Photocatalytic Degradation of Rhodamine B Dye in Aqueous Suspension by ZnO and M-ZnO (M = La3+, Ce3+, Pr3+ and Nd3+) Nanoparticles in the Presence of UV/H2O2
Abstract
1. Introduction
2. Materials and Experimental Procedures
2.1. Materials and Chemical Reagents
2.2. Chemical Synthesis of ZnO and M-ZnO (M = La3+, Ce3+, Pr3+ and Nd3+) Nanoparticles
2.3. Characterization Methods
2.4. Photocatalytic Degradation of Rhodamine B
3. Results and Discussion
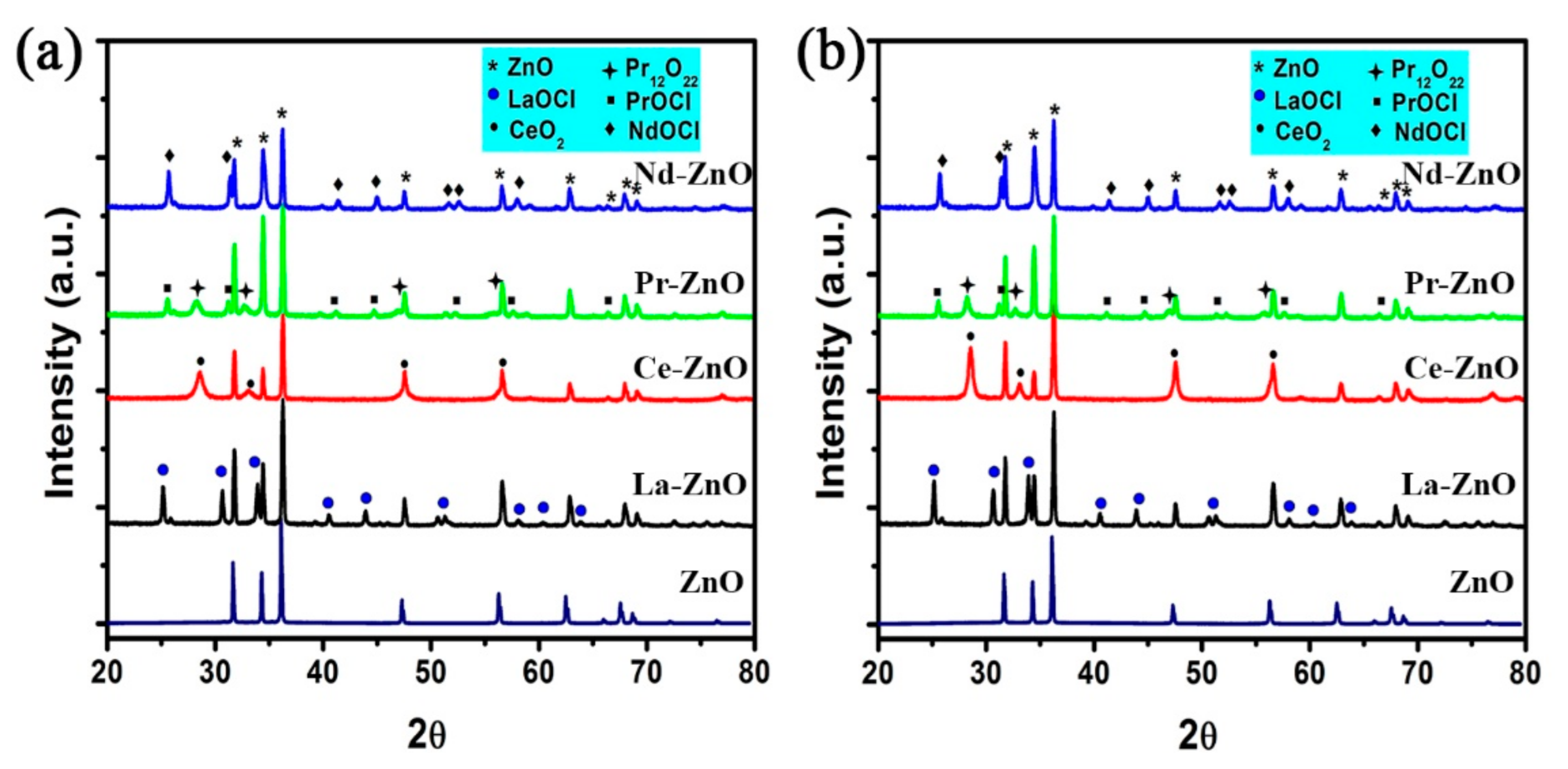
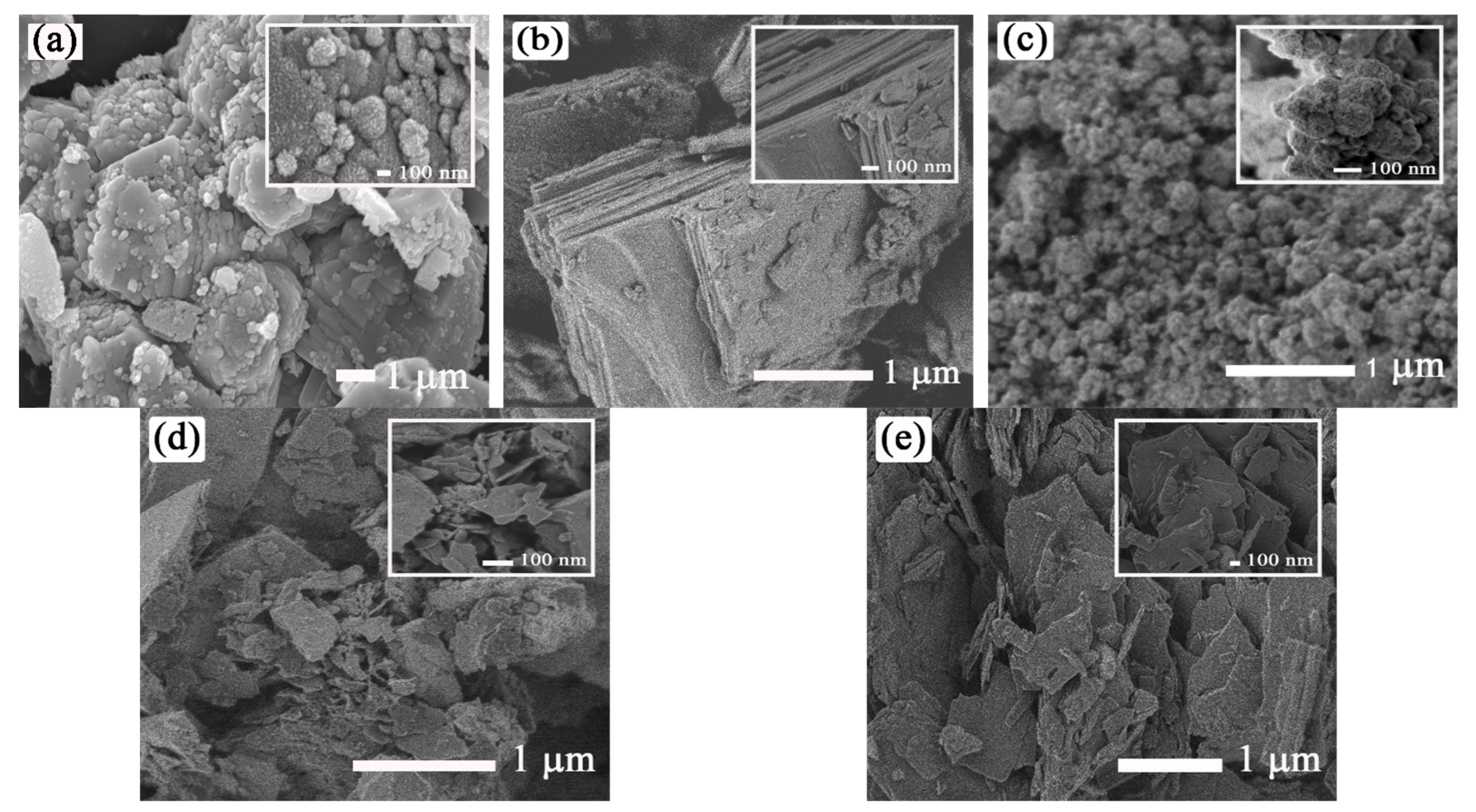
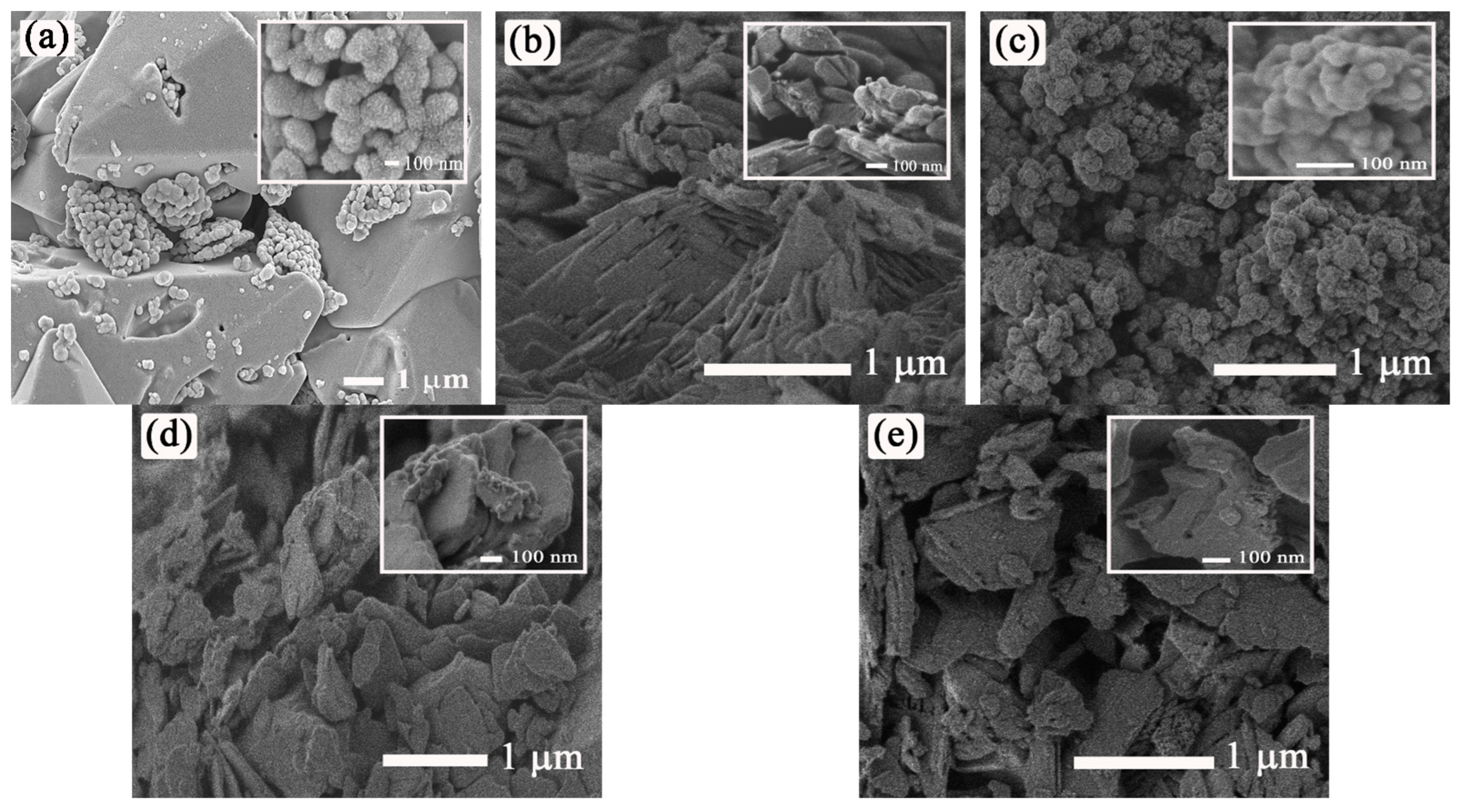
3.1. Characterization of ZnO and M-ZnO (M = La3+, Ce3+, Pr3+ and Nd3+) Photocatalysts
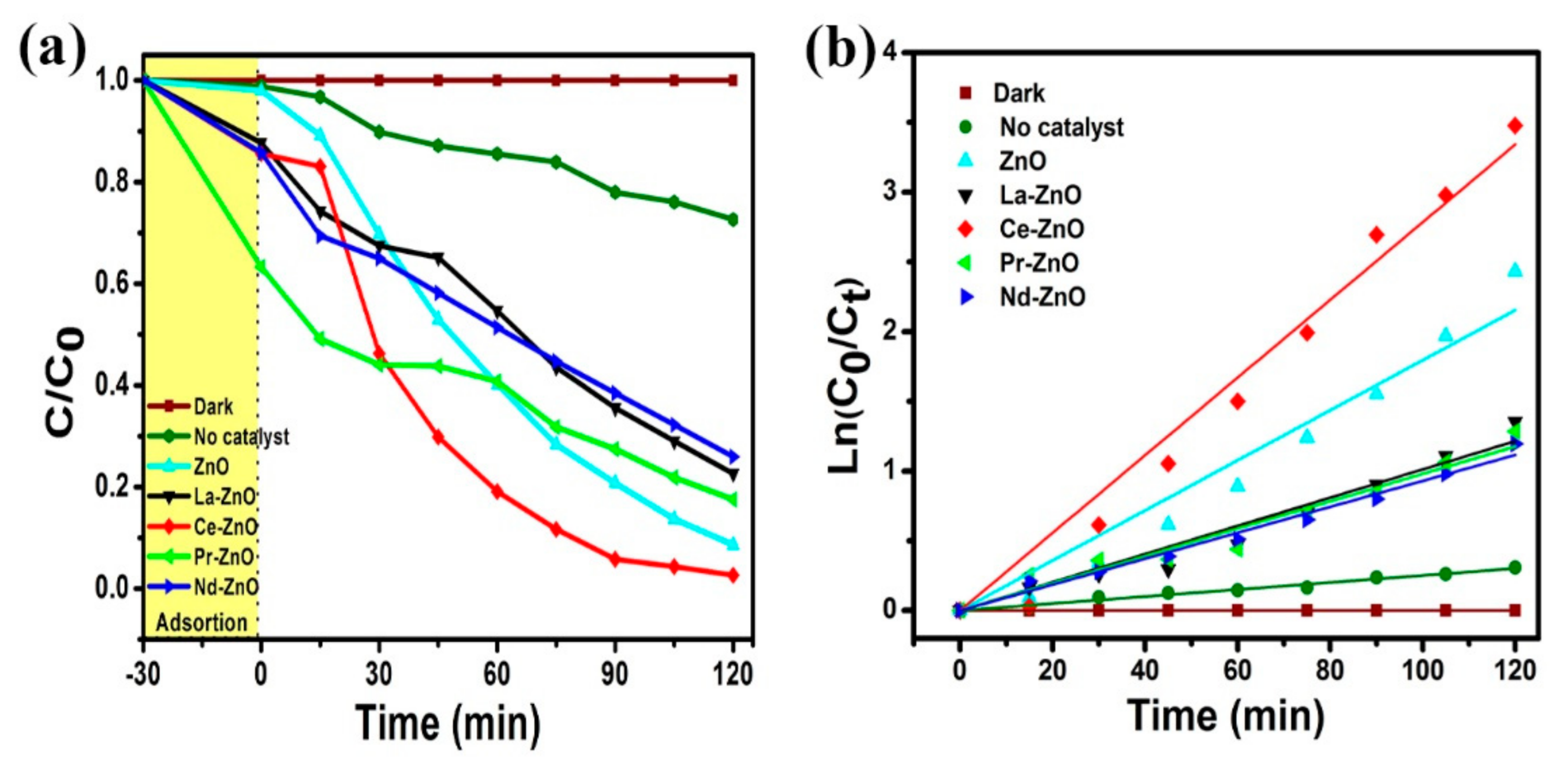
3.2. Photocatalytic Activity of ZnO and M-ZnO (M = La3+, Ce3+, Pr3+ and Nd3+) Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugimoto, T. Metal Oxides; CRC Press Fr. Gr.: Boca Raton, FL, USA, 2006; pp. 133–194. [Google Scholar] [CrossRef]
- Solozhenko, V.L.; Kurakevych, O.O.; Sokolov, P.S.; Baranov, A.N. Kinetics of the wurtzite-to-rock-salt phase transformation in Zno at high pressure. J. Phys. Chem. A 2011, 115, 4354–4358. [Google Scholar] [CrossRef]
- Marotti, R.E.; Guerra, D.N.; Bello, C.; Machado, G.; Dalchiele, E.A. Bandgap energy tuning of electrochemically grown ZnO thin films by thickness and electrodeposition potential. Sol. Energy Mater. Sol. Cells 2004, 82, 85–103. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Soares, N.D.F.F.; Coimbra, J.S.d.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Yu, L.; Hao, W.; Li, Z.; Ren, X.; Yang, H.; Ma, H. Synthesis of ZnO core/shell hollow microspheres to boost light harvesting capability in quantum dots-sensitized solar cell. Chem. Phys. Lett. 2021, 764, 138283. [Google Scholar] [CrossRef]
- Pradeep, N.; Venkatraman, U.; Grace, A.N. Flexible hydrogen gas sensor: ZnO decorated SnO2 nanowire on over head projector (OHP) sheet substrate. Mater. Today Proc. 2021, 45, 4073–4080. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, S.; Xue, X.; Lu, G. Unique approach to change ZnO appearance and its properties during its growth: Current. Mater. Lett. 2015, 139, 311–313. [Google Scholar] [CrossRef]
- Goulart, L.A.; Santos, G.O.S.; Eguiluz, K.I.B.; Salazar-Banda, G.R.; Lanza, M.R.V.; Saez, C.; Rodrigo, M.A. Towards a higher photostability of ZnO photo-electrocatalysts in the degradation of organics by using MMO substrates. Chemosphere 2021, 271, 129451. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Dutta, B.K. Photocatalytic degradation of model textile dyes in wastewater using ZnO as semiconductor catalyst. J. Hazard. Mater. 2004, 112, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Abdeldayem, H.M.; Al-Shihry, S.S.; Hassan, S.A. Selective methanol oxidation to hydrogen over Ag/ZnO catalysts doped with mono- and Bi-rare earth oxides. Ind. Eng. Chem. Res. 2014, 53, 19884–19894. [Google Scholar] [CrossRef]
- He, L.; Tong, Z.; Wang, Z.; Chen, M.; Huang, N.; Zhang, W. Effects of calcination temperature and heating rate on the photocatalytic properties of ZnO prepared by pyrolysis. J. Colloid Interface Sci. 2018, 509, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, H.; Deng, R.; Wang, X.; Sun, D.; Zheng, G. Engineering white light-emitting Eu-doped ZnO urchins by biopolymer-assisted hydrothermal method. Appl. Phys. Lett. 2006, 89, 123125. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphoslogies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- Shirdel, B.; Behnajady, M.A. Sol-gel synthesis of Ba-doped ZnO nanoparticles with enhanced photocatalytic activity in degrading Rhodamine B under UV-A irradiation. Optik (Stuttg) 2017, 147, 143–150. [Google Scholar] [CrossRef]
- Kalaiezhily, R.K.; Saravanan, G.; Asvini, V.; Vijayan, N.; Ravichandran, K. Tuning violet to green emission in luminomagnetic Dy, Er co-doped ZnO nanoparticles. Ceram. Int. 2018, 44, 19560–19569. [Google Scholar] [CrossRef]
- Wu, C.; Shen, L.; Yu, H.; Zhang, Y.C.; Huang, Q. Solvothermal synthesis of Cu-doped ZnO nanowires with visible light-driven photocatalytic activity. Mater. Lett. 2012, 74, 236–238. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Z.; Zhu, Y.; Jiang, L. Sonochemical synthesis and photocatalytic property of zinc oxide nanoparticles doped with magnesium(II). Mater. Res. Bull. 2011, 46, 1638–1641. [Google Scholar] [CrossRef]
- El Mir, L. Luminescence properties of calcium doped zinc oxide nanoparticles. J. Lumin. 2017, 186, 98–102. [Google Scholar] [CrossRef]
- Geburt, S.; Stichtenoth, D.; Müller, S.; Dewald, W.; Ronning, C.; Wang, J.; Jiao, Y.; Rao, Y.Y.; Hark, S.K.; Li, Q. Rare earth doped zinc oxide nanowires. J. Nanosci. Nanotechnol. 2008, 8, 244–251. [Google Scholar] [CrossRef]
- Röder, R.; Geburt, S.; Zapf, M.; Franke, D.; Lorke, M.; Frauenheim, T.; da Rosa, A.L.; Ronning, C. Transition Metal and Rare Earth Element Doped Zinc Oxide Nanowires for Optoelectronics. Phys. Status Solidi Basic Res. 2019, 256, 1800604. [Google Scholar] [CrossRef]
- Ji, S.; Yin, L.; Liu, G.; Zhang, L.; Ye, C. Synthesis of rare earth ions-doped ZnO nanostructures with efficient host-guest energy transfer. J. Phys. Chem. C 2009, 113, 16439–16444. [Google Scholar] [CrossRef]
- Manikandan, A.; Manikandan, E.; Meenatchi, B.; Vadivel, S.; Jaganathan, S.K.; Ladchumananandasivam, R.; Henini, M.; Maaza, M.; Aanand, J.S. Rare earth element (REE) lanthanum doped zinc oxide (La: ZnO) nanomaterials: Synthesis structural optical and antibacterial studies. J. Alloys Compd. 2017, 723, 1155–1161. [Google Scholar] [CrossRef]
- Zeng, X.; Yuan, J.; Zhang, L. Synthesis and photoluminescent properties of rare earth doped ZnO hierarchical microspheres. J. Phys. Chem. C 2008, 112, 3503–3508. [Google Scholar] [CrossRef]
- Khatamian, M.; Khandar, A.A.; Divband, B.; Haghighi, M.; Ebrahimiasl, S. Heterogeneous photocatalytic degradation of 4-nitrophenol in aqueous suspension by Ln (La3+, Nd3+ or Sm3+) doped ZnO nanoparticles. J. Mol. Catal. A Chem. 2012, 365, 120–127. [Google Scholar] [CrossRef]
- Ishizumi, A.; Kanemitsu, Y. Structural and luminescence properties of Eu-doped ZnO nanorods fabricated by a microemulsion method. Appl. Phys. Lett. 2005, 86, 253106. [Google Scholar] [CrossRef]
- Suwanboon, S.; Amornpitoksuk, P.; Sukolrat, A.; Muensit, N. Optical and photocatalytic properties of La-doped ZnO nanoparticles prepared via precipitation and mechanical milling method. Ceram. Int. 2013, 39, 2811–2819. [Google Scholar] [CrossRef]
- Deng, S.H.; Duan, M.Y.; Xu, M.; He, L. Effect of la doping on the electronic structure and optical properties of ZnO. Phys. B Condens. Matter 2011, 406, 2314–2318. [Google Scholar] [CrossRef]
- Kumar, V.; Ntwaeaborwa, O.M.; Soga, T.; Dutta, V.; Swart, H.C. Rare Earth Doped Zinc Oxide Nanophosphor Powder: A Future Material for Solid State Lighting and Solar Cells. ACS Photonics 2017, 4, 2613–2637. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, W.; Li, R.; Zhu, H.; Chen, X. ions doped ZnO nanocrystals. Opt. Express 2009, 17, 838–842. [Google Scholar]
- Lupan, O.; Chow, L.; Chai, G.; Roldan, B.; Naitabdi, A.; Schulte, A.; Heinrich, H. Nanofabrication and characterization of ZnO nanorod arrays and branched microrods by aqueous solution route and rapid thermal processing. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2007, 145, 57–66. [Google Scholar] [CrossRef]
- Kannadasan, N.; Shanmugam, N.; Cholan, S.; Sathishkumar, K.; Viruthagiri, G.; Poonguzhali, R. The effect of Ce4+ incorporation on structural, morphological and photocatalytic characters of ZnO nanoparticles. Mater. Charact. 2014, 97, 37–46. [Google Scholar] [CrossRef]
- Martynova, N.A.; Umedov, T.; Lepnev, L.S.; Komarova, M.Y.; Grigorieva, A.V. Electrochemicaly formed ZnO and Au/ZnO opal films. SN Appl. Sci. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Nguyen, H.T.T.; Le, T.H.; Nguyen, L.T.H.; Nguyen, H.Q.; Pham, T.T.H.; Bui, N.D.; Tran, N.T.K.; Nguyen, D.T.C.; Van Lam, T.; et al. Enhanced photocatalytic activity of spherical Nd3+ substituted ZnFe2O4 nanoparticles. Materials 2021, 14, 2054. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Deng, X.; Feng, J.; Yu, B.; Zhu, H.; Li, X.; Zheng, X.; Bai, J.; Peng, Y. Nonhydrolytic colloidal synthesis of ligand-capped single-crystalline NdOCl nanocubes and their magnetic properties. J. Alloys Compd. 2015, 619, 681–685. [Google Scholar] [CrossRef]
- Li, X.; Deng, X.; Zhu, H.; Feng, J.; Peng, Y.; Bai, J.; Zheng, X.; Fan, H.; Wang, M.; Chen, H. Well-Defined Flowerlike NdOCl Nanostructures: Nonaqueous Sol-Gel Synthesis, Nanoscale Characterization and Their Magnetic and Photoluminescence Properties. Chem. Asian J. 2014, 9, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, S.; Zeljkovic, V.; Obradovic, N.; Labus, N. Investigation of sintering kinetics of ZnO by observing reduction of the specific surface area. Sci. Sinter. 2007, 39, 259–265. [Google Scholar] [CrossRef][Green Version]
- Sakhaei, Z.; Rezaei, M. Mechanochemical synthesis of ZnO.Al2O3 powders with various Zn/Al molar ratios and their applications in reverse water-gas shift reaction. Environ. Sci. Pollut. Res. 2021, 28, 13790–13799. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Fan, R.; Yu, J.; Li, L. Improving the efficiency of ZnO-based dye-sensitized solar cells by Pr and N co-doping. J. Mater. Chem. A 2013, 1, 12066–12073. [Google Scholar] [CrossRef]
- Xie, Y.; Yuan, C. Photocatalysis of neodymium ion modified TiO2 sol under visible light irradiation. Appl. Surf. Sci. 2004, 221, 17–24. [Google Scholar] [CrossRef]
- Lv, L.; Wang, T.; Li, S.; Su, Y.; Wang, X. Tuning the optical, electronic and luminescence properties of LaOCl:Eu3+ via structural and lattice strain modulation. CrystEngComm 2016, 18, 907–916. [Google Scholar] [CrossRef]
- Chen, H.I.; Chang, H.Y. Synthesis of nanocrystalline cerium oxide particles by the precipitation method. Ceram. Int. 2005, 31, 795–802. [Google Scholar] [CrossRef]
- Anandan, S.; Miyauchi, M. Ce-doped ZnO (CexZn1-xO) becomes an efficient visible-light-sensitive photocatalyst by co-catalyst (Cu2+) grafting. Phys. Chem. Chem. Phys. 2011, 13, 14937–14945. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.S.; Ahmed, E.; Ahmad, M. Facile synthesis of Pr-doped ZnO photocatalyst using sol–gel method and its visible light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2020, 31, 1084–1093. [Google Scholar] [CrossRef]
- Byrappa, K.; Subramani, A.K.; Ananda, S.; Lokanatha Rai, K.M.; Dinesh, R.; Yoshimura, M. Photocatalytic degradation of rhodamine B dye using hydrothermally synthesized ZnO. Bull. Mater. Sci. 2006, 29, 433–438. [Google Scholar] [CrossRef]
- AlHamedi, F.H.; Rauf, M.A.; Ashraf, S.S. Degradation studies of Rhodamine B in the presence of UV/H2O2. Desalination 2009, 239, 159–166. [Google Scholar] [CrossRef]
- Liang, C.; Liu, C.; Li, F.; Wu, F. The effect of Praseodymium on the adsorption and photocatalytic degradation of azo dye in aqueous Pr3+-TiO2 suspension. Chem. Eng. J. 2009, 147, 219–225. [Google Scholar] [CrossRef]
- Vaiano, V.; Matarangolo, M.; Sacco, O.; Sannino, D. Photocatalytic treatment of aqueous solutions at high dye concentration using praseodymium-doped ZnO catalysts. Appl. Catal. B Environ. 2017, 209, 621–630. [Google Scholar] [CrossRef]
- Rauf, M.A.; Bukallah, S.B.; Hamadi, A.; Sulaiman, A.; Hammadi, F. The effect of operational parameters on the photoinduced decoloration of dyes using a hybrid catalyst V2O5/TiO2. Chem. Eng. J. 2007, 129, 167–172. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Photocatalytic decolourisation and degradation of Reactive Orange 4 by TiO2-UV process. Dyes Pigments 2006, 68, 133–142. [Google Scholar] [CrossRef]






| Samples | Amount of Hexahydrate Nitrates (Grams) | ||||
|---|---|---|---|---|---|
| Zn2+ | La3+ | Ce3+ | Pr3+ | Nd3+ | |
| ZnO | 10.97 | - | - | - | - |
| La-ZnO | 6.84 | 3.49 | - | - | - |
| Ce-ZnO | 6.81 | - | 3.48 | - | - |
| Pr-ZnO | 6.78 | - | - | 3.48 | - |
| Nd-ZnO | 6.75 | - | - | - | 3.49 |
| Samples | Calcination Temperature (°C) | Diffraction Peak (101) (2θ) | FWHM (in Degrees) (β) | τ (nm) | Lattice Parameters (Å) | Volume (Å) | ||
|---|---|---|---|---|---|---|---|---|
| a = b | c | c/a | ||||||
| ZnO | 500 a | 36.26 a | ----- | 50 a | 3.250 a | 5.214 a | 1.6043 a | 47.70 a |
| 500 | 36.24 | 0.120 | 72 | 3.250 | 5.208 | 1.6025 | 47.64 | |
| 700 | 36.26 | 0.121 | 72 | 3.250 | 5.205 | 1.6016 | 47.61 | |
| La-ZnO | 500 | 36.26 | 0.204 | 43 | 3.250 | 5.205 | 1.6017 | 47.61 |
| 700 | 36.26 | 0.208 | 42 | 3.249 | 5.206 | 1.6022 | 47.59 | |
| Ce-ZnO | 500 | 36.27 | 0.197 | 44 | 3.249 | 5.204 | 1.6016 | 47.57 |
| 700 | 36.25 | 0.218 | 40 | 3.249 | 5.205 | 1.6019 | 47.58 | |
| Pr-ZnO | 500 | 36.27 | 0.205 | 43 | 3.249 | 5.204 | 1.6018 | 47.47 |
| 700 | 36.26 | 0.201 | 43 | 3.249 | 5.205 | 1.6020 | 47.58 | |
| Nd-ZnO | 500 | 36.24 | 0.212 | 41 | 3.250 | 5.202 | 1.6003 | 47.58 |
| 700 | 36.25 | 0.209 | 42 | 3.251 | 5.200 | 1.5995 | 47.59 | |
| Samples | Calcination Temperature (°C) | Surface Area (m2/g) | Cumulative Surface Area of Pores (m2/g) | Total in Pores DFT | Average Pore Width (4 V/A) Å | ||||
|---|---|---|---|---|---|---|---|---|---|
| Single Point P/Po = 0.2655 | BET | Adsorption | Desorption | Area (m2/g) | Volume (cm3/g) (×10−4) | Adsorption | Desorption | ||
| La-ZnO | 500 | 1.0 | 0.9 | 0.5 | 0.5 | 0.1 | 7.7 | 55.2 | 114.5 |
| 700 | 0.9 | 0.8 | 0.5 | 0.4 | 0.1 | 7.3 | 55.6 | 92.8 | |
| Ce-ZnO | 500 | 12.1 | 12.8 | 14.6 | 16.1 | 10.2 | 198.7 | 53.9 | 56.6 |
| 700 | 5.8 | 6.1 | 6.0 | 6.1 | 4.8 | 110.0 | 72.0 | 86.3 | |
| Pr-ZnO | 500 | 2.4 | 2.4 | 2.2 | 1.4 | 0.9 | 24.1 | 52.5 | 90.6 |
| 700 | 1.8 | 1.7 | 1.9 | 0.9 | 0.6 | 17.9 | 42.4 | 92.5 | |
| Nd-ZnO | 500 | 3.3 | 3.2 | 3.1 | 2.2 | 1.3 | 36.5 | 61.0 | 100.1 |
| 700 | 2.2 | 2.1 | 1.9 | 1.0 | 0.8 | 21.9 | 46.0 | 100.4 | |
| Samples | Calcination Temperature (°C) | Band Gap (eV) | Degradation Efficiency(%) | κ (min−1) | R2 |
|---|---|---|---|---|---|
| ZnO | 500 | 3.12 | 82.70 | 0.0147 | 0.9968 |
| 700 | 3.12 | 91.41 | 0.0180 | 0.9919 | |
| La-ZnO | 500 | 3.00 | 64.00 | 0.0076 | 0.9969 |
| 700 | 3.06 | 77.36 | 0.0101 | 0.9927 | |
| Ce-ZnO | 500 | 3.05 | 97.72 | 0.0363 | 0.9691 |
| 700 | 3.13 | 97.36 | 0.0279 | 0.9953 | |
| Pr-ZnO | 500 | 2.58 | 96.81 | 0.0203 | 0.9957 |
| 700 | 2.61 | 82.49 | 0.0098 | 0.9932 | |
| Nd-ZnO | 500 | 3.08 | 76.57 | 0.0097 | 0.9889 |
| 700 | 3.13 | 74.03 | 0.0093 | 0.9978 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Crisostomo, J.C.; López-Juárez, R.; Petranovskii, V. Photocatalytic Degradation of Rhodamine B Dye in Aqueous Suspension by ZnO and M-ZnO (M = La3+, Ce3+, Pr3+ and Nd3+) Nanoparticles in the Presence of UV/H2O2. Processes 2021, 9, 1736. https://doi.org/10.3390/pr9101736
González-Crisostomo JC, López-Juárez R, Petranovskii V. Photocatalytic Degradation of Rhodamine B Dye in Aqueous Suspension by ZnO and M-ZnO (M = La3+, Ce3+, Pr3+ and Nd3+) Nanoparticles in the Presence of UV/H2O2. Processes. 2021; 9(10):1736. https://doi.org/10.3390/pr9101736
Chicago/Turabian StyleGonzález-Crisostomo, José C., Rigoberto López-Juárez, and Vitalii Petranovskii. 2021. "Photocatalytic Degradation of Rhodamine B Dye in Aqueous Suspension by ZnO and M-ZnO (M = La3+, Ce3+, Pr3+ and Nd3+) Nanoparticles in the Presence of UV/H2O2" Processes 9, no. 10: 1736. https://doi.org/10.3390/pr9101736
APA StyleGonzález-Crisostomo, J. C., López-Juárez, R., & Petranovskii, V. (2021). Photocatalytic Degradation of Rhodamine B Dye in Aqueous Suspension by ZnO and M-ZnO (M = La3+, Ce3+, Pr3+ and Nd3+) Nanoparticles in the Presence of UV/H2O2. Processes, 9(10), 1736. https://doi.org/10.3390/pr9101736






