Effect of Ni(NO3)2 Pretreatment on the Pyrolysis of Organsolv Lignin Derived from Corncob Residue
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstocks
2.2. Pretreatment of OL with Ni(NO3)2
2.3. Pyrolysis Experiment
2.4. Methods of Analysis and Characterization
2.4.1. Characterization of Pretreated OL
2.4.2. Characterization of the Liquid Products
2.4.3. Characterization of the Gaseous Products
3. Results and Discussion
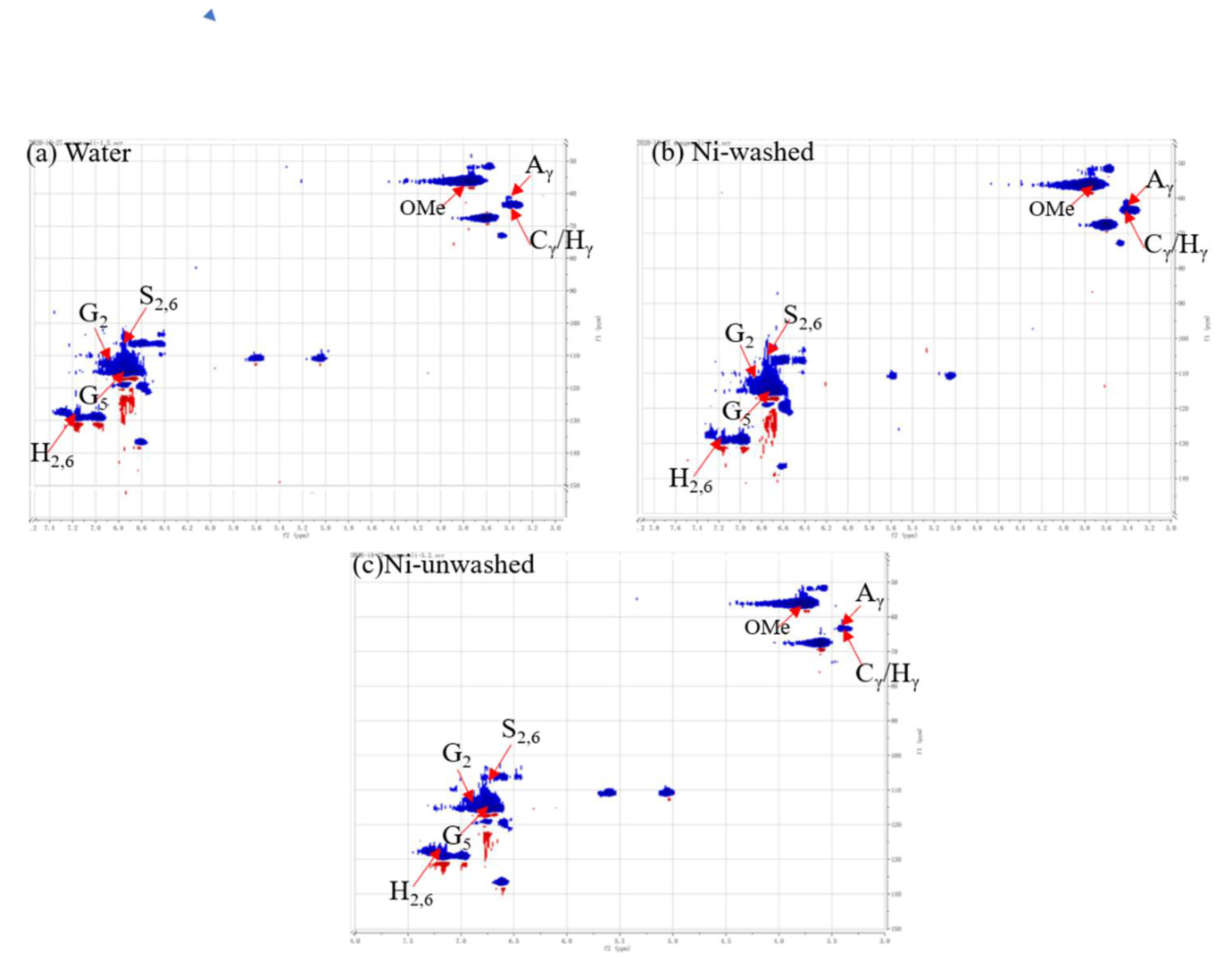
3.1. The Characteristics of Pretreated OL
3.2. Pyrolysis Products Distribution
3.3. The Characteristics of Bio-Oil
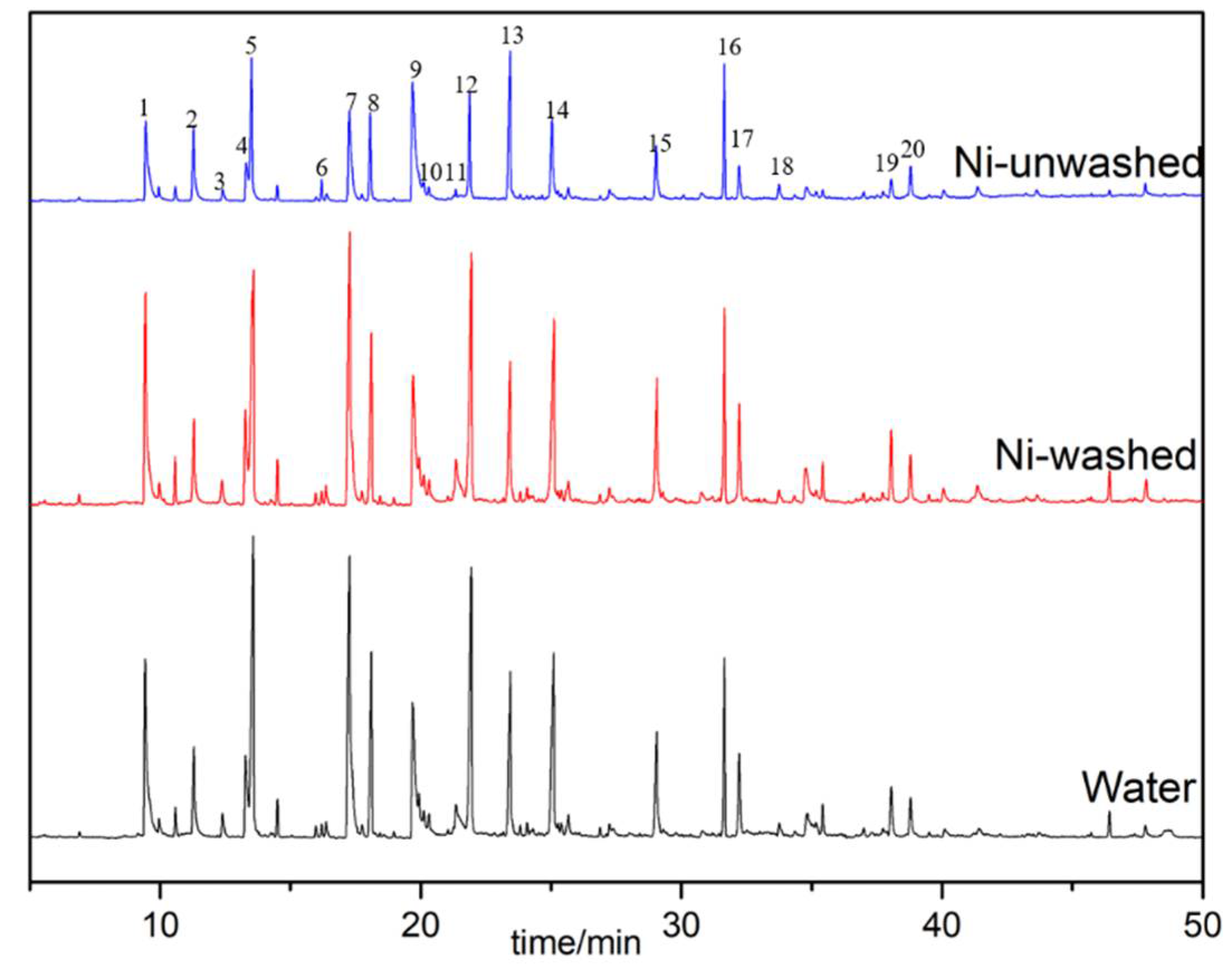
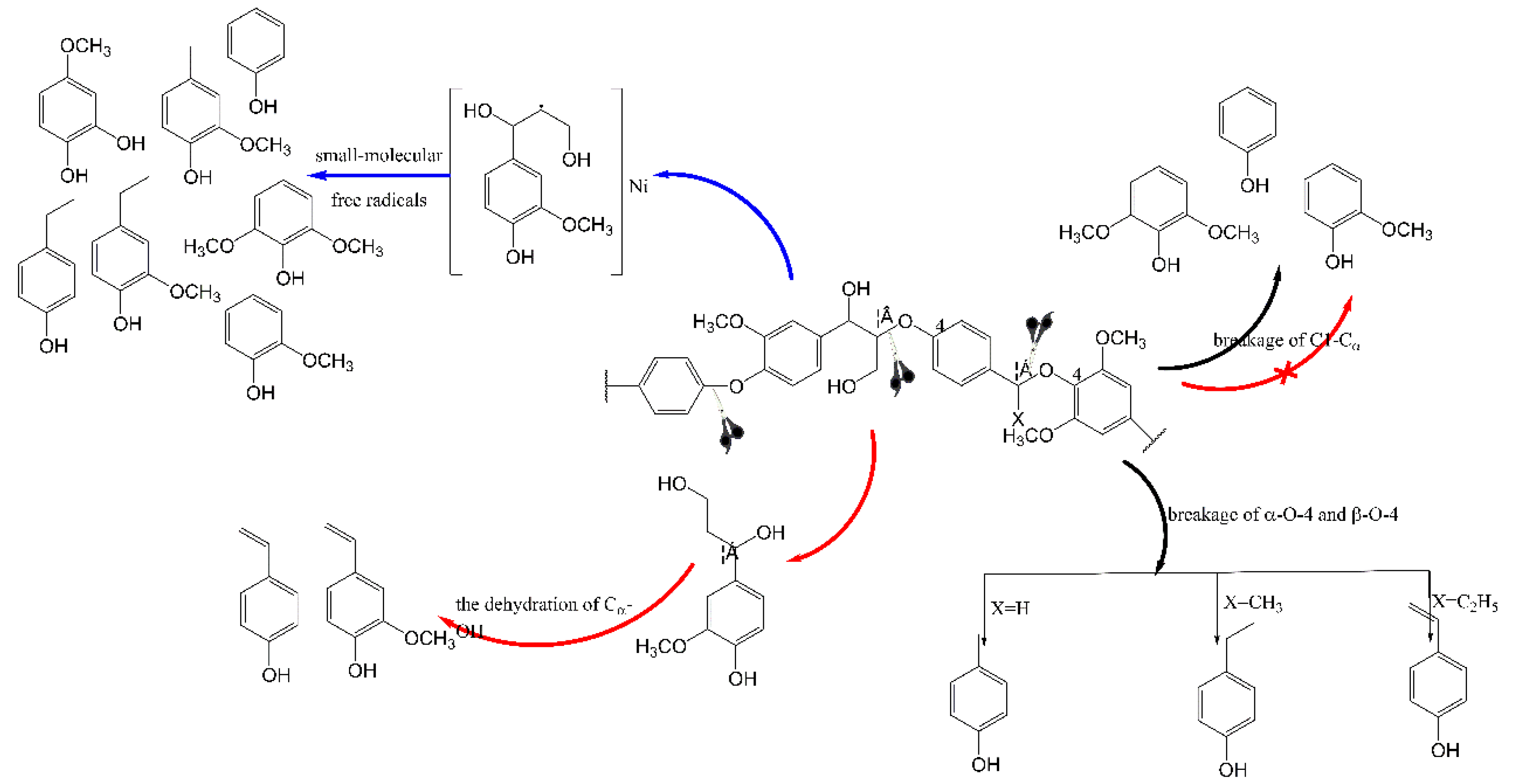
3.3.1. Monophenols in Bio-Oil
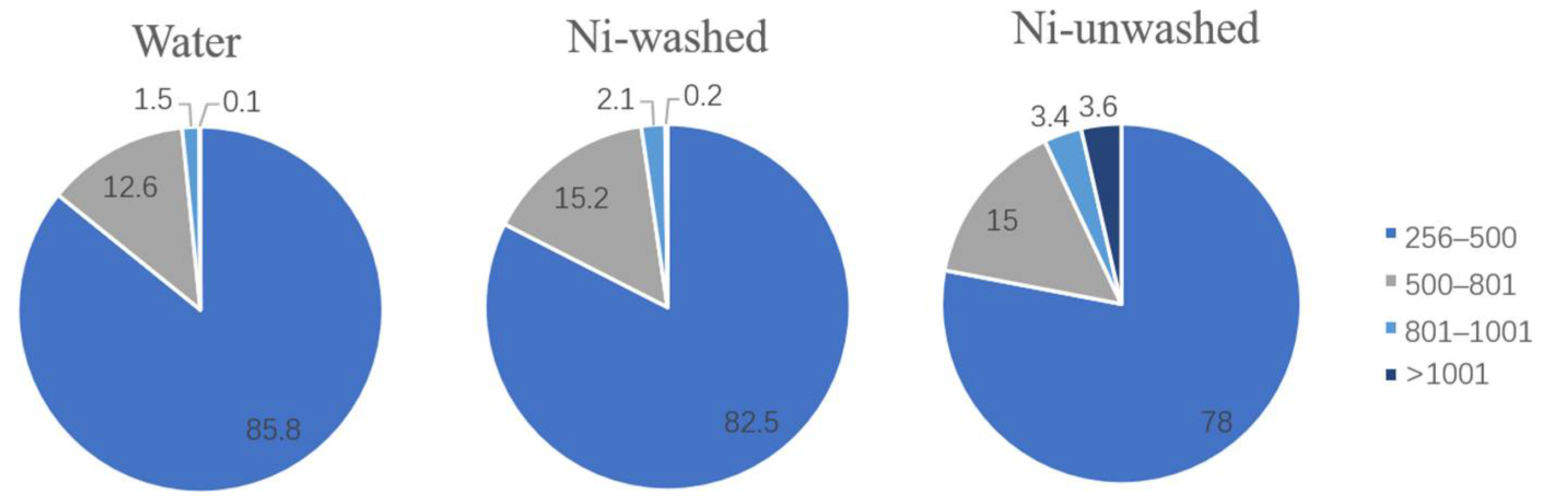
3.3.2. Phenolic Oligomers in Bio-Oil
3.4. The Characteristics of Gaseous Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Somerville, C.; Youngs, H. Feedstocks for Lignocellulosic Biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Li, W.W. Fates of Chemical Elements in Biomass during Its Pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef]
- Sun, Z.; Bottari, G. Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels. Nat. Catal. 2018, 1, 82–92. [Google Scholar] [CrossRef]
- Lynd, L.R.; Liang, X. Cellulosic ethanol: Status and innovation. Curr. Opin. Biotechnol. 2017, 45, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.-M.; Hwang, K.-R. Recent progress in the thermal and catalytic conversion of lignin. Renew. Sustain. Energy Rev. 2019, 111, 422–441. [Google Scholar] [CrossRef]
- Lahive, C.W.; Kamer, P.C.J. An Introduction to Model Compounds of Lignin Linking Motifs; Synthesis and Selection Considerations for Reactivity Studies. ChemSusChem 2020, 13, 4238–4265. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, C. Selective extraction and conversion of lignin in actual biomass to monophenols: A review. J. Eng. Chem. 2016, 25, 947–956. [Google Scholar] [CrossRef]
- Vispute, T.P.; Zhang, H. Renewable Chemical Commodity Feedstocks from Integrated Catalytic Processing of Pyrolysis Oils. Science 2010, 330, 1222–1227. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W. Relationship between the formation of oligomers and monophenols and lignin structure during pyrolysis process. Fuel 2020, 276, 118048. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y. Effects of MgCl2 Solution Pretreatment at Room Temperature on the Pyrolytic Behavior of Pubescens and the Properties of Bio-oil Obtained. Energy Fuels 2020, 34, 12665–12677. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z. Influence of inherent hierarchical porous char with alkali and alkaline earth metallic species on lignin pyrolysis. Bioresour. Technol. 2018, 268, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Suhag, M. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef]
- Kristiani, A.; Abimanyu, H. Effect of Pretreatment Process by Using Diluted Acid to Characteristic of oil Palm’s Frond. Energy Procedia 2013, 32, 183–189. [Google Scholar] [CrossRef]
- Chiesa, S.; Gnansounou, E. Use of Empty Fruit Bunches from the Oil Palm for bioethanol production: A thorough comparison between dilute acid and dilute alkali pretreatment. Bioresour. Technol. 2014, 159, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Cara, C.; Ruiz, E. Production, purification and characterisation of oligosaccharides from olive tree pruning autohydrolysis. Ind. Crops Prod. 2012, 40, 225–231. [Google Scholar] [CrossRef]
- Tan, H.T.; Lee, K.T. Understanding the impact of ionic liquid pretreatment on biomass and enzymatic hydrolysis. Chem. Eng. J. 2012, 183, 448–458. [Google Scholar] [CrossRef]
- Loow, Y.-L.; Wu, T.Y. Recent Advances in the Application of Inorganic Salt Pretreatment for Transforming Lignocellulosic Biomass into Reducing Sugars. J. Agric. Food. Chem. 2015, 63, 8349–8363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, X. Room temperature pretreatment of pubescens by AlCl3 aqueous solution. J. Eng. Chem. 2019, 31, 138–147. [Google Scholar] [CrossRef]
- Wu, X.; Huang, C. Use of metal chlorides during waste wheat straw autohydrolysis to overcome the self-buffering effect. Bioresour. Technol. 2018, 268, 259–265. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y. Torrefaction at 200 °C of Pubescens Pretreated with AlCl3 Aqueous Solution at Room Temperature. ACS Omega 2020, 5, 27709–27722. [Google Scholar] [CrossRef]
- Zhou, S.; Brown, R.C. The use of calcium hydroxide pretreatment to overcome agglomeration of technical lignin during fast pyrolysis. Green Chem. 2015, 17, 4748–4759. [Google Scholar] [CrossRef]
- Milovanović, J.; Luque, R. Study on the pyrolysis products of two different hardwood lignins in the presence of NiO contained-zeolites. Biomass Bioenergy 2017, 103, 29–34. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Choi, J.W. Effect of molecular size of lignin on the formation of aromatic hydrocarbon during zeolite catalyzed pyrolysis. Fuel 2019, 240, 92–100. [Google Scholar] [CrossRef]
- Guo, D.-L.; Wu, S.-B. Catalytic effects of NaOH and Na2CO3 additives on alkali lignin pyrolysis and gasification. Appl. Energy 2012, 95, 22–30. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A. Catalytic pyrolysis-GC/MS of lignin from several sources. Fuel Process. Technol. 2010, 91, 1446–1458. [Google Scholar] [CrossRef]
- Eibner, S.; Broust, F. Catalytic effect of metal nitrate salts during pyrolysis of impregnated biomass. J. Anal. Appl. Pyrolysis 2015, 113, 143–152. [Google Scholar] [CrossRef]
- Custodis, V.B.F.; Karakoulia, S.A. Catalytic Fast Pyrolysis of Lignin over High-Surface-Area Mesoporous Aluminosilicates: Effect of Porosity and Acidity. ChemSusChem 2016, 9, 1134–1145. [Google Scholar] [CrossRef]
- Zhang, H. Performances of Several Solvents on the Cleavage of Interand. ChemSusChem 2018, 11, 1494–1504. [Google Scholar] [CrossRef]
- Lv, X.; Li, Q. Structure characterization and pyrolysis behavior of organosolv lignin isolated from corncob residue. J. Anal. Appl. Pyrolysis 2018, 136, 115–124. [Google Scholar] [CrossRef]
- Bru, K.; Blin, J. Pyrolysis of metal impregnated biomass: An innovative catalytic way to produce gas fuel. J. Anal. Appl. Pyrolysis 2007, 78, 291–300. [Google Scholar] [CrossRef]
- Wang, L.; Li, J. Investigation of the pyrolysis characteristics of guaiacol lignin using combined Py-GC×GC/TOF-MS and in-situ FTIR. Fuel 2019, 251, 496–505. [Google Scholar] [CrossRef]
- Tan, S.S.Y.; MacFarlane, D.R. Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzenesulfonate ionic liquid. Green Chem. 2009, 11, 339–345. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X. Study on pyrolysis behaviors of non-woody lignins with TG-FTIR and Py-GC/MS. J. Anal. Appl. Pyrolysis 2015, 113, 499–507. [Google Scholar] [CrossRef]
- Tayier, M.; Zhao, Y. Bamboo biochar-catalytic degradation of lignin under microwave heating. J. Wood Chem. Technol. 2020, 40, 190–199. [Google Scholar] [CrossRef]
- Norouzi, O.; Kheradmand, A. Superior activity of metal oxide biochar composite in hydrogen evolution under artificial solar irradiation: A promising alternative to conventional metal-based photocatalysts. Int. J. Hydrogen Energy 2019, 44, 28698–28708. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. Influence of impregnated metal on the pyrolysis conversion of biomass constituents. J. Anal. Appl. Pyrolysis 2012, 95, 213–226. [Google Scholar] [CrossRef]
- Collard, F.-X.; Bensakhria, A. Influence of impregnated iron and nickel on the pyrolysis of cellulose. Biomass Bioenergy 2015, 80, 52–62. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J. Thermal conversion of lignin to phenols: Relevance between chemical structure and pyrolysis behaviors. Fuel 2016, 182, 864–870. [Google Scholar] [CrossRef]
- Terakado, O.; Amano, A. Explosive degradation of woody biomass under the presence of metal nitrates. J. Anal. Appl. Pyrolysis 2009, 85, 231–236. [Google Scholar] [CrossRef]
- Fu, X.; Li, Q. Identification and structural characterization of oligomers formed from the pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2019, 144, 104696.1–104696.10. [Google Scholar] [CrossRef]
- del Rio, J.C.; Rencoret, J. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food. Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yuan, T.-Q. Structural Elucidation of Whole Lignin in Cell Walls of Triploid of Populus tomentosa Carr. ACS Sustain. Chem. Eng. 2016, 4, 1006–1015. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L. Low-Temperature Catalytic CO2 Dry Reforming of Methane on Ni-Si/ZrO2 Catalyst. ACS Catal. 2018, 8, 6495–6506. [Google Scholar] [CrossRef]




| Content/(wt.%) | Water | Ni-Washed | Ni-Unwashed |
|---|---|---|---|
| Ni | 0.00 ± 0.00 | 0.03 ± 0.01 | 4.00 ± 0.71 |
| Ni(NO3)2·6H2O | 0.00 ± 0.00 | 0.10 ± 0.01 | 19.82 ± 0.71 |
| Lignin Samples | Conversion | Char | Bio-Oil | Gas |
|---|---|---|---|---|
| Water | 54.60 ± 0.42 | 45.44 ± 0.48 | 36.50 ± 0.14 | 18.15 ± 0.35 |
| Ni-washed | 55.05 ± 0.78 | 44.95 ± 0.78 | 35.55 ± 0.346.5 | 19.50 ± 0.62 |
| Ni-unwashed | 52.34 ± 0.64 | 47.66 ± 0.96 | 32.81 ± 0.78 | 19.52 ± 0.26 |
| C/wt.% | H/wt.% | O a/wt.% | N/wt.% | HHV (mJ/kg) b | |
|---|---|---|---|---|---|
| Water | 66.38 ± 1.42 | 7.07 ± 0.12 | 25.65 ± 1.55 | 0.90 ± 0.01 | 27.94 ± 0.93 |
| Ni-washed | 66.81 ± 0.16 | 6.98 ± 0.01 | 25.38 ± 0.17 | 0.83 ± 0.01 | 27.87 ± 0.11 |
| Ni-unwashed | 67.48 ± 0.89 | 7.02 ± 0.08 | 24.11 ± 0.98 | 1.39 ± 0.01 | 28.46 ± 0.58 |
| Compound | Water | Ni-Washed | Ni-Unwashed | |
|---|---|---|---|---|
| H | phenol | 3.2 | 4.4 | 2.8 |
| o-cresol | 0.3 | 0.3 | 0.2 | |
| p-cresol | 1.2 | 1.5 | 1.1 | |
| 2-ethylphenol | 0.0 | 0.1 | 0.1 | |
| 4-ethylphenol | 3.4 | 4.5 | 1.9 | |
| 4-vinylphenol | 2.7 | 3.0 | 4.4 | |
| 4-isopropylphenol | 0.1 | 0.2 | 0.1 | |
| 3-methoxycatechol | 1.0 | 1.6 | 0.8 | |
| ∑H | 11.9 | 15.6 | 11.4 | |
| G | Guaiacol | 3.1 | 4.1 | 2.0 |
| 4-methylguaiacol | 1.5 | 1.9 | 1.1 | |
| 4-ethylguaiacol | 2.1 | 2.7 | 1.0 | |
| 4-vinylguaiacol | 1.3 | 1.5 | 2.1 | |
| ∑G | 8.0 | 10.2 | 6.2 | |
| S | Syringol | 2.2 | 3.0 | 1.6 |
| 4-methylsyringol | 1.2 | 1.6 | 1.1 | |
| ∑S | 3.4 | 4.6 | 2.7 | |
| Total (wt%) | 23.3 | 30.2 | 20.3 |
| H2 | CO | CH4 | CO2 | |
|---|---|---|---|---|
| Water | 2.0 | 31.1 | 29.3 | 37.6 |
| Ni-washed | 7.6 | 48.3 | 14.7 | 29.4 |
| Ni-unwashed | 17.1 | 8.5 | 15.3 | 59.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liu, Y.; Wang, Y.; Liu, L.; Hu, C. Effect of Ni(NO3)2 Pretreatment on the Pyrolysis of Organsolv Lignin Derived from Corncob Residue. Processes 2021, 9, 23. https://doi.org/10.3390/pr9010023
Wang W, Liu Y, Wang Y, Liu L, Hu C. Effect of Ni(NO3)2 Pretreatment on the Pyrolysis of Organsolv Lignin Derived from Corncob Residue. Processes. 2021; 9(1):23. https://doi.org/10.3390/pr9010023
Chicago/Turabian StyleWang, Wenli, Yichen Liu, Yue Wang, Longfei Liu, and Changwei Hu. 2021. "Effect of Ni(NO3)2 Pretreatment on the Pyrolysis of Organsolv Lignin Derived from Corncob Residue" Processes 9, no. 1: 23. https://doi.org/10.3390/pr9010023
APA StyleWang, W., Liu, Y., Wang, Y., Liu, L., & Hu, C. (2021). Effect of Ni(NO3)2 Pretreatment on the Pyrolysis of Organsolv Lignin Derived from Corncob Residue. Processes, 9(1), 23. https://doi.org/10.3390/pr9010023




