Abstract
To initiate our research into the development of biocompatiîle gelled-microemulsions based on essential oils (EOs) and sucrose esters (SEs) for the topical delivery of fluconazole, this formulation study investigated the usefulness of two relatively harmless natural non-ionic surfactants from the group of SEs (sucrose laurate and stearate) to form, in the presence of antifungal EOs, stable, isotropic microemulsions effective on fluconazole solubilization. Fluconazole’s solubility in EO significantly depended on their chemical composition, showing higher values for cinnamon, oregano and clove essential oils, further selected as oil phase components for microemulsion formulations. The phase behavior of several EO–isopropyl miristate/SE–isopropanol/water systems was assessed through pseudo-ternary phase diagrams constructed by microplate dilution technique. The hydrocarbon chain length of the SE and EO type strongly influenced the size of the microemulsion region in the pseudo-ternary phase diagrams. Ten microemulsion formulations containing 2% fluconazole, 6% or 10% oil mixture of EO–isopropyl myristate in 1:1 ratio, 45% SE-isopropanol mixture and water, were selected and evaluated for physicochemical properties (droplet size, polydispersity, viscosity, refractive index, zeta potential and pH). All formulations were physicochemically acceptable, but viscosity enhancement and further in vitro and in vivo tests are required for the development of biocompatible, clinically safe and effective fluconazole topical preparations.
1. Introduction
For the treatment of mild or localized forms of superficial and cutaneous fungal infections, topical preparations containing antifungal drugs, including azoles, are of first choice, allowing to restrict the therapeutic effect on the affected skin area and minimalize the systemic adverse effects [1]. The currently available topical antifungal formulations are conventional ointments, creams, gels, lotions and sprays containing the drug in free form [2]. The major drawback of conventional topical antifungal formulations is poor drug penetration through the stratum corneum, the outermost skin layer with excellent barrier properties. Consequently, their clinical efficacy and effectiveness is limited because the antifungal molecule cannot reach deeper skin layers, such as viable epidermis, in effective concentrations [3]. This inconvenience is mainly determined by the physicochemical properties of the drug (solubility, partition coefficient and molecular weight) and its vehicle (chemical nature, hydrophilic–lipophilic and mechanical properties). Therefore, the poor solubility and cutaneous permeability of most antifungals, including azole derivatives, represent pharmaceutical formulation challenges in developing clinically effective topical preparations.
Among the synthetic azole compounds, fluconazole (FZ) is a fluorinated derivative frequently orally administered for the treatment of systemic, cutaneous and mucous candidiasis, despite its inherent adverse effects especially gastric disorders [4]. Nevertheless, several fluconazole properties (broad spectrum of activity including various Candida species and dermatophytes, low toxicity and high efficacy due to fungistatic effect) make it a good candidate for topical administration. However, FZ has disadvantageous physicochemical properties for cutaneous administration, namely moderate lipophilicity (log p = 0.5) and poor water solubility (1 mg/mL), which are responsible for its low skin penetration [5]. To enhance the FZ ability to penetrate the stratum corneum and deliver it into deeper skin layers, modern drug nanocarriers and chemical penetration enhancers were recently proposed [6,7,8,9,10,11,12,13].
Among the colloidal carriers, microemulsions (MEs) have attracted great interest due to their following advantageous properties: spontaneous formation and accordingly easy preparation, transparency which confers them pharmaceutical elegance, thermodynamic stability leading to a long shelf life, high solubilization capacity for both lipophilic and hydrophilic compounds and drug skin permeation enhancing effect. MEs are colloidal dispersions of micellar type, based on oil, water, surfactant and often cosurfactant, with a size of the dispersed particles in the range of 10–100 nm, which makes them transparent, isotropic and fluid [14,15,16]. Nevertheless, when formulating topical MEs, a restraint arises from the acceptability criteria for the excipients and also from high concentrations of surfactant and/or cosurfactant, resulting in skin sensitization potential. Thus, to develop topical MEs highly effective in drug skin penetration, while maintaining a satisfying safety profile, different natural non-ionic, biodegradable, nontoxic, effective as penetration enhancers and low skin irritant surfactants have been increasingly studied recently, as stabilizers in ME formulations. For this study, from the group of natural non-ionic surfactants, sucrose esters were selected as they possess all the aforementioned features, as well as high environmental compatibility [17,18,19,20]. Moreover, the intrinsic antifungal effect of sucrose esters, scientifically demonstrated [21,22], was considered when they were selected for developing FZ-loaded ME formulations, based on the hypothesis that they could potentiate through synergism the antifungal effect of FZ.
Extending this hypothesis for other natural products that are described in the literature also as skin-compatible penetration enhancers, in order to accomplish the abovementioned formulation aims of dermal antifungal MEs, essential oils were of interest for the present study. As complex mixtures of lipophilic volatile and non-volatile compounds, essential oils have demonstrated antifungal activity and even synergistic effects with synthetic antifungal drugs like fluconazole [23,24]; also, they are effective penetration enhancers, GRAS (generally recognized as safe) certified by FDA [25,26]. Considering these properties, several essential oils were combined as components of the oil phase with sucrose esters as surfactants (stabilizers), isopropyl alcohol as cosurfactant and water in order to form stable ME systems for topical FZ delivery. Although numerous published works investigated the use of sucrose esters as stabilizers in microemulsions [17,20,27], to the best of our knowledge, only few systems contained essential oils as oil phase [28].
Therefore, as a formulation study within the research literature on developing topical biocompatible FZ-loaded microemulsions based on essential oils and sucrose esters, the present work aimed to evaluate the capacity of two sucrose esters (sucrose laurate and sucrose stearate) to form hydrophilic microemulsions in combination with some essential oils and isopropyl myristate as the oil phase, isopropyl alcohol as the cosurfactant and water, using pseudo-ternary phase diagrams. The effect of hydrocarbon chain length of sucrose ester and its concentration, and of the essential oil type and its concentration on the phase behavior, were also evaluated. Moreover, solubility studies of fluconazole and sucrose esters in essential and synthetic oils have also been performed, keeping in mind the following generally accepted strategies of ME formulation: (a) currently, the formulation study of a poorly water-soluble drug as ME is preceded by the experimental determination of its solubility in various oils to select the most powerful oily solubilizer as a lipophilic phase of the ME [13,14,17,27]; (b) when a solid surfactant is selected as the ME stabilizer, the determination of its solubility in the lipophilic components is required, because this property significantly affects the phase behavior and the size of the microemulsion area in the ternary oil–surfactant/cosurfactant–water systems [13,20]. Consequently, the study of drug and solid surfactant solubility in lipophilic components represents the starting point to address the formulation of stable and safe microemulsion vehicles with a minimum content of surfactant and cosurfactant.
2. Materials and Methods
2.1. Materials
Fluconazole (FZ) was received as a gift sample from S.C. Vim Spectrum S.R.L (Romania). Essential oils (cinnamon, clove, eucalyptus, lavender, lemongrass, mint, oregano, sweet fennel and thyme essential oils) were purchased from Elemental (Romania). Isopropyl myristate was kindly donated by Cognis (Germany) and ethyl oleate was purchased from Sigma Aldrich (Germany). Natural surfactants sucrose laurate (D1216) and sucrose palmitate (D1616) were received as free samples from Mitsubishi Chemical Foods Corporation (Japan). Isopropyl alcohol (IPA) and ethyl alcohol were provided by S.C. Chemical Company S.A. (Romania). Distilled water was used to prepare the microemulsions. All the materials used in the study were of pharmaceutical or analytical purity and were used as such.
2.2. Solubility Studies
Due to its low solubility in water, fluconazole was subjected to solubility tests in two synthetic fatty oils (isopropyl myristate and ethyl oleate) and nine essential oils (eucalyptus, lemongrass, clove, cinnamon, lavender, mint, sweet fennel and oregano), for which the antimycotic activity was reported in the literature. In the case of essential oils, a preliminary screening was initially performed, evaluating, by visual observation, their ability to dissolve FZ; a qualitative criterion was used, based on the compendial recommendations on “Solubility” [29]. Briefly, an accurately weighted amount (Kern ABJ-NM/ABS-N analytical balance, Kern&Sohn GmbH, Germany) of fluconazole (1 mg) was added to 1 mL of essential oil, then the systems were sonicated for 15 min to accelerate the drug dissolution and the FZ solubility was visually evaluated after 24 h. For the essential oils, in which the entire added amount of FZ was dissolved, the technique described above was repeated consecutively until a precipitate appeared. The apparent solubility was expressed as the total amount of drug (in mg) added in 1 mL essential oil until saturation occurred.
Essential oils in which fluconazole exhibited greater apparent solubility (cinnamon essential oil, CIN, clove essential oil, CLV and oregano essential oil, ORG) were selected for quantitative solubility assessment, by the saturation shake-flask method. For each experiment, an excess amount of fluconazole was added to each vial containing 2 mL of essential oil. After closure, the vial was shaken vigorously for 10 min to facilitate the proper dispersion of the fluconazole powder in the essential oil. The obtained dispersions were shaken for 48 h at room temperature (25 ± 1 °C) to reach equilibrium, then centrifuged at 3000 rpm for 5 min. The saturated solution was separated from the precipitate by filtration (0.45 μm membrane filter, 25 mm, Teknokroma, Germany). A sample of each saturated solution (0.1 mL) was diluted with the respective essential oil and then with a 9:1 mixture of acetonitrile–aqueous CuCl2 solution. The concentration of the cupric chloride aqueous solution was 0.53 g/mL.
The quantification of fluconazole in these solutions was based on the formation of a colored (green) metal complex of Cu2+ ion with FZ in the 1:9 water–acetonitrile mixture. Consequently, the quantitative determination of FZ was performed by spectrophotometry in VIS (T70+ spectrophotometer, PG Instruments, UK) at the wavelength of 800 nm (for cinnamon and clove essential oils) and of 762 nm (for oregano essential oil), based on the equations of the calibration curves obtained for each volatile oil under the same dilution conditions. Three determinations were performed in parallel for each indicated solubility data.
It has been chosen to quantify fluconazole by spectrophotometry in the visible domain as described above and not in the conventionally used ultraviolet domain, because of the interferences between the UV absorption spectra of the fluconazole and essential oils in the organic solvents commonly used for drug solubility determination (ethanol, methanol and acetonitrile) observed in our preliminary solubility studies. These interferences hinder the correct quantification of fluconazole in studied essential oils.
Moreover, the saturation shake-flask method was used to determine the solubility of fluconazole in synthetic fatty oils (isopropyl myristate and ethyl oleate), following the aforementioned technique, but the FZ concentration in the filtrate after dilution with ethanol, was measured spectrophotometrically in the UV domain at 253 nm.
In addition, using the qualitative criterion based on the official standards, the apparent solubility of sucrose laurate and sucrose palmitate was determined in essential oils of clove, cinnamon and oregano as well as in isopropyl myristate; the technique described above for fluconazole was followed.
2.3. Selection of the Formulation Components
2.3.1. Selection of the Oil Phase
The selection of the essential oil, as a component of the oily phase of the fluconazole-loaded microemulsions, was based on the maximum solubilization capacity of the drug. The second component of the oily phase was selected from the group of synthetic lipophilic solvents, based on its miscibility with the essential oil and its ability to dissolve fluconazole. The essential oil was diluted with the synthetic fatty oil in a 1:1 mass ratio.
2.3.2. Selection of Surfactant and Cosurfactant
Among the sucrose esters used as emulsifiers for pharmaceutical formulations, two surfactants, namely sucrose laurate (D1216) and sucrose palmitate (D1616), were selected for this study. Screening was based on their capacity to solubilize the oily phase, indicated by the ability to form a large area of microemulsion in the pseudo-ternary phase diagram [17,20]. It is worth mentioning that surfactants having the same HLB value (16) were chosen, although the fatty acid chain length was different (12 and 16 carbon atoms, respectively).
The only criterion for the cosurfactant selection, in developing the experimental biocompatible microemulsions, was its capability to dissolve the hydrophilic surfactant (sucrose ester), which is solid at room temperature.
2.4. Construction of Pseudo-Ternary Phase Diagrams
In order to determine the concentration domain of the components (oil phase, aqueous phase and surfactant/cosurfactant mixture, Smix) corresponding to the microemulsion region, pseudo-ternary phase diagrams were constructed. The essential oil and isopropyl myristate were mixed in a 1:1 mass ratio, and the surfactant (sucrose laurate or sucrose palmitate) was dissolved in the cosurfactant (isopropyl alcohol) in two mass ratios (1:1.5 and 1:2) by stirring. Thereafter, various mixtures of oily phase and Smix were prepared in 9 weight ratios (1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:2 and 9:1), to cover the maximum mass ratios, which allows the boundary between the phases formed in the pseudo-ternary phase diagram to be precisely delineated.
Pseudo-ternary phase diagrams were constructed using a technique based on the conventional water titration method, namely the microplate dilution. This technique, described by Schmidts T. et al. [30] has the advantage of saving time and materials, because the water titration of oil–Smix blends is carried out in microtitration plate. Briefly, the individual blends of oil–Smix were gradually diluted with water in a microtitration plate (96 wells, with a volume of 350 μL each). The microtitration plate was filled with a microsyringe, according to the following pattern: the oil phase containing the mixture of surfactant–cosurfactant (Smix) was added starting from A1 with 200 μL to D4 with 5 μL, decreasing with 5 μL in each well and then adding the aqueous phase starting with A2 with 5 μL to D5 with 200 μL, increasing with 5 μL for each well. Wells E1 to H5 were completed with the following batches using the same procedure. Plates thus filled were then sealed with an adhesive film and shaken with a thermo-agitator (equipped with a temperature control system) at 25 °C, to ensure proper mixing and to maintain the constant temperature of the system. Subsequently, each plate was visually checked for transparency and isotropy, as well as for boundaries between homogeneous and heterogeneous mixtures. These were classified as L/H microemulsions only for the single-phase, clear, fluid and homogeneous systems.
2.5. Preparation of O/W Fluconazole-Loaded Microemulsions
Among the constructed pseudo-ternary phase diagrams, only five were used to select the ME formulations for further studies. From each of these pseudo-ternary phase diagrams, two ME formulations with 6% or 10% oily phase and 45% Smix were selected. Taking into account that both the sucrose ester/IPA mixture (Smix) and the essential oil can influence the rheological characteristics of the ME-based formulations and drug release/permeation from the vehicle through the membrane, the proportion of Smix has been kept constant in order to assess the effect of the essential oils on these features. The composition of the ten experimental MEs loaded with fluconazole is shown in Table 1 and Table 2.

Table 1.
Composition (%, w/w) of fluconazole-loaded microemulsions based on sucrose palmitate.

Table 2.
Composition (%, w/w) of fluconazole-loaded microemulsions based on sucrose laurate.
Fluconazole was dissolved in the mixture formed by the oily phase (volatile oil and isopropyl myristate) with the sucrose ester (sucrose palmitate or laurate) isopropyl alcohol solution, under stirring. To the resulting solution, the appropriate amount of aqueous phase (2% acetic acid aqueous solution) was added, dropwise and under stirring. Stirring the mixture during the preparation was carried out using a magnetic stirrer (Magnetic Stirrer with Hot Plate, Model 4803-02, Cole Parmer Instrument Company, Vernon Hills, IL, USA). The fluconazole content in the prepared MEs was 2% (w/w). All prepared MEs were stored for 24 h at room temperature, to equilibrate before being subjected to further testing.
2.6. Physicochemical Characterization of O/W Fluconazole-Loaded Microemulsions
The macroscopic examination was performed by the visual and olfactory inspection of the formulations, in order to evaluate the organoleptic characters (appearance, color, odor, fluidity). The distinction between liquid and viscous systems was based on the flow capacity of the dispersion placed at a 45° inclination.
2.6.1. Droplet Size and Zeta Potential Determination
The mean diameter of the droplets, polydispersity index and zeta potential of the FZ-loaded microemulsions were measured by photon correlation spectroscopy using the Zetasizer Nano-ZS instrument (Malvern Instruments, UK). The measurements were performed in triplicate at a fixed angle of 173° and at 25 °C.
2.6.2. Refractive Index Determination
The refractive index value of the FZ-loaded microemulsions was measured using a refractometer (Digital ABBE Mark II-REICHERT, Depew, NY, USA). The measurements were performed in triplicate at 25 °C.
2.6.3. Viscosity Measurements
The viscosity of the ME samples was measured using a rotary viscometer (Brookfield DV-I +, UK) with a S-05 probe. Each determination was carried out in triplicate at 25 ± 2 °C.
2.6.4. pH Measurements
The pH value of FZ-loaded microemulsions was measured at 25 °C, using a portable pH-meter (SensionTM, Hach Company, USA). The experiments were performed in triplicate for each sample.
2.7. Statistical Analysis
The experimental data were expressed as the means ± standard deviation (SD) and their regression analysis was performed with Microsoft Excel (2016). When statistical analysis was carried out, the software Statistica 7.0 was used and p < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Solubility Studies
The solubility data reported in the literature, including those of a previous own study [13], showed that fluconazole is very slightly soluble in water (5.551 mg/mL), slightly soluble or soluble in fatty oils, soluble or freely soluble in isopropyl alcohol, propyleneglycol and ethanol (60.614, 103.436, and 126.991 mg/mL, respectively).
The results of a preliminary evaluation for the maximum fluconazole dissolution capacity of the selected essential oils are presented in Table 3 as apparent solubility. It can be observed that fluconazole is freely soluble in clove and oregano essential oils and soluble in cinnamon essential oil.

Table 3.
Apparent solubility of fluconazole in the selected essential oils.
Due to the fact that fluconazole had the highest apparent solubility values in the essential oils of clove, oregano and cinnamon, these were selected for the more accurate quantification of their dissolution capacity for fluconazole. From the analysis of the solubility data obtained for the three essential oils (Table 4), it was found that oregano essential oil had the highest dissolution capacity of FZ (242.47 ± 0.91 mg/mL), followed by clove essential oil (140.56 ± 0.52 mg/mL) and cinnamon essential oil (90.18 ± 0.73 mg/mL) (Table 4).

Table 4.
Values of fluconazole solubility in various essential and synthetic fixed oils.
By comparing the fluconazole solubility values in the two fatty oils (Table 4), it can be observed that isopropyl myristate showed a higher dissolution capacity of the drug than ethyl oleate.
It is known that the surfactant, due to its properties (HLB value, structure, solubility in the microemulsion components), influences the phase behavior and the size of the microemulsion region in the ternary oil–surfactant/cosurfactant–water systems [13,20]. Therefore, a study was conducted on the solubility of the two sucrose ester surfactants in the lipophilic phases, in which fluconazole had the highest solubility coefficients, since these oils were expected to be components of the oily phase of microemulsion formulations.
The solubility data of sucrose laurate and sucrose palmitate in the three essential oils (CLV, CIN and ORG) and isopropyl myristate are listed in Table 5 in the form of apparent solubility and therefore they were used only for guidance.

Table 5.
The apparent solubility of sucrose laurate and sucrose palmitate in the oil phase components of the experimental microemulsions.
It can be observed that sucrose laurate is freely soluble in all three tested essential oils, and sucrose palmitate is freely soluble in CLV and ORG and soluble in CIN. The amounts of sucrose palmitate dissolved in CLV and ORG were close and about 1.7 times 4.4 times higher than those of sucrose laurate, respectively. However, sucrose palmitate was dissolved in CIN in an amount of about 5.3 times greater than palmitate. These results partially confirmed our expectations, namely that the sucrose esters solubility in the essential oils increased with the increase in the hydrocarbon chain length of the sucrose ester, only for CLV and ORG. The controversial results obtained for CIN suggest that the sucrose esters solubility also depends on the polarity, and consequently on the chemical composition of the essential oil.
Solubility of sucrose palmitate and laurate in water and several hydrophilic solvents (ethanol, isopropyl alcohol and tetraglycol) are presented in Table 6. In the case of D1616, it can be observed that the lowest solubility value was obtained for water, while the solubility in tetraglycol and ethanol was 178 times and 139 times higher, respectively. Isopropyl alcohol exhibited the highest dissolution capacity of D1616, with a calculated growth factor of 367 (compared to its water solubility).

Table 6.
Solubility of sucrose palmitate and sucrose laurate in the tested solvents, at 25 ± 2 °C.
For D1216, the lowest solubility value was obtained for water, and the highest value for ethanol (2.45 times higher than for water). Moreover, the solubility of sucrose laurate in isopropyl alcohol and tetraglycol was higher than in water by 1.28 and 1.57 times, respectively, but the increase was more modest than in the case of ethanol, which can thus be considered the preferred solvent for this sucrose ester. Comparing the solubility data obtained for the two sucrose esters, D1216 was more soluble than D1616 in all tested solvents.
3.2. Selection of the Microemulsions Components
3.2.1. Selection of the Oil Phase
When choosing the oily phase of the MEs, it was taken into account that the incorporation of essential oils into pharmaceutical preparations presents several technological limitations because of their insolubility in water, and the reactivity and volatility of their bioactive components. Although they are stable thermodynamic systems, MEs can be easily destabilized by dilution with an aqueous phase, if the oil phase was composed of volatile oils. Recent studies have demonstrated that by mixing the essential oil with a fatty oil, the resulting MEs present high stability over dilution with the aqueous phase [31,32]. Consequently, this strategy was approached in our study, that of mixing the essential oil with a fatty oil, selected from the group of synthetic fatty oils with a determined chemical composition and chemical stability (to light, temperature and humidity), higher than that of essential oils.
The essential oils of oregano, clove and cinnamon have been selected as components of the oily phase for the development of microemulsions, as they produced the highest fluconazole solubility values.
Of the two fatty oils selected for screening, isopropyl myristate was selected as the second component of the oily phase of the microemulsions, due to its higher dissolution capacity for fluconazole. In addition, isopropyl myristate, a saturated fatty acid ester (myristic acid) shows higher stability to oxidation than ethyl oleate, the ester of an unsaturated fatty acid (oleic acid).
In order to obtain stable microemulsions with a suitable concentration of essential oil, at which this could promote the percutaneous penetration of fluconazole, IPM and essential oil were mixed in a 1:1 mass ratio.
3.2.2. Selection of Surfactant and Cosurfactant
Selecting the appropriate surfactants is essential, because they should possess some desirable properties, namely: (i) low toxicity—the most critical issue in formulating topical ME systems is the toxicity of their components, which can cause skin irritation. In this study, two sucrose esters (sucrose laurate and sucrose palmitate) belonging to the group of non-ionic natural hydrophilic surfactants have been used. In addition to low toxicity and reduced skin sensitization potential, these surfactants also showed other valuable properties, such as biocompatibility, biodegradability, percutaneous penetration enhancing capacity and high compatibility with the environment; (ii) appropriate HLB value—the efficacy of the surfactant is correlated with the appropriate HLB value, which results in the spontaneous formation of a stable ME. The HLB of 16 of the two sucrose esters ensured the spontaneous formation of oil-in-water (L/H) stable MEs; (iii) increased solubilization capacity of drug and oil—previous studies have shown the ability of sucrose esters to solubilize fatty oils (like isopropyl myristate) and essential oils, as well as various poorly water-soluble substances [17,20]. However, it was also observed that not always, the surfactant having a good solubilization capacity of the drug will have at the same time a good affinity for the oil phase.
In addition, the sucrose esters selected as surfactants also possess pharmacological properties of interest for the formulations to be further evaluated, namely antimicrobial and antimycotic activity [33,34].
The selection of the cosurfactant for the MEs containing sucrose ester was based on the fact that, generally, the addition of cosurfactants in the formulation of these microemulsified systems is favorable because it provides an additional reduction in the interfacial tension, and increases the fluidity of the surfactant film, which curves in different ways, thus extending the area of the microemulsified system [14,20,35,36,37,38]. Since sucrose esters alone are not capable of reducing the interfacial tension insofar, as to favor the formation of MEs [31], the use of a cosurfactant was necessary.
In the present study, the co-surfactant selection criterion was not the usual one, namely its drug dissolution potential, but its dissolution capacity of the surfactant (sucrose ester), because the homogeneous dispersion of the solid hydrophilic surfactant upon mixing with the oily phase was possible only by dissolving the surfactant in a solvent miscible with the lipophilic phase. Therefore, isopropyl alcohol has been selected as cosurfactant, due to its higher dissolution capacity of sucrose esters surfactants [20,34], although the solubility of fluconazole in this solvent is lower than in other commonly polar solvents used as cosurfactants in ME formulations (ethanol, propylene glycol, tetraglycol, PEG 400) [13]. A series of studies have suggested that isopropyl alcohol in the sucrose ester microemulsion plays the role of cosurfactant, as it interacts with the surfactant molecules at the oil–water interface, influencing a great extent their arrangement (wrapping). All these lead to an increased flexibility (fluidity) of the interfacial film, to which the cosurfactant confers an optimal curvature required for spontaneous ME formation [20,39].
Considering that the fluidity of ME limits their topical application, it is commonly recommended to use a thickening agent. Therefore, the 2% acetic acid aqueous solution was selected as the aqueous phase of the ME systems in view of using a natural, biocompatible polymer like chitosan as a gelling agent, which requires an acidic medium for gelation.
3.3. Pseudo-Ternary Phase Diagrams and the Effect of Sucrose Ester Hydrocarbon Chain Length on the Microemulsion Formation
In the formulation of a microemulsion, the construction of the pseudo-ternary phase diagram is an important step, because it allows to determine the concentration range of the components (oil phase, aqueous phase and surfactant/cosurfactant mixture) corresponding to the microemulsion region.
Figure 1, Figure 2 and Figure 3 present the pseudo-ternary phase diagrams for five essential oils/IPM–sucrose ester/IPA–water systems for both Smix ratios 1:1.5 and 1: 2 in the case of cinnamon and oregano essential oils, and only the Smix ratio 1: 2 in the case of clove essential oil.
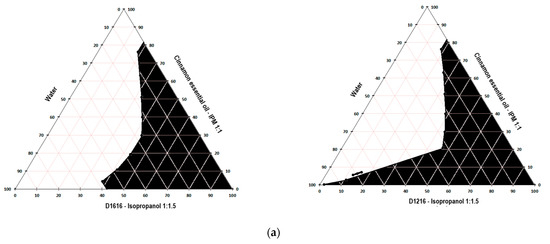
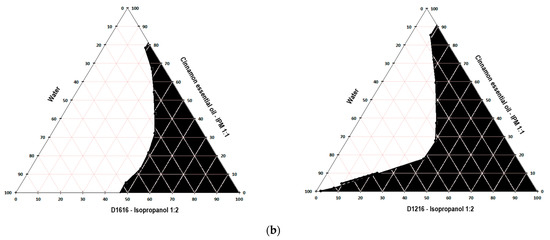
Figure 1.
Pseudo-ternary phase diagrams for the cinnamon essential oil/IPM–D1616/IPA–water and cinnamon essential oil/IPM–D1216/IPA–water systems, for Smix 1:1.5 (a) and 1:2 (b).
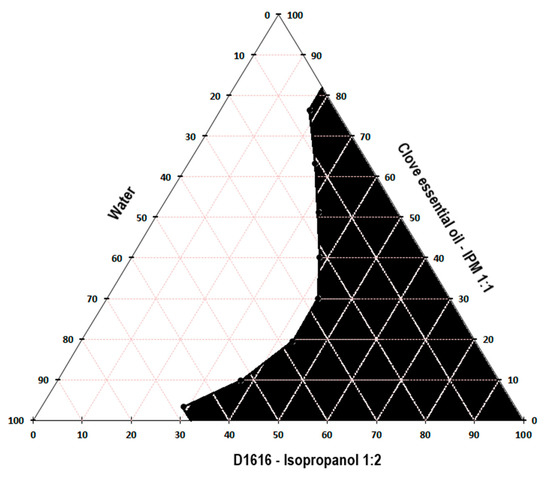
Figure 2.
Pseudo-ternary phase diagram for the clove essential oil/IPM–D1616/IPA–water system, for Smix ratio 1:2.
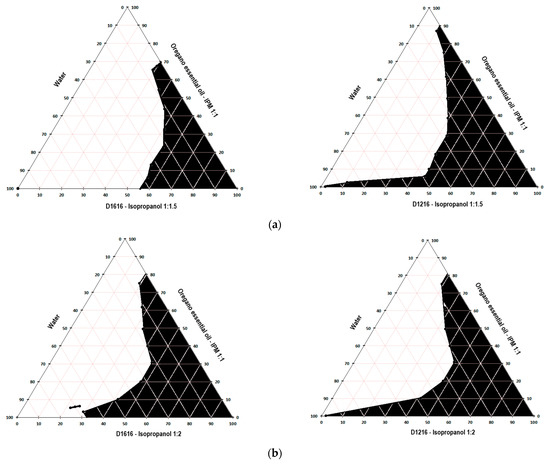
Figure 3.
Pseudo-ternary phase diagrams for the oregano essential oil/IPM–D1616/IPA–water and oregano essential oil/IPM–D1216/IPA–water systems, for Smix 1:1.5 (a) and 1:2 (b).
The black colored area marked in the diagrams represent the microemulsion region. It was not possible to construct the pseudo-ternary phase diagrams for the clove essential oil/IPM–D1616/IPA–water system, where the Smix ratio was 1:1.5 and the clove essential oil/IPM–D1216/IPA–water system for both Smix ratios (1:1.5 and 1:2), because the oil phase-Smix mixtures were opalescent prior to the dilution with water in the microtitration plate. The opacity of these mixtures was most likely produced by the much lower solubility of the surfactant in the oily phase (essential oil–isopropyl myristate 1:1) and Smix. Thus, based on the data indicated in Table 5, it can be stated that the solubility of sucrose palmitate in the mixture of clove essential oil–IPM (1:1) is lower than its solubility in the pure essential oil (approximately 658 mg/mL), caused by the addition of isopropyl myristate, in which D1616 is poorly soluble (about 1.82 mg/mL). Similarly, sucrose laurate, very poorly soluble in isopropyl myristate (Table 5), shows less solubility in the oily phase than in the clove essential oil. However, the Smix of D1616/IPA in the 1:2 ratio was clear, allowing the construction of the pseudo-ternary phase diagram, because the solubility of sucrose palmitate in the clove essential oil is considerable higher than that of sucrose laurate (Table 5), and the presence of isopropanol in a larger proportion contributed to maintain D1616 dissolved.
Considering that the two sucrose esters possess the same HLB value, a similar reduction in the oil–water interfacial tension would be expected for the microemulsion formulations with the same oil phase and having the same surfactant content. However, the pseudo-ternary phase diagrams depicted in Figure 1, Figure 2 and Figure 3 show differences among the microemulsion regions, which suggest the influence of the surfactant’s chemical structure (especially the hydrocarbon chain length of the fatty acid) on the phase behavior of the essential oil/sucrose ester–isopropanol/water mixtures, probably by modifying the arrangement of the surfactant molecules at the oil–water interface, as mentioned before.
Comparing the size of the microemulsion zones, from the obtained pseudo-ternary phase diagrams, it can be seen that sucrose laurate produced slightly larger microemulsion areas than sucrose palmitate. Thus, in the series of the systems containing cinnamon essential oil, the microemulsion regions produced by sucrose laurate for both Smix ratios (1:1.5 and 1:2) occupied 49.16% and 56.49% of the diagram surface, respectively, while the microemulsion regions produced by sucrose palmitate at the same Smix ratios were lower (43.49% and 35.94%, respectively). Similarly, in the series of the systems containing oregano essential oil, D1216 produced for both Smix ratios larger microemulsion areas (45.32% and 45.12%, respectively) than D1616 (28.44% and 42.39%, respectively). Additionally, it can be seen that by increasing the concentration of the cosurfactant versus that of the surfactant in the Smix (from 1:1.5 to 1:2), the size of the microemulsion area produced by D1216 increased slightly (1.15 fold) or remained constant for the systems with essential oils of cinnamon or oregano. In contrast, in the case of D1616, the increase in the Smix of the cosurfactant concentration versus that of the surfactant produced different effects on the size of the microemulsion area, which decreased 1.2 times for the system with cinnamon essential oil and increased 1.5 times for the system with oregano essential oil.
These results can be attributed to the ability of sucrose laurate to form a less sensitive interface film to water-dilution. It is well known that the emulsification capacity of surfactants decreases with the increase in the hydrocarbon chain length, because a long chain makes the interface more rigid and reduces the surfactant’s ability to bind the aqueous phase [40,41]. Because the sucrose esters possess the same polar group, but differ in hydrocarbon chain lengths, the steric repulsions of the polar group should not affect the size of the microemulsion region. However, it has been observed that increasing the chain length of the sucrose esters will reduce the area of the microemulsion [20,40]. Moreover, these results can be explained also based on the sucrose esters solubility in water, isopropyl myristate and cosurfactant (isopropyl alcohol). As indicated in Table 6, the solubility of sucrose palmitate in water and isopropyl alcohol was approximately 585 and 2.5 times lower, respectively, than the solubility of sucrose laurate in the same solvents. In a previous study, Todosijević et al. showed that the two sucrose esters have comparable solubility values in isopropyl myristate [20]. As expected, the solubility in water has decreased with the increase in the alkyl chain length in the surfactant structure. Consequently, the decrease in the microemulsion region in the case of D1616 can be a consequence of its lower solubility in water, which will play an important role in determining the amount of the incorporated water.
In addition, significant differences can be observed with regard to the size of the microemulsion area obtained in the pseudo-ternary phase diagram for the systems containing the same surfactant, the same Smix ratio, but different essential oils. Thus, D1216 produced, for both Smix ratios, slightly larger microemulsion regions in the presence of cinnamon essential oil than in the case of oregano essential oil. Instead, D1616 at Smix 1:1.5 led to a 1.53-fold larger microemulsion area in the presence of cinnamon essential oil than that of oregano essential oil. In addition, for the MEs containing D1616 and Smix 1:2, the system containing clove essential oil produced the largest microemulsion area (45.47%) in the phase diagram, followed closely by the systems with oregano essential oil (42.39%), while the area occupied by the microemulsion region for the system with cinnamon essential oil was only 35.94%. The coefficient of their solubility and implicitly the affinity of the two sucrose esters for the essential oil contribute most likely, together with other factors to the occurrence of these differences. Thus, sucrose laurate, with an apparent solubility in the cinnamon essential oil about 2.8 times higher than that of oregano (Table 6), exhibits greater affinity than the former, as well as a greater binding/solubilizing capacity for the essential oil. Similarly, sucrose palmitate, being easily soluble in the essential oils of clove and oregano (Table 6), has higher solubility in these essential oils than in cinnamon essential oil. Consequently, these results can be included in the group of those who emphasize the importance of the simultaneous evaluation of three factors, namely the surfactants’ HLB value, structure and solubility in the system components, factors which influence the formation of MEs.
Considering all the above, the microemulsion formulations intended for further evaluation were selected from the L/H microemulsion regions of the pseudo-ternary phase diagrams constructed for the following systems: cinnamon essential oil/IPM–D1616/IPA–water, with the Smix ratio 1:1.5; cinnamon essential oil/IPM–D1216/IPA–water, with the Smix ratio 1:2; oregano essential oil/IPM–D1616/IPA–water, with the Smix ratio 1:2; oregano essential oil/IPM–D1216/IPA–water, with the Smix ratio 1:1.5; clove essential oil/IPM–D1616/IPA–water, with the Smix ratio of 1:2. In the pseudo-ternary phase, the diagrams obtained for these systems, the L/H microemulsion region occupied the largest areas.
3.4. Formulation of FZ-Loaded Microemulsions Based on Essential Oils and Sucrose Esters
The criteria on which the component proportions (%) established in the microemulsion formulations were as follows: (i) the internal oily phase should dissolve the desired concentration of the drug; (ii) the oily phase should permit a concentration of 3% and 5% of volatile oil in the final microemulsion, respectively, since for this concentration range the effect of increasing the percutaneous penetration of some drugs has been reported in the literature [42,43,44]; (iii) the surfactant–cosurfactant mixture should be at the minimum concentration required for the solubilization of the oily phase and for obtaining stable and well tolerated systems for topical application; this criterion was also established on the basis of the results of previous studies, that when MEs are used as enhancers of percutaneous penetration, the formulations containing the largest amount of surfactant, do not usually provide the maximum drug transfer rate [13,15,20,31,32,35]. Based on these criteria, from each pseudo-ternary phase diagram constructed for the five ternary systems listed above, two formulations, for each essential oil, containing 6% or 10% internal oily phase, 45% Smix and 49% or 45% water, were selected. It was considered that selecting the 10 formulations would permit the further study of the components effect, especially of the essential oil and surfactant, on microemulsion characteristics.
3.5. Characterization of the FZ-Loaded Microemulsions Based on Essential Oils and Sucrose Esters
At macroscopic examination, the studied MEs were homogeneous, fluid, transparent, colorless or slightly yellowish systems, with the characteristic odor of the strong odorants (essential oil and acetic acid). The eventual coloration was due to the presence of the essential oil and sucrose palmitate in their composition.
The results of the physical characterization of the experimental MEs are presented in Table 7.

Table 7.
Mean droplet size, polydispersity index (PDI), viscosity, refractive index, zeta potential and pH of the experimental fluconazole-loaded biocompatible microemulsions.
To assess the size, size distribution and polydispersity of ME oil droplets, dynamic light diffusion (DLS) technique can be used. The mean droplet size for the experimental MEs is shown in Table 7, and the droplet size distribution, as a function of the relative intensity of the light scattered by the particles, is shown in Figure 4, Figure 5 and Figure 6.
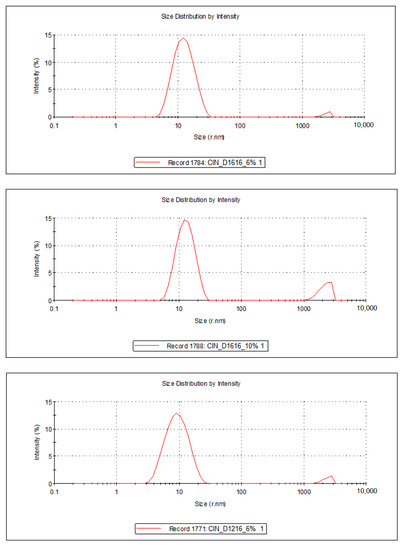
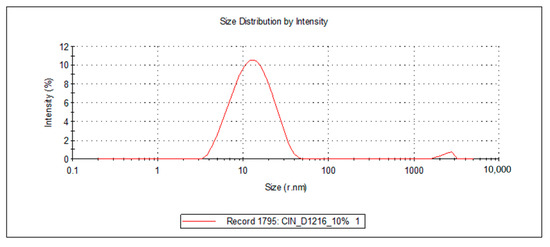
Figure 4.
Droplet size distribution of the FZ-loaded microemulsions based on cinnamon essential oil and sucrose ester (sucrose palmitate or laurate).
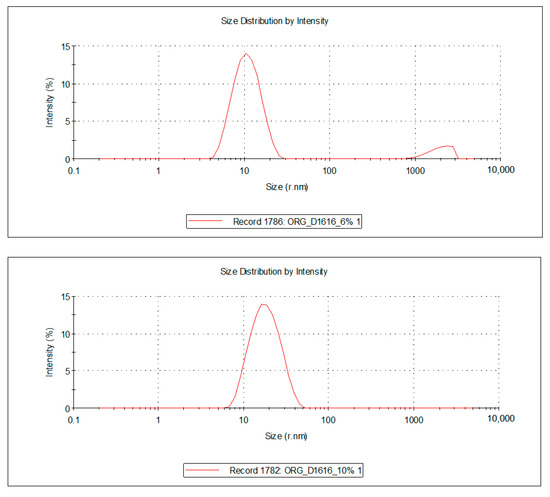
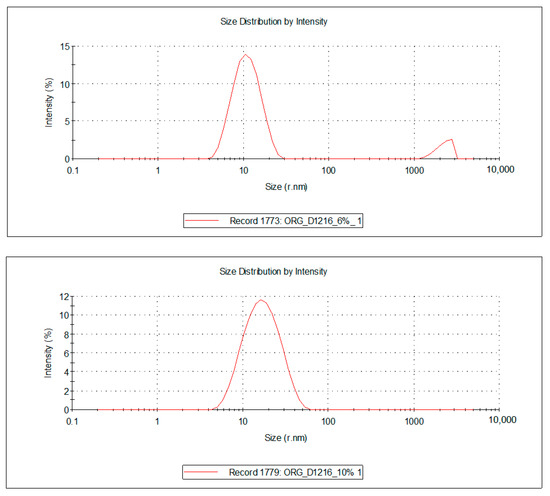
Figure 5.
Droplet size distribution of the FZ-loaded microemulsions based on oregano essential oil and sucrose ester (sucrose palmitate or laurate).
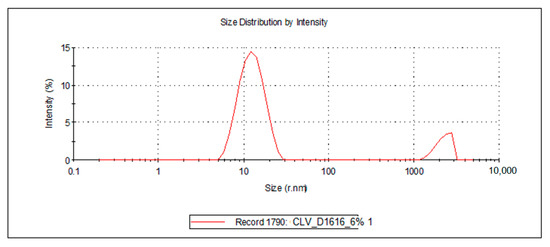
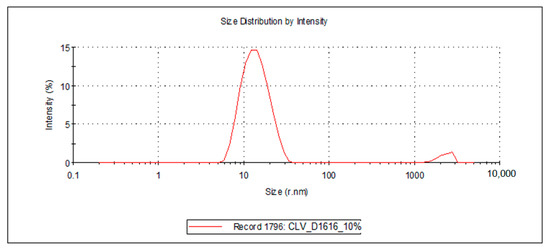
Figure 6.
Droplet size distribution of the FZ-loaded microemulsions based on clove essential oil and sucrose palmitate.
For the 10 microemulsion formulations, the PDI values ranged from 0.129 to 0.363, and the hydrodynamic rays, rh (through intensity, I), were relatively low, having values ranging from 9.9 to 19.2 nm. The lower the PDI, the more stable the system is. In addition, due to the small values of the mean droplet size in all studied MEs, it can be stated that their specific surface area is high; therefore, a better contact between the oil droplets and membrane/skin is achieved, resulting in an increased concentration gradient and improved fluconazole permeability.
For the ten samples of analyzed MRs, the following hierarchy of hydrodynamic rays, rh (I), can be stated: rh ME-FZ-D1616-ORG 10% (19.2 nm) > rh ME-FZ-D1216-ORG 10% (18.2 nm) > rh ME-FZ-D1616-CLV 10% (14.3 nm) > rh ME-FZ-D1216-CIN 10% (14.2 nm) > rh ME-FZ-D1616-CIN 10% (13.3 nm) > rh ME-FZ-D1616-CIN 6% (13.0 nm) > rh ME-FZ-D1616-CLV 6% (12.9 nm) > rh ME-FZ-D1216-ORG 6% (11.5 nm) > rh ME-FZ-D1616-ORG 6% (11.3 nm) > rh ME-FZ-D1216-CIN 6% (9.9 nm).
It can be observed that the MEs with a 10% oil phase containing oregano essential oil presented the largest oil droplets (rh = 19.2 nm for ME-FZ-D1616-ORG 10% formulation; 18.2 nm for ME-FZ-D1216-ORG 10% formulation) (Table 7). Size distribution for these systems exhibits a monomodal behavior (Figure 5) and a small PDI (0.129 for ME-FZ-D1616-ORG 10%; 0.171 for ME-FZ-D1216-ORG 10%) (Table 7). The fact that the PDI for the ME-FZ-D1616-ORG 10% formulation showed the smallest value indicates the highest homogeneity of the oil droplets. For the other microemulsion formulations, the size distribution showed a bimodal behavior (Figure 4, Figure 5 and Figure 6). On the one hand, a majority population (I = 87.1%–98.1%) of small oil droplets (rh = 9.9–14.3 nm) is obtained, depending on the nature of the surfactant or essential oil, as well as on the essential oil concentration. On the other hand, there is a minor population (I = 1.9%–13.0%) of large size droplets (rh = 2007–2515 nm), that may be attributed to the presence of an impurity in the system (i.e., dust). For this reason, the PDI values (PDI = 0.197–0.363) of the samples showing bimodal distribution are higher than those of the systems with monomodal distribution.
The zeta potential values measured for the ten experimental MEs ranged between +0.05 and +1.05 mV (Table 7). These results indicate the formation of MEs with an electrical charge density close to zero. Generally, a colloidal system containing cationic or anionic surfactants is stable if the zeta potential is greater than +30 or −30 mV. Since the analyzed microemulsion systems contain non-ionic surfactants, the zeta potential was near zero. Considering only the values of this parameter, it might be suggested that these systems are unstable. However, the results of particle size determination on clear, homogeneous samples that have maintained this status throughout the 6 month follow-up period (including the time of DLS measurements), demonstrate the formation of stable colloidal systems. In conclusion, it can be stated that the analyzed MEs are stabilized by dispersion forces, steric interactions or other weak forces.
The viscosity of all analyzed systems did not change with the change in shear stress, confirming the Newtonian character of the formulations. For all the experimental MEs, the viscosity values were small, ranging from 4 to 12 mPa·s, which was expected for this fluid pharmaceutical form. The MEs based on clove essential oil and sucrose palmitate (ME-FZ-D1616-CLV 6% and ME-FZ-D1616-CLV 10%) and the formulation with oregano essential oil and sucrose laurate, FZ-D1216-ORG 10%, produced the highest viscosity value (12 mPa·s), followed by the MEs based on essential cinnamon or oregano oil and sucrose laurate, with 6% oily phase (formulations ME-FZ-D1216-CIN 6% and ME-FZ-D1216-ORG 6%), whose viscosity was 1.5 times lower (Table 7). Unlike the MEs based on clove essential oil and sucrose palmitate, the formulations with cinnamon or oregano essential oil and the same surfactant (ME-FZ-D1616-CIN 6% and ME-FZ-D1616-CIN 10%; and ME-FZ-D1616-ORG 6% and ME-FZ-D1616-ORG 10%, respectively) exhibited of 1.8-fold lower (6.67 mPa·s) and 3-fold lower (4 mPa·s) viscosity values, respectively (Table 7). These results indicate the influence of the nature (composition) of the essential oil and the phase behavior (association structures) of the surfactant on the viscosity of the systems. It is known, that at concentrations between 10% and 35%, sucrose esters form anisotropic systems (liquid crystals) as a result of intermicellar interaction, and the micelles formation and the interactions among them are influenced by the fatty acid chain length from the sucrose ester structure.
The refractive index is the parameter that shows the isotropic character of the formulations. For all tested MEs, the mean refractive index values were very close, falling within the narrow range of 1.3862–1.3948 (Table 7). These values were slightly higher than the water refraction index (1.333), most likely due to the presence of the volatile oils, for which this parameter has higher values (1.532 for clove essential oil, 1.59–1.62 for cinnamon essential oil and 1.509 for oregano essential oil).
The pH of the studied fluconazole-loaded microemulsions varied in a very narrow range, from 3.47 ± 0.06 to 3.72 ± 0.03 (Table 7), with insignificant differences between formulations, due to the presence of 2% acetic acid in the aqueous phase of the systems.
4. Conclusions
In conclusion, to the best of our knowledge, this work reports for the first time the successful formulation of oil-in-water biocompatibile microemulsions for the topical delivery of fluconazole, using natural, antifungal essential oils (cinnamon, oregano and clove essential oil) in combination with synthetic isopropyl myristate as oil phase, natural sucrose ester (sucrose laurate or sucrose stearate) as surfactant and isopropyl alcohol as cosurfactant. The solubility studies provided new solubility data for fluconazole and sucrose esters in several essential oils and commonly used pharmaceutical solvents. The ability of sucrose esters to solubilize the essential oil-based lipophilic phase and to form microemulsions has been indicated by large isotropic areas labeled in the pseudoternary-phase diagrams constructed by using the microplate dilution technique. The comparative analysis of pseudoternary-phase diagrams produced by different systems of essential oil/IPM–sucrose ester/IPA–water revealed that both hydrocarbon chain length and the concentration of sucrose ester, as well as the type and concentration of essential oil are important formulation variables when developing stable microemulsions. Biocompatible fluconazole-loaded microemulsion formulations showed acceptable physicochemical properties (droplet size, polydispersity index, refractive index, zeta potential and pH), but their viscosity needs to be increased so that they can be applied on the skin. Moreover, prospective in vitro and in vivo studies regarding their performance, bioavailablity and effectiveness in the treatment of skin fungal infections are justified.
Author Contributions
Conceptualization, L.V., G.C., A.M.M., I.O. and V.V.; methodology, L.V., G.C., A.M.M., I.O., V.V., D.F.A. and M.E.M.; software, I.O., V.V. and A.D.; validation, V.V., A.M.M. and M.H.; writing—original draft preparation, L.V., G.C.; writing—review and editing, I.O., L.V. and G.C.; visualization, A.M.M.; supervision, D.L., L.V. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Robertson, D.B.; Maibach, H.I. Dermatologic Pharmacology. In Basic & Clinical Pharmacology, 14th ed.; Katzung, B.G., Ed.; McGraw-Hill Education: New York, NY, USA, 2018; pp. 1072–1073. [Google Scholar]
- Dias, M.F.; Filho, F.B.; Santos, M.V.Q.; Amorim, A.G.; Schechtman, R.C.; Azulay, D.R. Treatment of superficial mycoses: Review Part II. An. Bras. Dermatol. 2013, 88, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Güngör, S.; Erdal, M.; Aksu, B. New Formulation Strategies in Topical Antifungal Therapy. J. Chem. Dermatol. Sci. Appl. 2013, 3, 56–65. [Google Scholar]
- Cleary, J.D.; Lewis, R.E. Fungal infections. In Applied Therapeutics. The Clinical Use of Drugs, 11th ed.; Zeind, C.S., Carvalho, M.G., Eds.; Wolters Kluwer Health: Philadelphia, PA, USA, 2018; pp. 4179–4231. [Google Scholar]
- Lampiris, H.W.; Maddix, D.S. Antifungal agents. In Basic & Clinical Pharmacology, 14th ed.; Katzung, B.G., Ed.; McGraw-Hill Education: New York, NY, USA, 2018; pp. 853–863. [Google Scholar]
- Zhao, S.S.; Du, Q.; Cao, D.Y. Preparation of liposomal fluconazole gel and in vitro transdermal delivery. J. Chin. Pharm. Sci. 2007, 16, 116–118. [Google Scholar]
- Bhalaria, M.K.; Naik, S.; Misra, A.N. Ethosomes: A novel delivery system for antifungal drugs in the treatment of topical fungal diseases. Indian J. Exp. Biol. 2009, 47, 368–375. [Google Scholar] [PubMed]
- Zakir, F.; Vaidya, B.; Goyal, A.K.; Malik, B.; Vyas, S.P. Development and characterization of oleic acid vesicles for the topical delivery of fluconazole. Drug Deliv. 2010, 17, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Vyas, S.P. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem. Phys. Lipids 2012, 165, 454–461. [Google Scholar] [CrossRef]
- Jadhav, K.R.; Kadam, V.J.; Pisal, S.S. Formulation and evaluation of lecithin organogel for topical delivery of fluconazole. Curr. Drug Deliv. 2009, 6, 174–183. [Google Scholar] [CrossRef]
- Salerno, C.; Carlucci, A.M.; Bregni, C. Study of in vitro drug release and percutaneous absorption of fluconazole from topical dosage forms. AAPS P PharmSciTech 2010, 11, 986–993. [Google Scholar] [CrossRef]
- Lalit, S.K.; Panwar, A.S.; Darwhekar, G.; Jain, D.K. Formulation and evaluation of fluconazole amphiphilogel. Der. Pharm. Lett. 2011, 3, 125–131. [Google Scholar]
- Coneac, G.; Vlaia, V.; Olariu, I.; Muţ, A.M.; Anghel, D.F.; Ilie, C.; Popoiu, C.; Lupuleasa, D.; Vlaia, L. Development and Evaluation of New Microemulsion-Based Hydrogel Formulations for Topical Delivery of fluconazole. AAPS PharmSciTech 2015, 16, 889–904. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2012, 64, 175–193. [Google Scholar] [CrossRef]
- Pappinen, S.; Urtti, A. Microemulsions. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement. Nanocarriers; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin, Germany, 2016; pp. 253–261. [Google Scholar]
- Nastiti, C.M.R.R.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics. 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.C.; Klang, V.; Hoppel, M.; Mahrhauser, D.; Valenta, C. Natural microemulsions: Formulation design and skin interaction. Eur. J. Pharm. Biopharm. 2012, 81, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Szüts, A.; Révész, P.S. Sucrose esters as natural surfactants in drug delivery systems—A mini-review. Int. J. Pharm. 2012, 433, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arzaluz, M.G.N.; Segundo, E.P.; Rondero, A.G. Sucrose Esters as Transdermal Permeation Enhancers. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Dragicevic, N., Maibach, H., Eds.; Springer: Berlin, Germany, 2015; pp. 273–290. [Google Scholar]
- Todosijević, M.N.; Cekić, N.D.; Savić, M.M.; Gašperlin, M.; Ranđelović, M.R.; Savić, S.D. Sucrose ester-based biocompatible microemulsions as vehicles for aceclofenac as a model drug: Formulation approach using D-optimal mixture design. Colloid Polym. Sci. 2014, 292, 3061–3076. [Google Scholar] [CrossRef]
- Marshall, D.L.; BullermanL, B. Antimicrobial Activity of Sucrose Fatty Acid Ester Emulsifiers. J. Food Sci. 1986, 51, 468–470. [Google Scholar] [CrossRef]
- Zhang, X.; Song, F.; Taxipalati, M.; Wei, W.; Feng, F. Comparative Study of Surface-Active Properties and Antimicrobial Activities of Disaccharide Monoesters. PLoS ONE 2014, 9, e114845. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Ahmad, I. Antifungal activity of essential oils and their synergy with fluconazole against drug-resistant strains of Aspergillus fumigatus and Trichophyton rubrum. Appl. Microbiol. Biotechnol. 2011, 90, 1083–1094. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, Y.; Zhang, H.; Liu, P.; Yao, J.; Yao, P.; Chen, J.; Duan, J. Development of essential oils as skin permeation enhancers: Penetration enhancement effect and mechanism of action. Pharm. Biol. 2017, 55, 1592–1600. [Google Scholar] [CrossRef]
- Vostinaru, O.; Heghes, S.O.; Filip, L. Safety Profile of Essential Oils. In Essential Oils. Bioactive Compounds, New Perspectives and Applications; Oliveira, M.S., Silva, S., da Costa, W.A., Eds.; IntechOpen: London, UK, 2020; pp. 45–68. [Google Scholar]
- Pajic, N.B.; Nikolic, I.; Mitsou, E.; Papadimitriou, V.; Xenakis, A.; Randjelovic, D.; Dobricic, V.; Smitran, A.; Cekic, N.; Calija, B.; et al. Biocompatible microemulsions for improved dermal delivery of sertaconazole nitrate: Phase behavior study and microstructure influence on drug biopharmaceutical properties. J. Mol. Liq. 2018, 272, 746–758. [Google Scholar] [CrossRef]
- Fanum, M. Sugar Esters Microemulsions; Elsevier Inc.: Cambridge, UK, 2017. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 10th ed.; Counsil of Europe: Strasbourg, France, 2020; p. 3. [Google Scholar]
- Schmidts, T.; Nocker, P.; Lavi, G.; Kuhlmann, J.; Czermak, P.; Runkel, F. Development of an alternative, time and cost saving method of creating pseudoternary diagrams using the example of microemulsion. Colloid Surf. A Physicochem. Eng. Asp. 2009, 340, 187–192. [Google Scholar] [CrossRef]
- Garti, N.; Aserin, A.; Tiunova, I.; Fanun, M. A DSC study of water behavior in water-in-oil microemulsions stabilized by sucrose esters and butanol. Colloid Surf. A Physicochem. Eng. Asp. 2000, 170, 1–18. [Google Scholar] [CrossRef]
- Ma, Q.; Zhong, Q. Incorporation of soybean oil improves the dilutability of essential oil microemulsions. Food Res. Int. 2015, 71, 118–125. [Google Scholar] [CrossRef]
- Polat, T.; Linhardt, R.J. Syntheses and applications of sucrose-based esters. J. Surfact. Deterg. 2001, 4, 415–421. [Google Scholar] [CrossRef]
- Toro, C.; Sanchez, S.A.; Zanocco, A.; Lemp, E.; Gratton, E.; Gunther, G. Solubilization of lipid bilayers by myristyl sucrose ester: Effect of cholesterol and phospholipid head group size. Chem. Phys. Lipids. 2009, 157, 104–112. [Google Scholar] [CrossRef][Green Version]
- Fanun, M. Phase behavior, transport, diffusion and structural parameters of nonionic surfactants microemulsions. J. Mol. Liq. 2008, 139, 14–22. [Google Scholar] [CrossRef]
- Popovici, I. Microemulsii. In Tehnologie Farmaceutică; Popovici, I., Lupuleasa, D., Eds.; Polirom: Iaşi, Romania, 2017; Volume 2, pp. 286–309. [Google Scholar]
- Heuschkel, S.; Goebel, A.; Neubert, R.H.H. Microemulsions—Modern Colloidal Carrier for Dermal and Transdermal Drug Delivery. J. Pharm. Sci. 2008, 97, 603–631. [Google Scholar] [CrossRef]
- Friberg, S.E.; Aikens, P.A. A Phase Diagram Approach to Microemulsions. In Microemulsions. Properties and Applications; Fanum, M., Ed.; CRC Press Taylor & Francis Group: Bocca Raton, FL, USA, 2009; Volume 144, pp. 1–17. [Google Scholar]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Antimicrobial properties of microemulsions formulated with essential oils, soybean oil, and Tween 80. Int. J. Food Microbiol. 2016, 226, 20–25. [Google Scholar] [CrossRef]
- Fanun, M. Surfactant chain length effect on the structural parameters of nonionic microemulsions. J. Dispers. Sci. Technol. 2008, 29, 289–296. [Google Scholar] [CrossRef]
- Fanun, M. Oil type effect on diclofenac solubilization in mixed nonionic surfactants microemulsions. Colloids Surf. A 2009, 343, 75–82. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2014, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Aqil, M.; Ahad, A.; Ali, A.; Khar, R.K. Basil oil is a promising skin penetration enhancer for transdermal delivery of labetolol hydrochloride. Drug Dev. Ind. Pharm. 2008, 34, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.A. Essential Oils and Terpenes. In Percutaneous Penetration Enhancers; Smith, E.W., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 159–173. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).