Hg2+ Detection with Rational Design of DNA-Templated Fluorescent Silver Nanoclusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ag-DNA Nanoclusters
2.3. Fluorescence Measurements
3. Results and Discussion
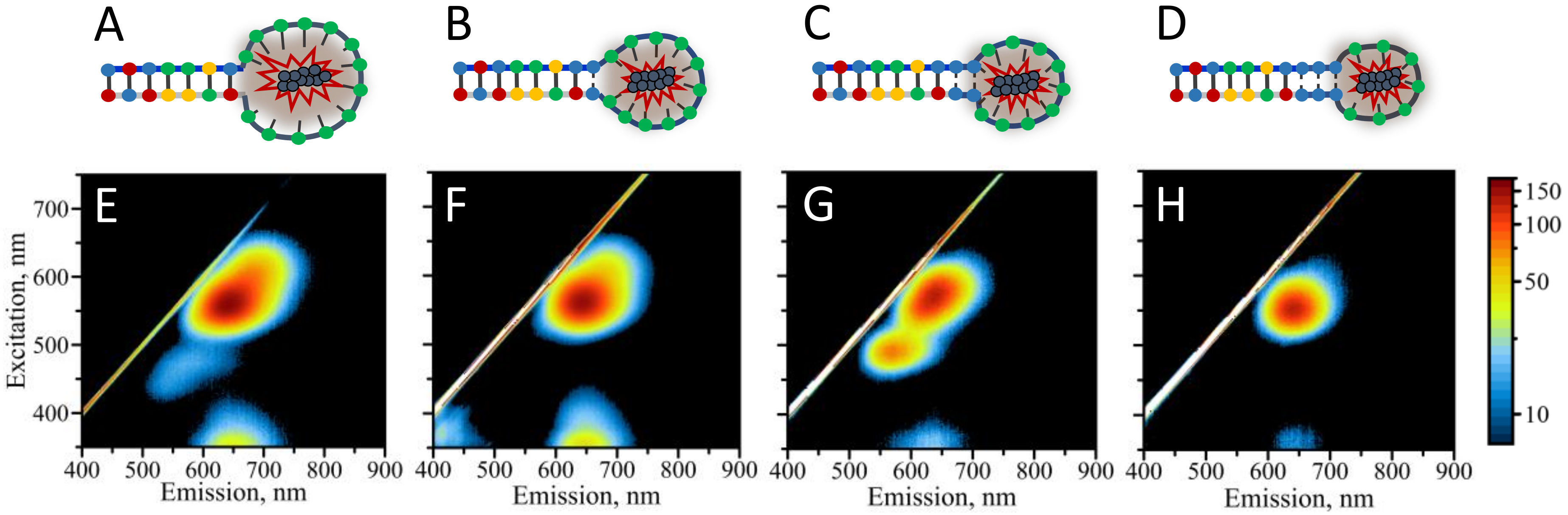
3.1. Design of the Hairpin-Loop AgNC DNA Template
3.2. Optical Properties of AgNCs Templated by Various Hairpin-Loop DNA Templates
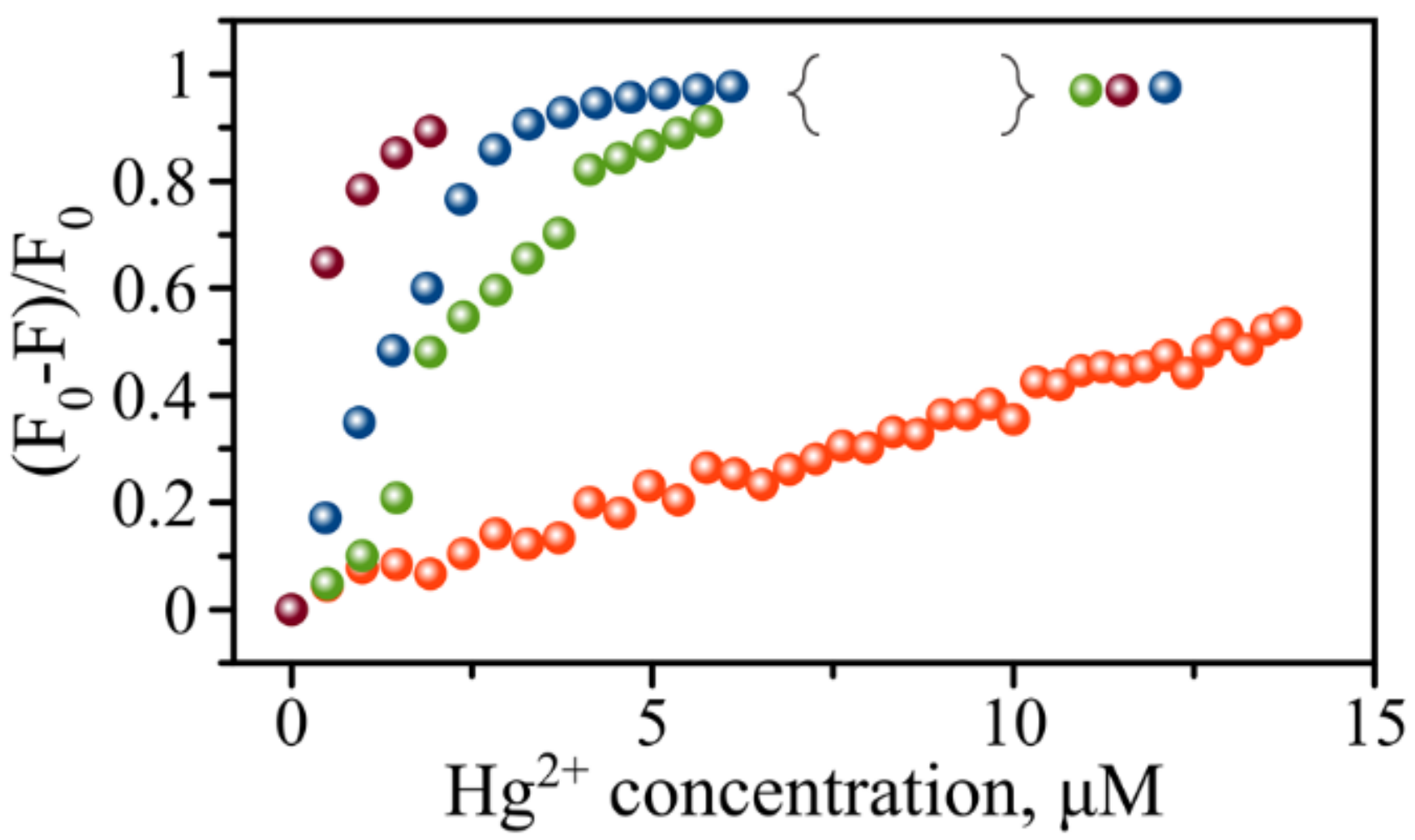
3.3. Hg2+ Sensing with AgNC’s Fluorescence
3.4. Extending the Range of Detectable Hg2+ Concentrations Using Rational Design of HL Template
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, H.; Yu, H. A Mini Review on Controlling the Size of Ag Nanoclusters by Changing the Stabilizer to Ag Ratio and by Changing DNA Sequence. Adv. Nat. Sci. 2016, 8, 1–9. [Google Scholar] [CrossRef]
- Geczy, R.; Christensen, N.J.; Rasmussen, K.K.; Kálomista, I.; Tiwari, M.K.; Shah, P.; Yang, S.W.; Bjerrum, M.J.; Thulstrup, P.W. Formation and Structure of Fluorescent Silver Nanoclusters at Interfacial Binding Sites Facilitating Oligomerization of DNA Hairpins. Angew. Chem. Int. Ed. 2020, 59, 16091–16097. [Google Scholar] [CrossRef]
- Latorre, A.; Somoza, Á. DNA-Mediated Silver Nanoclusters: Synthesis, Properties and Applications. ChemBioChem 2012, 13, 951–958. [Google Scholar] [CrossRef]
- Gonzàlez-Rosell, A.; Cerretani, C.; Mastracco, P.; Vosch, T.; Copp, S.M. Structure and luminescence of DNA-templated silver clusters. Nanoscale Adv. 2021, 3, 1230–1260. [Google Scholar] [CrossRef]
- New, S.Y.; Lee, S.T.; Su, X.D. DNA-templated silver nanoclusters: Structural correlation and fluorescence modulation. Nanoscale 2016, 8, 17729–17746. [Google Scholar] [CrossRef] [PubMed]
- Yourston, L.E.; Lushnikov, A.Y.; Shevchenko, O.A.; Afonin, K.A.; Krasnoslobodtsev, A.V. First Step Towards Larger DNA-Based Assemblies of Fluorescent Silver Nanoclusters: Template Design and Detailed Characterization of Optical Properties. Nanomaterials 2019, 9, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yourston, L.; Rolband, L.; Lushnikov, A.; West, C.; Afonin, K.; Krasnoslobodtsev, A. Tuning properties of silver nanoclusters with RNA nanoring assemblies. Nanoscale 2020, 12, 16189–16200. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ou, X.; Cheng, Y.; Zhai, T.; Liu, B.; Lou, X.; Xia, F. Coordination-induced structural changes of DNA-based optical and electrochemical sensors for metal ions detection. Dalton Trans. 2019, 48, 5879–5891. [Google Scholar] [CrossRef]
- MacLean, J.L.; Morishita, K.; Liu, J. DNA stabilized silver nanoclusters for ratiometric and visual detection of Hg2+ and its immobilization in hydrogels. Biosens. Bioelectron. 2013, 48, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Xu, J.; Chen, Y.; Zhang, L.; Lu, Y.; Qing, Z. DNA-Templated Fluorescent Nanoclusters for Metal Ions Detection. Molecules 2019, 24, 4189. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef] [Green Version]
- Tangahu, B.V.; Sheikh Abdullah, S.R.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef] [Green Version]
- Nolan, E.M.; Lippard, S.J. Tools and Tactics for the Optical Detection of Mercuric Ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef]
- Busairi, N.; Syahir, A. Recent Advances in Mercury Detection: Towards Enabling a Sensitive and Rapid Point-of-Check Measurement. J. Toxicol. Risk Assess. 2018, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Salek Maghsoudi, A.; Hassani, S.; Mirnia, K.; Abdollahi, M. Recent Advances in Nanotechnology-Based Biosensors Development for Detection of Arsenic, Lead, Mercury, and Cadmium. Int. J. Nanomed. 2021, 16, 803–832. [Google Scholar] [CrossRef]
- Wang, G.-L.; Zhu, X.-Y.; Jiao, H.-J.; Dong, Y.-M.; Li, Z.-J. Ultrasensitive and dual functional colorimetric sensors for mercury (II) ions and hydrogen peroxide based on catalytic reduction property of silver nanoparticles. Biosens. Bioelectron. 2012, 31, 337–342. [Google Scholar] [CrossRef]
- Yu, C.-J.; Tseng, W.-L. Colorimetric Detection of Mercury(II) in a High-Salinity Solution Using Gold Nanoparticles Capped with 3-Mercaptopropionate Acid and Adenosine Monophosphate. Langmuir 2008, 24, 12717–12722. [Google Scholar] [CrossRef]
- Sun, Z.; Li, S.; Jiang, Y.; Qiao, Y.; Zhang, L.; Xu, L.; Liu, J.; Qi, W.; Wang, H. Silver Nanoclusters with Specific Ion Recognition Modulated by Ligand Passivation toward Fluorimetric and Colorimetric Copper Analysis and Biological Imaging. Sci. Rep. 2016, 6, 20553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Zhou, Z.; Li, J.; Li, T.; Dong, S. Fluorescent silver nanoclusters in hybridized DNA duplexes for the turn-on detection of Hg2+ ions. Chem. Commun. 2011, 47, 11065–11067. [Google Scholar] [CrossRef]
- Petty, J.T.; Zheng, J.; Hud, N.V.; Dickson, R.M. DNA-Templated Ag Nanocluster Formation. J. Am. Chem. Soc. 2004, 126, 5207–5212. [Google Scholar] [CrossRef]
- O’Neill, P.R.; Velazquez, L.R.; Dunn, D.G.; Gwinn, E.G.; Fygenson, D.K. Hairpins with Poly-C Loops Stabilize Four Types of Fluorescent Agn:DNA. J. Phys. Chem. C 2009, 113, 4229–4233. [Google Scholar] [CrossRef]
- Rolband, L.; Yourston, L.; Chandler, M.; Beasock, D.; Danai, L.; Kozlov, S.; Marshall, N.; Shevchenko, O.; Krasnoslobodtsev, A.V.; Afonin, K.A. DNA-Templated Fluorescent Silver Nanoclusters Inhibit Bacterial Growth While Being Non-Toxic to Mammalian Cells. Molecules 2021, 26, 4045. [Google Scholar] [CrossRef]
- Svoboda, P.; Cara, A.D. Hairpin RNA: A secondary structure of primary importance. Cell. Mol. Life Sci. CMLS 2006, 63, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Hunter, P. Nucleic acid-based nanotechnology: The ability of DNA and RNA to fold into precise and complex shapes can be exploited for applications both in biology and electronics. EMBO Rep. 2018, 19, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, X. MicroRNA-21 and microRNA-30c as diagnostic biomarkers for prostate cancer: A meta-analysis. Cancer Manag. Res. 2019, 11, 2039–2050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkov, I.L.; Reveguk, Z.V.; Serdobintsev, P.Y.; Ramazanov, R.R.; Kononov, A.I. DNA as UV light–harvesting antenna. Nucleic Acids Res. 2018, 46, 3543–3551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copp, S.M.; Gorovits, A.; Swasey, S.M.; Gudibandi, S.; Bogdanov, P.; Gwinn, E.G. Fluorescence Color by Data-Driven Design of Genomic Silver Clusters. ACS Nano 2018, 12, 8240–8247. [Google Scholar] [CrossRef] [PubMed]
- Ramazanov, R.R.; Kononov, A.I. Excitation Spectra Argue for Threadlike Shape of DNA-Stabilized Silver Fluorescent Clusters. J. Phys. Chem. C 2013, 117, 18681–18687. [Google Scholar] [CrossRef]
- Brown, S.L.; Hobbie, E.K.; Tretiak, S.; Kilin, D.S. First-Principles Study of Fluorescence in Silver Nanoclusters. J. Phys. Chem. C 2017, 121, 23875–23885. [Google Scholar] [CrossRef]
- Swasey, S.M.; Karimova, N.; Aikens, C.M.; Schultz, D.E.; Simon, A.J.; Gwinn, E.G. Chiral electronic transitions in fluorescent silver clusters stabilized by DNA. ACS Nano 2014, 8, 6883–6892. [Google Scholar] [CrossRef] [PubMed]
- Jabed, M.A.; Dandu, N.; Tretiak, S.; Kilina, S. Passivating Nucleobases Bring Charge Transfer Character to Optically Active Transitions in Small Silver Nanoclusters. J. Phys. Chem. A 2020, 124, 8931–8942. [Google Scholar] [CrossRef]
- Ma, H.; Xue, N.; Wu, S.; Li, Z.; Miao, X. Fluorometric determination of mercury(II) using positively charged gold nanoparticles, DNA-templated silver nanoclusters, T-Hg(II)-T interaction and exonuclease assisted signal amplification. Microchim. Acta 2019, 186, 317. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, P.; Xiong, W.; Qi, B.; Shi, R.; Xiang, D.; Zhai, K. Simultaneous detection of mercury (II), lead (II) and silver (I) based on fluorescently labelled aptamer probes and graphene oxide. Environ. Technol. 2021, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Banerjee, A. Facile Synthesis of Water-Soluble Fluorescent Silver Nanoclusters and HgII Sensing. Chem. Mater. 2010, 22, 4364–4371. [Google Scholar] [CrossRef]
- Yourston, L.E.; Krasnoslobodtsev, A.V. Micro RNA Sensing with Green Emitting Silver Nanoclusters. Molecules 2020, 25, 3026. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Zhang, Z.; Zhang, F.; He, P.; Zhang, X.; Song, W. Fluorometric determination of mercury(II) by using thymine-thymine mismatches as recognition elements, toehold binding, and enzyme-assisted signal amplification. Microchim. Acta 2019, 186, 551. [Google Scholar] [CrossRef]

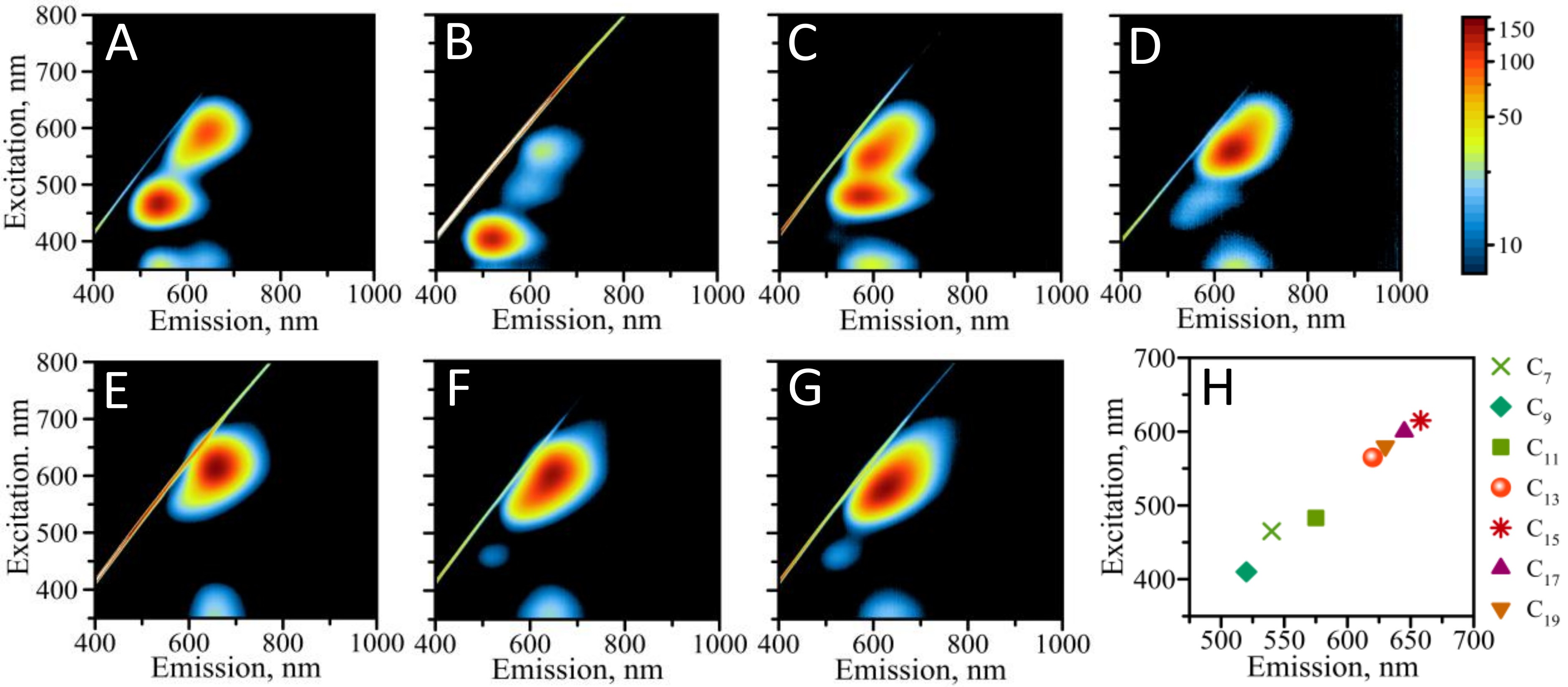

| HL-T0C13 | HL-T2C11 | HL-T4C9 | HL-T6C7 | |
|---|---|---|---|---|
| λEXC, nm | 560 | 562 | 568 | 550 |
| λEM, nm | 640 | 642 | 636 | 640 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yourston, L.; Dhoqina, P.; Marshall, N.; Mahmud, R.; Kuether, E.; Krasnoslobodtsev, A.V. Hg2+ Detection with Rational Design of DNA-Templated Fluorescent Silver Nanoclusters. Processes 2021, 9, 1699. https://doi.org/10.3390/pr9101699
Yourston L, Dhoqina P, Marshall N, Mahmud R, Kuether E, Krasnoslobodtsev AV. Hg2+ Detection with Rational Design of DNA-Templated Fluorescent Silver Nanoclusters. Processes. 2021; 9(10):1699. https://doi.org/10.3390/pr9101699
Chicago/Turabian StyleYourston, Liam, Polikron Dhoqina, Nolan Marshall, Rujani Mahmud, Ethen Kuether, and Alexey Viktorovich Krasnoslobodtsev. 2021. "Hg2+ Detection with Rational Design of DNA-Templated Fluorescent Silver Nanoclusters" Processes 9, no. 10: 1699. https://doi.org/10.3390/pr9101699
APA StyleYourston, L., Dhoqina, P., Marshall, N., Mahmud, R., Kuether, E., & Krasnoslobodtsev, A. V. (2021). Hg2+ Detection with Rational Design of DNA-Templated Fluorescent Silver Nanoclusters. Processes, 9(10), 1699. https://doi.org/10.3390/pr9101699







