Evaluation of Pb, Mg, Al, Zn, and Cu as Electrode Materials in the Electrocoagulation of Microalgae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Cultivation
2.2. Electrode Preparation
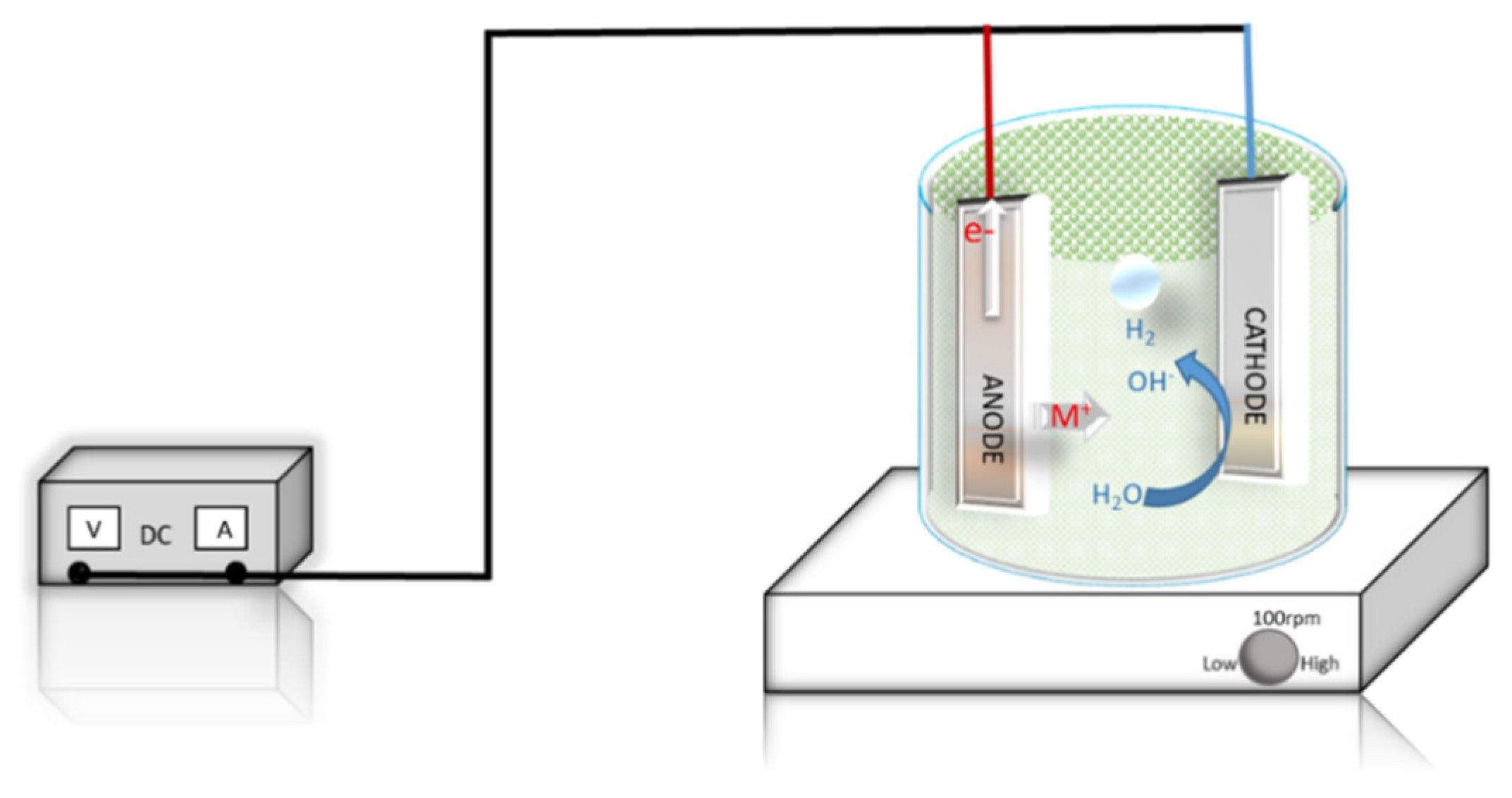
2.3. Electrocoagulation Experiments
2.4. Analytical Methods
3. Results and Discussion
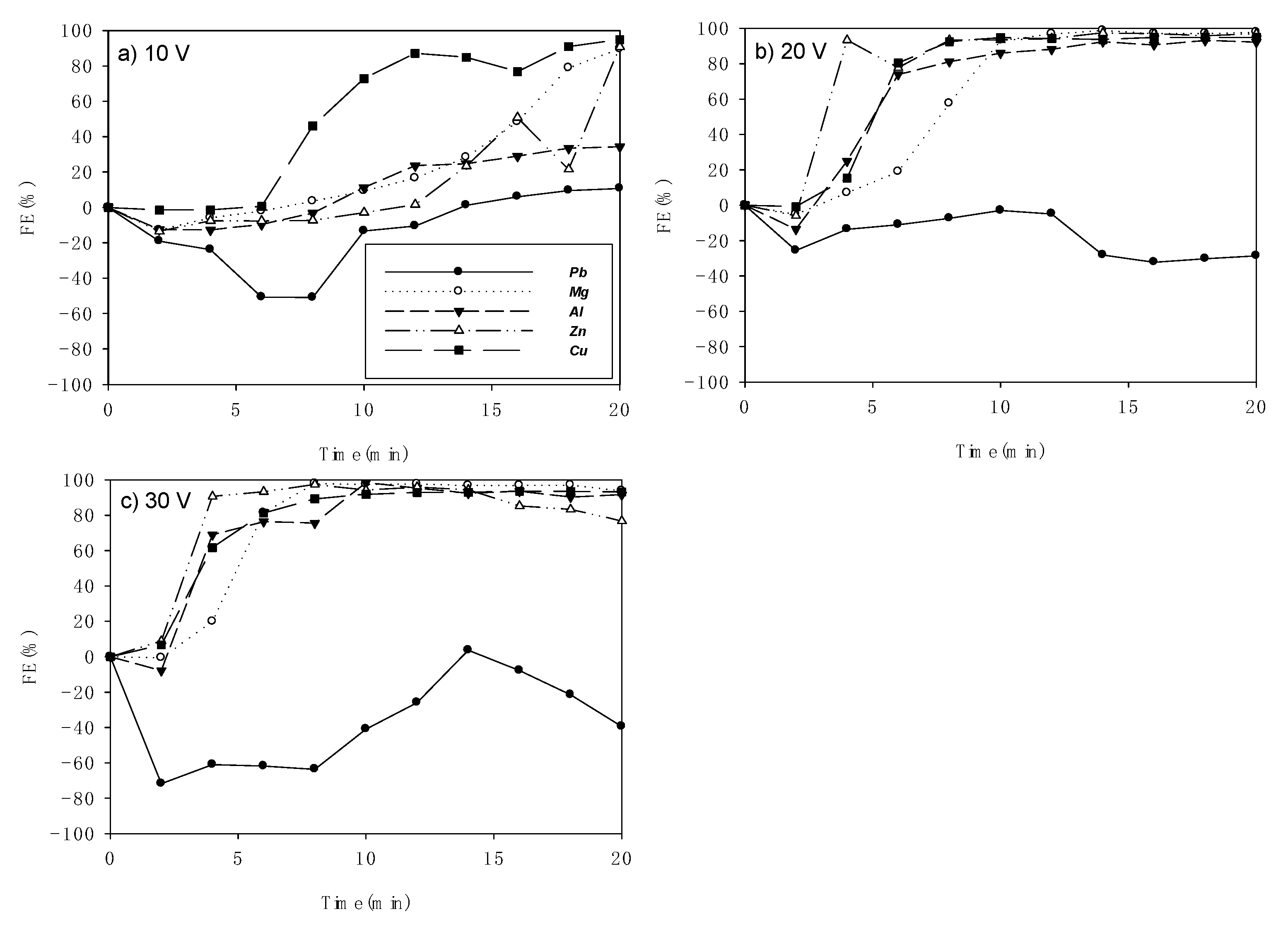
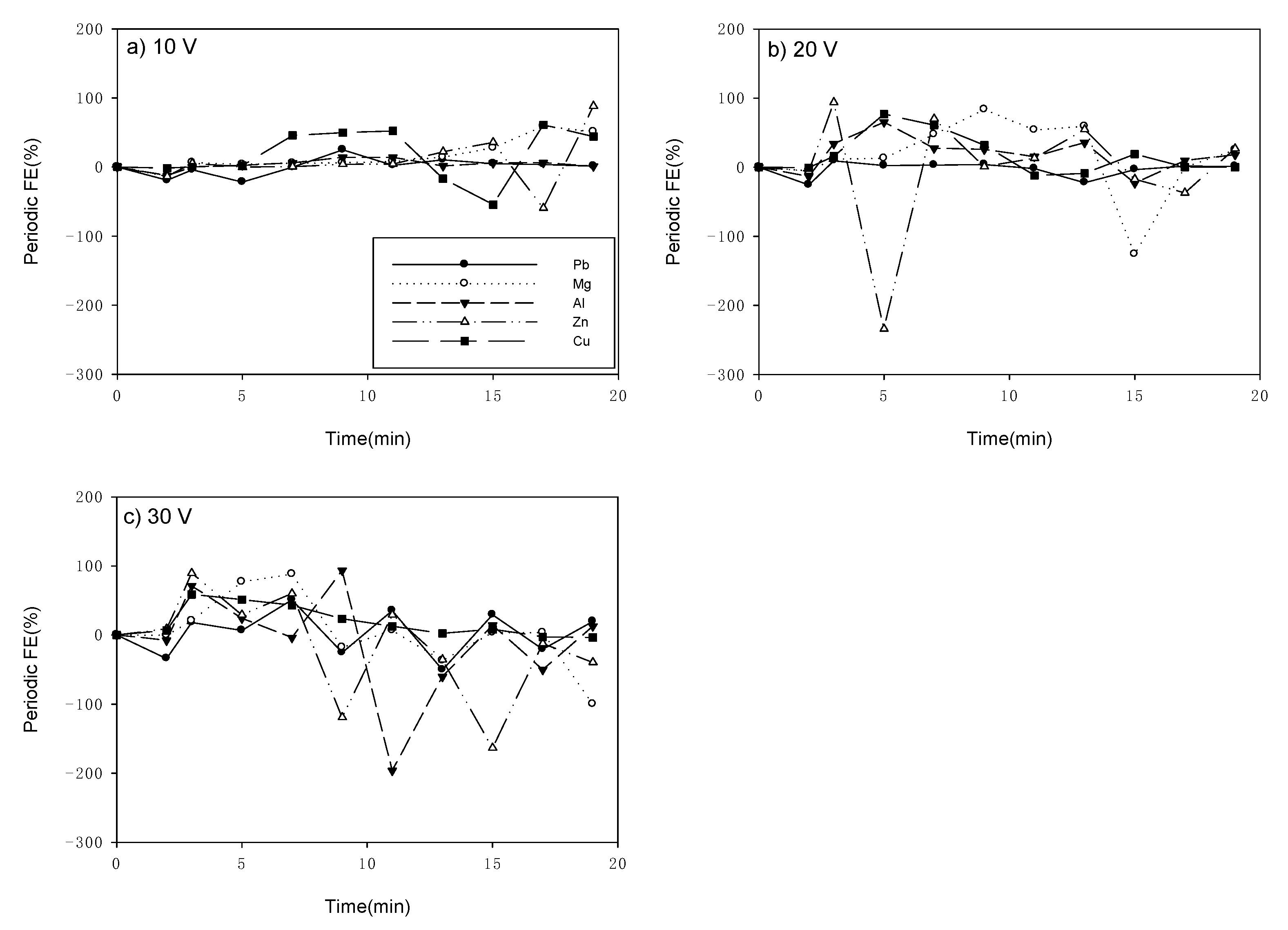
3.1. Flocculation Efficiency (FE)
3.2. Temperature
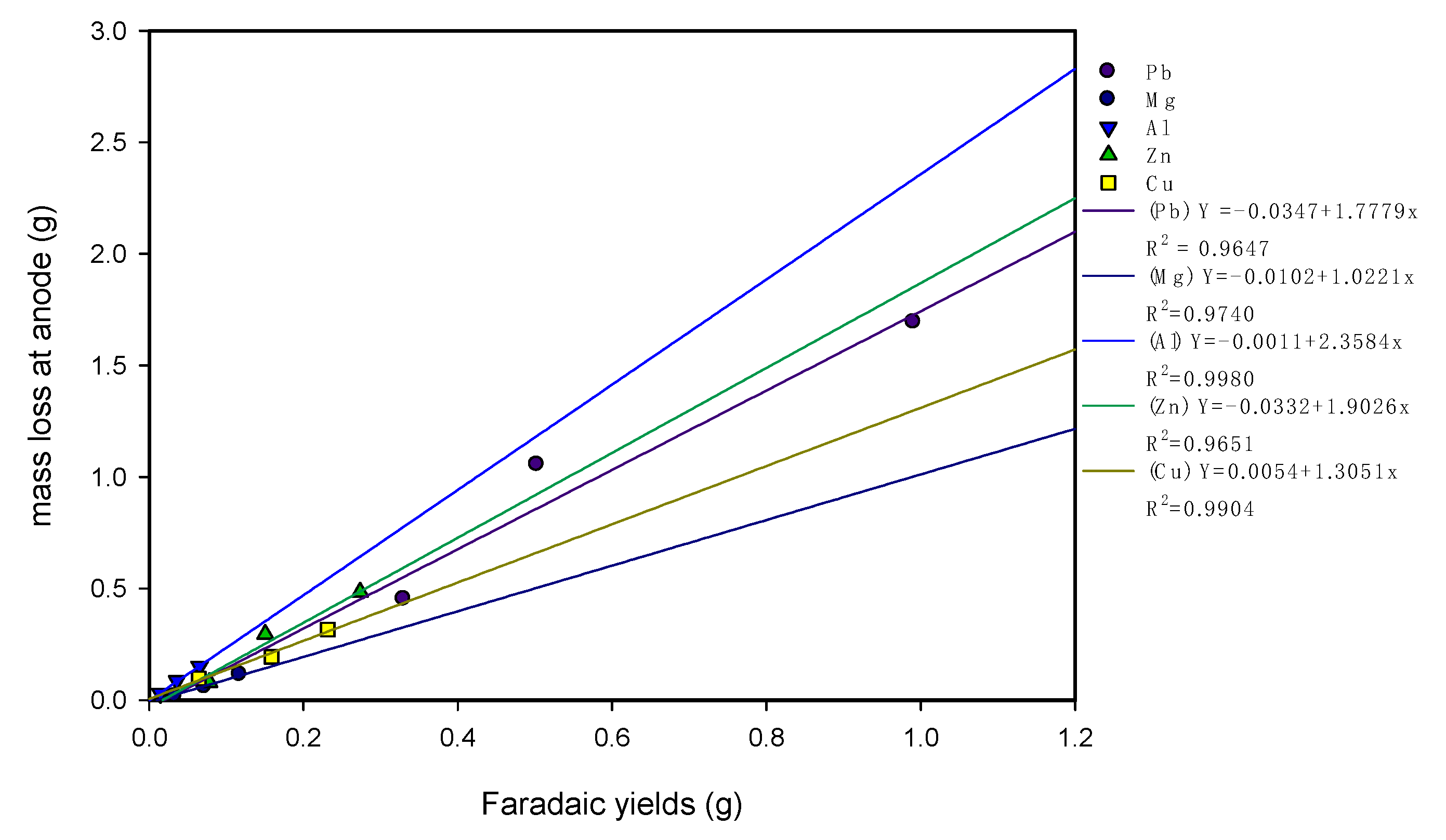
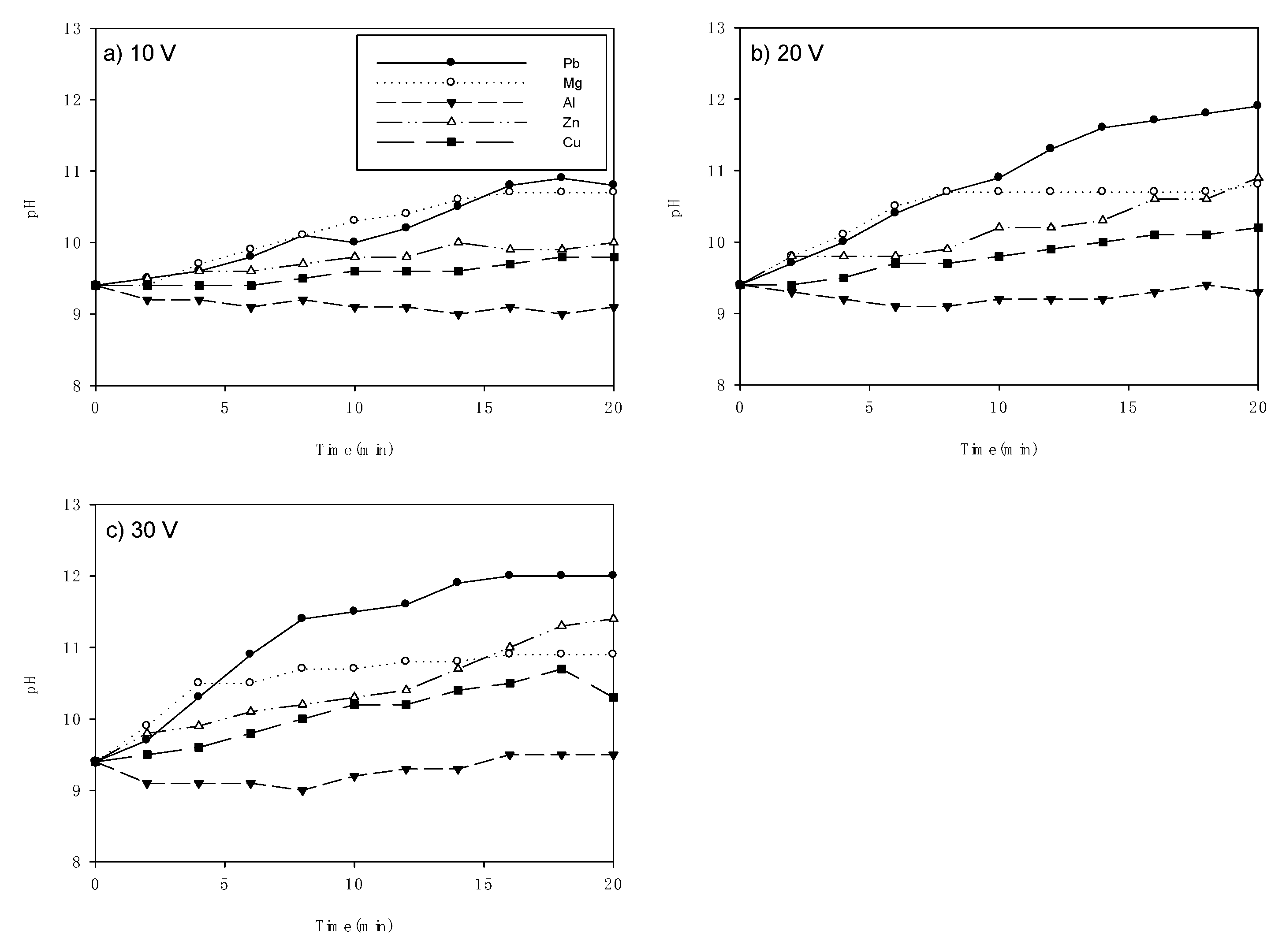
3.3. Consumption of Electrode Material and pH Variations
3.4. Metallic Content in the Effluents after Electrocoagulation
3.5. Practical Implications of This Study
4. Conclusions
- In addition to Al electrodes, Pb, Mg, Zn, and Cu electrodes can be used as electrode materials in the harvesting of microalgae.
- Higher voltages increase the flocculation efficiency of microalgae as well as the pH of the supernatants, temperature, and consumption of the electrode materials.
- Other variables such as the rate of metal passivation, corrosion, bubble nucleation, and the electrode material’s affinity for water contribute to the determination of a material’s viability as an electrode in the harvesting of microalgae.
- Pb is not an appropriate electrode material to employ in the harvesting of microalgae when the clarity of the supernatant (effluent) is crucial.
- All the electrode materials showed metallic concentrations in their effluents.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Chong, W.T.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgae Biofuels as an Alternative to Fossil Fuel for Power Generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Vale, M.A.; Ferreira, A.; Pires, J.C.M.; Gonçalves, A.L. CO2 capture using microalgae. Adv. Carbon Capture 2020, 381–405. [Google Scholar]
- Sun, H.; Zhao, W.; Mao, X.; Li, Y.; Wu, T.; Chen, F. High-Value Biomass from Microalgae Production Platforms: Strategies and Progress Based on Carbon Metabolism and Energy Conversion. Biotechnol. Biofuels 2018, 11, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randrianarison, G.; Ashraf, M.A. Microalgae: A Potential Plant for Energy Production. Geol. Ecol. Landsc. 2017, 1, 104–120. [Google Scholar] [CrossRef]
- Kucmanová, A.; Gerulová, K. Microalgae Harvesting: A Review. J. Environ. Manag. 2019, 27, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Chlorella Vulgaris—Microbewiki. Available online: https://microbewiki.kenyon.edu/index.php/Chlorella_vulgaris (accessed on 22 June 2021).
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A Comprehensive Review of Electrocoagulation for Water Treatment: Potentials and Challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a Low-Cost Method for Harvesting Microalgae for Bulk Biomass Production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Crittenden, J.C.; Trussell, R.R.; Hand, W.D.; Howe, K.J.; Tchobanoglous, G. MWH’s Water Treatment Principles and Design, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; p. 554. [Google Scholar]
- Visigalli, S.; Barberis, M.G.; Turolla, A.; Canziani, R.; Berden Zrimec, M.; Reinhardt, R.; Ficara, E. Electrocoagulation–Flotation (ECF) for Microalgae Harvesting—A Review. Sep. Purif Technol. 2021, 271, 118684. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—Science and Applications. J. Hazard. Mater. 2001, 84, 29–41. [Google Scholar] [CrossRef]
- Bartley, M.L.; Boeing, W.J.; Dungan, B.N.; Holguin, F.O.; Schaub, T. PH Effects on Growth and Lipid Accumulation of the Biofuel Microalgae Nannochloropsis Salina and Invading Organisms. J. Appl. Phycol. 2013, 26, 1431–1437. [Google Scholar] [CrossRef]
- Li, W.K.W. Temperature Adaptation in Phytoplankton: Cellular and Photosynthetic Characteristics. Prim. Product. Sea 1980, 259–279. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of Photosynthesis by Heat Stress: The Activation State of Rubisco as a Limiting Factor in Photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ras, M.; Steyer, J.P.; Bernard, O. Temperature Effect on Microalgae: A Crucial Factor for Outdoor Production. Rev. Environ. Sci. Bio/Technol. 2013, 12, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Jamshaid, M.; Khan, A.A.; Ahmed, K.; Saleem, M. Heavy Metal in Drinking Water Its Effect on Human Health and Its Treatment Techniques—A Review. Int. J. Biosci. 2018, 12, 223–240. [Google Scholar] [CrossRef]
- Baierle, F.; John, D.K.; Souza, M.P.; Bjerk, T.R.; Moraes, M.S.A.; Hoeltz, M.; Rohlfes, A.L.B.; Camargo, M.E.; Corbellini, V.A.; Schneider, R.C.S. Biomass from Microalgae Separation by Electroflotation with Iron and Aluminum Spiral Electrodes. Chem. Eng. J. 2015, 267, 274–281. [Google Scholar] [CrossRef]
- Fayad, N.; Yehya, T.; Audonnet, F.; Vial, C. Harvesting of Microalgae Chlorella Vulgaris Using Electro-Coagulation-Flocculation in the Batch Mode. Algal Res. 2017, 25, 1–11. [Google Scholar] [CrossRef]
- Bleeke, F.; Quante, G.; Winckelmann, D.; Klöck, G. Effect of Voltage and Electrode Material on Electroflocculation of Scenedesmus Acuminatus. Bioresour. Bioprocess. 2015, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, A.; Zerrouki, D.; Djafer, L.; Ayral, A. Hydrogen Recovery from the Photovoltaic Electroflocculation-Flotation Process for Harvesting Chlorella Pyrenoidosa Microalgae. Int. J. Hydrog. Energy 2017, 42, 19591–19596. [Google Scholar] [CrossRef]
- Sanchez-Galvis, E.M.; Cardenas-Gutierrez, I.Y.; Contreras-Ropero, J.E.; García-Martínez, J.B.; Barajas-Solano, A.F.; Zuorro, A. An Innovative Low-Cost Equipment for Electro-Concentration of Microalgal Biomass. Appl. Sci. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Can, O.T.; Bayramoglu, M.; Kobya, M. Decolorization of Reactive Dye Solutions by Electrocoagulation Using Aluminum Electrodes. Ind. Eng. Chem. Res. 2003, 42, 3391–3396. [Google Scholar] [CrossRef]
- Water Quality Standards for Drinking Water: Busan Water Authority. Available online: https://www.busan.go.kr/water_en/InstitutionalStandard (accessed on 10 September 2021).
- Standard Reduction Potential Charts for Chemistry. Available online: https://www.flinnsci.com/standard-reduction-potential-charts/ (accessed on 2 June 2021).
- Rayleigh, L. On the Pressure Developed in a Liquid during the Collapse of a Spherical Cavity. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1917, 34, 94–98. [Google Scholar] [CrossRef]
- Naje, A.S.; Chelliapan, S.; Zakaria, Z.; Ajeel, M.A.; Alaba, P.A. A Review of Electrocoagulation Technology for the Treatment of Textile Wastewater. Rev. Chem. Eng. 2017, 33, 263–292. [Google Scholar] [CrossRef]
- Sakuma, G.; Fukunaka, Y.; Matsushima, H. Nucleation and Growth of Electrolytic Gas Bubbles under Microgravity. International J. Hydrog. Energy 2014, 39, 7638–7645. [Google Scholar] [CrossRef]
- Volanschi, A.; Nijman, J.G.H.; Olthuis, W.; Bergveld, P. Microcavity Electrodes Used as Single-Nucleation Site Electrodes for the Electrolysis of Water. Sens. Mater. 1997, 9, 223–240. [Google Scholar]
- Fekete, É.; Lengyel, B.; Cserfalvi, T.; Pajkossy, T. Electrochemical Dissolution of Aluminium in Electrocoagulation Experiments. J. Solid State Electrochem. 2016, 20, 3107–3114. [Google Scholar] [CrossRef] [Green Version]
- Vandamme, D.; Pontes, S.C.V.; Goiris, K.; Foubert, I.; Pinoy, L.J.J.; Muylaert, K. Evaluation of Electro-Coagulation–Flocculation for Harvesting Marine and Freshwater Microalgae. Biotechnol. Bioeng. 2011, 108, 2320–2329. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Q.; Pang, Q.; Pan, X.; Chika, A.O.; Wang, L.; Shi, J.; Jia, L.; Chen, C.; Gao, Y. Facile Sand Enhanced Electro-Flocculation for Cost-Efficient Harvesting of Dunaliella Salina. Bioresour. Technol. 2015, 187, 326–330. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, L.; Cheng, W.; Chen, L.; Wang, J.; Wang, H.; Zhang, W.; Liu, T. Electro-Flotation of Chlorella Sp. Assisted with Flocculation by Chitosan. Algal Res. 2016, 18, 7–14. [Google Scholar] [CrossRef]
- Castellanos-Estupinan, M.; Sanchez-Galvis, M.; Garcia-Martinez, J.B.; Barajas-Ferreira, C.; Zuorro, A.; Barajas-Solano, A.F. Design of an Electroflotation System for the Concentration and Harvesting of Freshwater Microalgae. Chem Eng Trans 2018, 64, 1–6. [Google Scholar] [CrossRef]
- Resistivity and Conductivity—Temperature Coefficients for Common Materials. Available online: https://www.engineeringtoolbox.com/resistivity-conductivity-d_418.html (accessed on 28 May 2021).
- Gabrielli, C.; Huet, F.; Keddam, M.; Macias, A.; Sahar, A. Potential Drops Due to an Attached Bubble on a Gas-Evolving Electrode. J. Appl. Electrochem. 1989, 19, 617–629. [Google Scholar] [CrossRef]
- Licona Buelvas, W.; Cecilia, K.; Ávila, P.; Realpe Jiménez, Á. Temperature as a Factor Determining on Water Electrolysis. Int. J. Eng. Trends Technol. 2014, 7, 1. [Google Scholar]
- Cañizares, P.; Carmona, M.; Lobato, J.; Martínez, F.; Rodrigo, M.A. Electrodissolution of Aluminum Electrodes in Electrocoagulation Processes. Ind. Eng. Chem. Res. 2005, 44, 4178–4185. [Google Scholar] [CrossRef]
- Picard, T.; Cathalifaud-Feuillade, G.; Mazet, M.; Vandensteendam, C. Cathodic Dissolution in the Electrocoagulation Process Using Aluminium Electrodes. J. Environ. Monit. 2000, 2, 77–80. [Google Scholar] [CrossRef] [PubMed]



| Voltage (V) | Pb | Mg | Al | Zn | Cu | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| FEmax (%) | Time (min) | FEmax (%) | Time (min) | FEmax (%) | Time (min) | FEmax (%) | Time (min) | FEmax (%) | Time (min) | |
| 10 | 10.7 | 20 | 89.6 | 20 | 66.4 | 20 | 90.6 | 20 | 94.9 | 20 |
| 20 | −3.0 | 10 | 98.7 | 14 | 93.0 | 18 | 97.4 | 14 | 94.9 | 16 |
| 30 | 3.7 | 14 | 97.8 | 8 | 98.4 | 10 | 97.3 | 8 | 93.6 | 16 |
| Voltage (V) | FE Values (%) | ||||
|---|---|---|---|---|---|
| Pb | Mg | Al | Zn | Cu | |
| 10 | 10.7 | 89.6 | 66.4 | 90.6 | 94.9 |
| 20 | −28.6 | 97.8 | 92.1 | 96.9 | 94.9 |
| 30 | −39.4 | 93.7 | 91.5 | 76.7 | 93.2 |
| Species | Material | Time (min) | FE (%) | Reference |
|---|---|---|---|---|
| Phaeodactylum tricornutum | Al/Fe (anode) and IrO2/TiO2(cathode) | 30 | 94 | [30] |
| Chlorella vulgaris | Al/Fe (anode) and IrO2/TiO2(cathode) | 25 | 98 | [30] |
| Scenedesmus acuminatus | Al, Zn, Mg, Fe, Cu and Brass | 7.3–71.1 | 90 | [19] |
| Dunaliella salina | Al coupled with sand | 4.5 | 98 | [31] |
| Chlorella sp. 0217 | Graphite mixed with chitosan | 4 | 96 | [32] |
| Chlorella pyrenoidosa | Al, Zn, Fe, Cu and carbon | 5 | 71–96 | [20] |
| Chlorella vulgaris | Al and Cu | 25 | 95 and 85 | [33] |
| Electrode Material | Weight Loss/Gain at Anodes (%) | Weight Loss/Gain at Cathodes (%) | ||||
|---|---|---|---|---|---|---|
| 10 V | 20 V | 30 V | 10 V | 20 V | 30 V | |
| Pb | −1.004 | −2.345 | −3.855 | −0.002 | −0.004 | −0.001 |
| Mg | −0.506 | −1.708 | −3.435 | −0.010 | −0.011 | +0.003 |
| Al | −1.851 | −5.215 | −9.070 | −0.244 | −0.651 | −1.195 |
| Zn | −1.810 | −6.759 | −11.043 | +0.143 | +0.011 | −0.120 |
| Cu | −1.873 | −3.733 | −6.069 | −0.005 | −0.006 | −0.004 |
| Electrode Material | 10 V | 20 V | 30 V |
|---|---|---|---|
| Pb | 0.513 | 3.462 | 43.835 |
| Mg | 20.535 | 7.015 | - |
| Al | 1.336 | 4.563 | 9.087 |
| Zn | 0.089 | 0.079 | 0.081 |
| Cu | 0.434 | 0.363 | 0.252 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phiri, J.T.; Pak, H.; We, J.; Oh, S. Evaluation of Pb, Mg, Al, Zn, and Cu as Electrode Materials in the Electrocoagulation of Microalgae. Processes 2021, 9, 1769. https://doi.org/10.3390/pr9101769
Phiri JT, Pak H, We J, Oh S. Evaluation of Pb, Mg, Al, Zn, and Cu as Electrode Materials in the Electrocoagulation of Microalgae. Processes. 2021; 9(10):1769. https://doi.org/10.3390/pr9101769
Chicago/Turabian StylePhiri, Jesse T., Hun Pak, Junhyung We, and Sanghwa Oh. 2021. "Evaluation of Pb, Mg, Al, Zn, and Cu as Electrode Materials in the Electrocoagulation of Microalgae" Processes 9, no. 10: 1769. https://doi.org/10.3390/pr9101769
APA StylePhiri, J. T., Pak, H., We, J., & Oh, S. (2021). Evaluation of Pb, Mg, Al, Zn, and Cu as Electrode Materials in the Electrocoagulation of Microalgae. Processes, 9(10), 1769. https://doi.org/10.3390/pr9101769






