zhang, Q.-Y.; Cai, S.-Q.; Park, J.-H. Trollioside, a new compound from the flowers of Trollius chinensis. J. Asian Nat. Prod. Res. 2004, 6, 139–144. [Google Scholar] [CrossRef] [PubMed]

| Standard | Concentration (µg/mL) | Detected Concentration (µg/mL) | RSD (%) | Precision (%) |
|---|---|---|---|---|
| Orientin | 1.00 | 1.02 | 0.302 | 101.70 |
| 5.00 | 4.93 | 0.392 | 98.55 | |
| 10.00 | 10.07 | 0.112 | 100.66 | |
| 25.00 | 24.99 | 0.212 | 99.97 | |
| 50.00 | 50.00 | 0.045 | 100.00 | |
| Isoorientin | 1.00 | 0.99 | 0.047 | 98.75 |
| 5.00 | 4.93 | 0.072 | 98.69 | |
| 10.00 | 10.08 | 0.029 | 100.80 | |
| 25.00 | 25.02 | 0.045 | 100.06 | |
| 50.00 | 49.98 | 0.007 | 99.97 |
| Standard | Concentration (µg/mL) | Detected Concentration (µg/mL) | RSD (%) | Precision (%) |
|---|---|---|---|---|
| Orientin | 1.00 | 1.01 | 0.628 | 101.18 |
| 5.00 | 4.94 | 0.370 | 98.70 | |
| 10.00 | 10.07 | 0.103 | 100.69 | |
| 25.00 | 24.99 | 0.194 | 99.95 | |
| 50.00 | 50.00 | 0.043 | 100.00 | |
| Isoorientin | 1.00 | 0.99 | 0.058 | 98.77 |
| 5.00 | 4.94 | 0.035 | 98.70 | |
| 10.00 | 10.08 | 0.076 | 100.83 | |
| 25.00 | 25.02 | 0.056 | 100.07 | |
| 50.00 | 49.99 | 0.006 | 99.97 |
| Standard | Spiked Concentration (µg/mL) | Detected Concentration (µg/mL) | Recovery (%) | SD | RSD (%) |
|---|---|---|---|---|---|
| Orientin | 1.00 | 30.54 | 101.57 | 0.003 | 0.389 |
| 5.00 | 34.44 | 98.37 | 0.022 | 0.453 | |
| 10.00 | 39.35 | 98.30 | 0.082 | 0.837 | |
| Isoorientin | 1.00 | 36.05 | 98.43 | 0.011 | 1.168 |
| 5.00 | 40.06 | 97.81 | 0.008 | 0.172 | |
| 10.00 | 45.42 | 102.14 | 0.095 | 0.933 |
| Standard | Number | Retention Time | Content Average (mg/g) | RSD (%) |
|---|---|---|---|---|
| Orientin | 1 | 22.884 | 2.90 | 0.006 |
| 2 | 22.883 | |||
| 3 | 22.880 | |||
| Isoorientin | 1 | 23.693 | 3.45 | 0.484 |
| 2 | 23.691 | |||
| 3 | 23.689 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-G.; Ko, H.; Choi, E.-J.; Oh, D.-R.; Bae, D.; Choi, C. Isolation and Analytical Method Validation for Phytocomponents of Aqueous Leaf Extracts from Vaccinium bracteatum Thunb. in Korea. Processes 2021, 9, 1868. https://doi.org/10.3390/pr9111868
Lee S-G, Ko H, Choi E-J, Oh D-R, Bae D, Choi C. Isolation and Analytical Method Validation for Phytocomponents of Aqueous Leaf Extracts from Vaccinium bracteatum Thunb. in Korea. Processes. 2021; 9(11):1868. https://doi.org/10.3390/pr9111868
Chicago/Turabian StyleLee, Seul-Gi, Haeju Ko, Eun-Jin Choi, Dool-Ri Oh, Donghyuck Bae, and Chulyung Choi. 2021. "Isolation and Analytical Method Validation for Phytocomponents of Aqueous Leaf Extracts from Vaccinium bracteatum Thunb. in Korea" Processes 9, no. 11: 1868. https://doi.org/10.3390/pr9111868
APA StyleLee, S.-G., Ko, H., Choi, E.-J., Oh, D.-R., Bae, D., & Choi, C. (2021). Isolation and Analytical Method Validation for Phytocomponents of Aqueous Leaf Extracts from Vaccinium bracteatum Thunb. in Korea. Processes, 9(11), 1868. https://doi.org/10.3390/pr9111868






