Socheongryongtang Modulates Asthma-Related Changes via Modulation of TNF-α and T-bet as well as IFN-γ in an Asthma Murine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Socheongryongtang and Identification of Anti-Asthmatic Compounds
2.2. Animal Experiments
2.3. Ethic Statement
2.4. Broncheoalveolar Fluid (BALF) and Serum Analysis
2.5. Histopathological Analysis
2.6. Immunofluorescent Analysis
2.7. Immunohistochemical (IHC) Analysis
2.8. Statistical Analysis
3. Results
3.1. Identification of Anti-Asthmatic Markers in Socheongryongtang
3.2. Socheongryongtang Effectively Inhibited the Proliferation of WBC and Neutrophil and IgE Overexpression
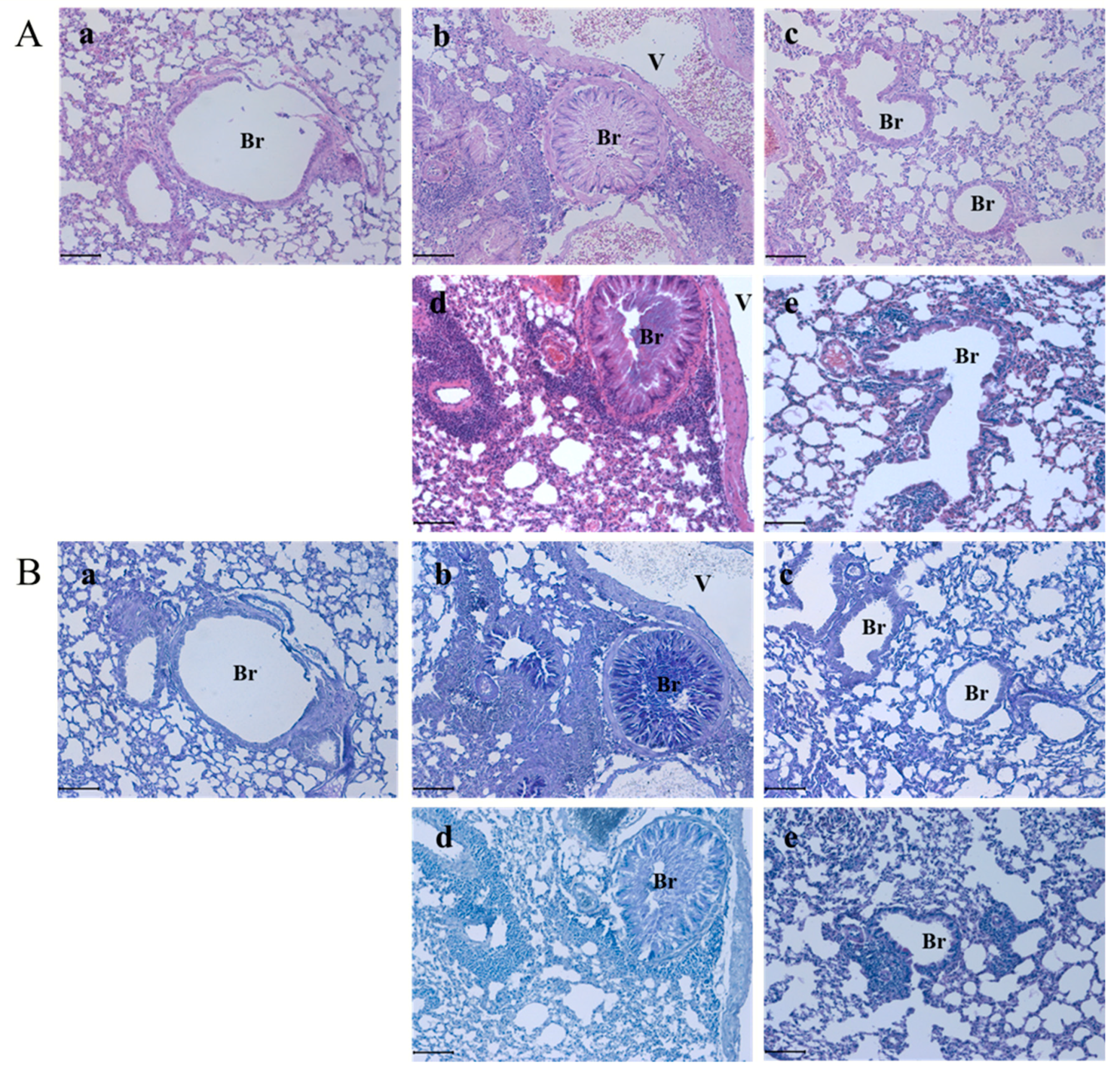
3.3. Socheongryongtang Effectively Prevented Typical Asthmatic Morphological Changes in the Pulmonary System
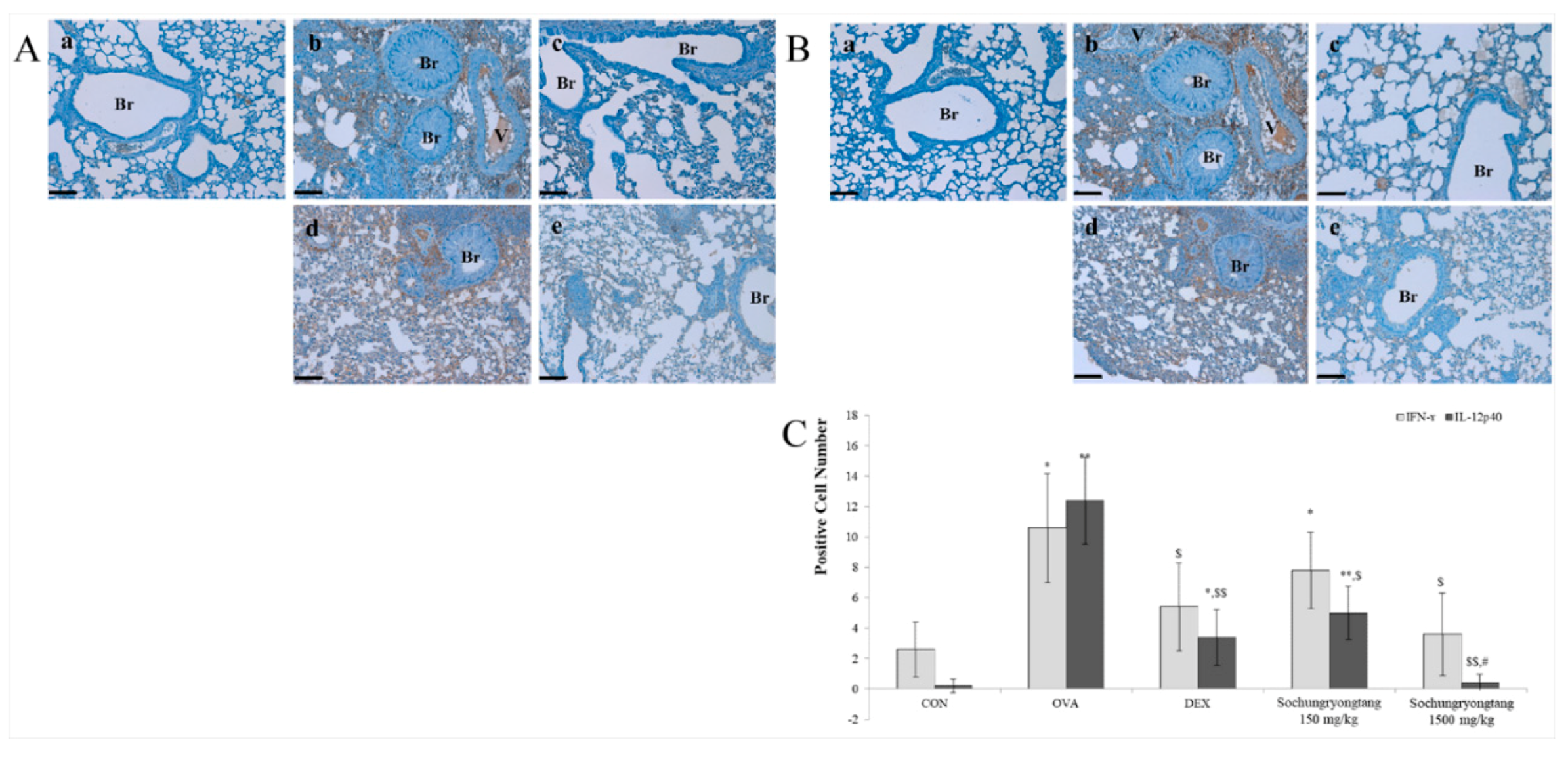
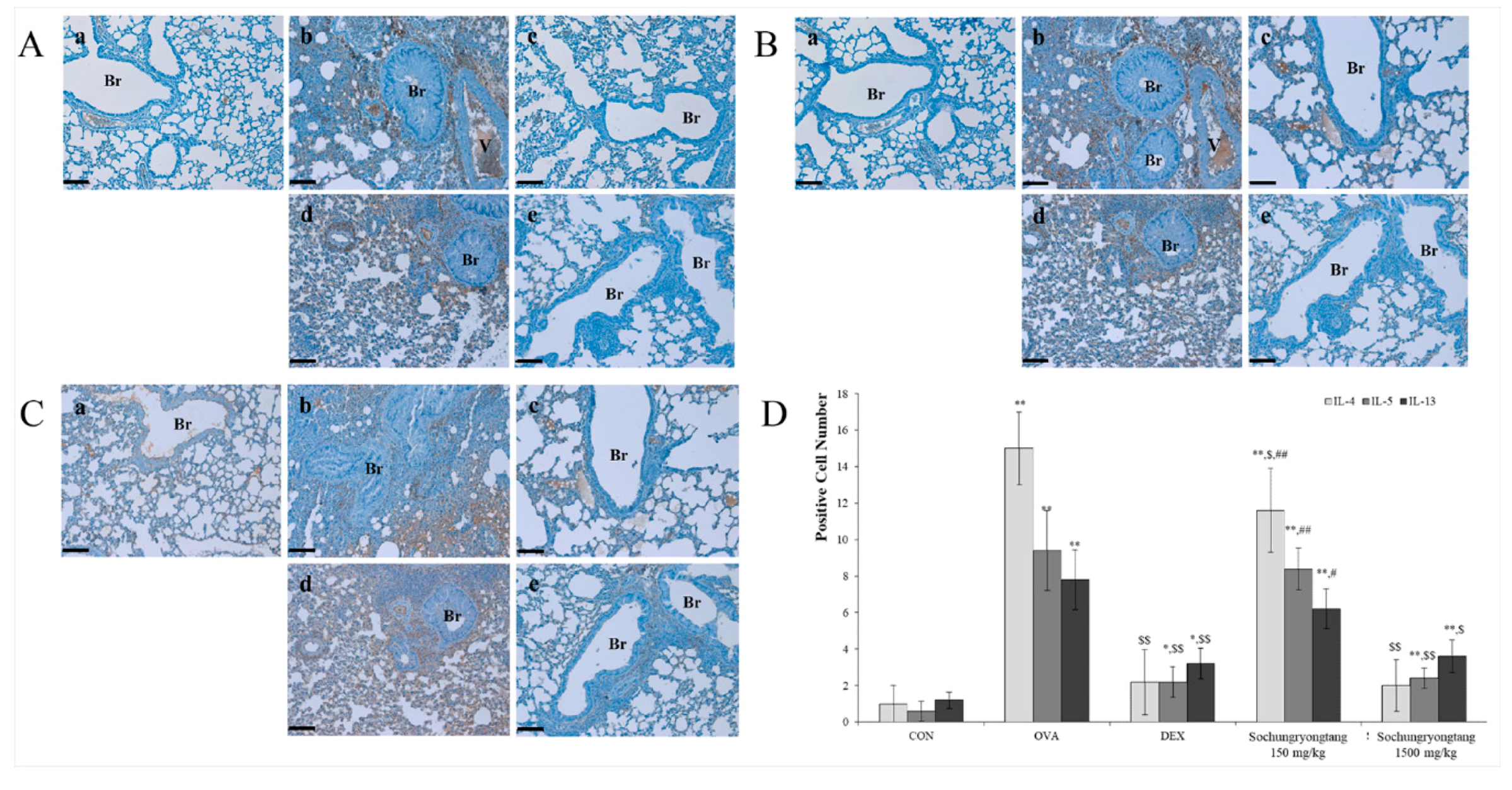
3.4. Socheongryongtang Inhibited Both Activations of Th1 Cell Transcription Factor, T-bet and Th2 Cell Transcription Factor, GATA-3
3.5. Socheongryongtang Suppressed Th1-Related Cytokine Expression
3.6. Socheongryongtang Dose-Dependently Modulated Th2-Related Cytokine: Interleukin 4 (IL-4), IL-5, and IL-13
3.7. Socheongryongtang Dose-Dependently Controlled the Expression Levels of TNF-α but not IL-6
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Asthma Fact Sheet N°307; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Kay, A.B. Allergy and allergic diseases. First of two parts. N. Engl. J. Med. 2001, 344, 30–37. [Google Scholar] [CrossRef] [PubMed]
- National Asthma Education and Prevention Program. Expert panel report: Guidelines for the diagnosis and management of asthma update on selected topics. J. Allergy Clin. Immunol. 2002, 110, S141–S219. [Google Scholar]
- Platts-Mills, T.A.; Vervloet, D.; Thomas, W.R.; Aalberse, R.C.; Chapman, M.D. Indoor allergens and asthma: Report of the third international workshop. J. Allergy Clin. Immunol. 1997, 100, S1–S24. [Google Scholar]
- Burge, H.A.; Rogers, C.A. Outdoor allergens. Environ. Health Perspect. 2000, 108, 653–659. [Google Scholar] [PubMed] [Green Version]
- World Health Organization. The selection and used of essential medicines (2011: Geneva, Switzerland) & World health organization. In World Model List of Essential Medicines: Essential Medicines 17th List; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Wise, J. Corticosteroids for asthma may suppress growth in children in first year of treatment, researchers say. BMJ 2014, 349, g4623. [Google Scholar] [CrossRef]
- Ciriaco, M.; Ventrice, P.; Russo, G.; Scicchitano, M.; Mazzitello, G.; Scicchitano, F.; Russo, E. Corticosteroid-related central nervous system side effects. J. Pharmacol. Pharmacother. 2013, 4, s94–s98. [Google Scholar]
- Lee, S.Y.; Bae, C.S.; Choi, Y.H.; Seo, N.S.; Na, C.S.; Yoo, J.C.; Cho, S.S.; Park, D.H. Opuntia humifusa modulates morphological changes characteristic of asthma via IL-4 and IL-13 in an asthma murine model. Food Nutr. Res. 2017, 61, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Bae, C.S.; Seo, J.H.; Cho, S.S.; Oh, D.S.; Park, D.H. Mycoleptodonoides aitchisoii suppresses asthma via IL-6 and IL-13 in ovalbumin-induced asthma mouse model. Mol. Med. Rep. 2018, 17, 11–20. [Google Scholar]
- Seo, J.W.; Cho, S.C.; Park, S.J.; Lee, E.J.; Lee, J.H.; Han, S.S.; Pyo, B.S.; Park, D.H.; Kim, B.H. 1′-Acetoxychavicol acetate isolated from Alpinia galanga ameliorates ovalbumin-induced asthma in mice. PLoS ONE 2013, 8, e56447. [Google Scholar] [CrossRef]
- Seo, J.H.; Bang, M.A.; Cho, S.S.; Park, D.H. Erythronium japonicum significantly suppresses OVA-induced asthma via upregulation the IFN-γ expression and downregulation the expression of TNF-α and IL-4. Int. J. Mol. Med. 2016, 37, 1221–1228. [Google Scholar] [CrossRef] [Green Version]
- Huh, J. Donguibogam; Bubinmunhwasa: Seoul, Korea, 2002. [Google Scholar]
- Kumar, R.K.; Webb, C.C.; Herbert, C.; Foster, P.S. Interferon-gamma as a possible target in chronic asthma. Inflamm. Allergy Drug Targets 2006, 5, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Hamaza, T.; Barnett, J.B.; Li, B. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int. J. Mol. Sci. 2010, 11, 789–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busse, W.; Banks-Schlegel, S.P.; Larsen, G.L. Childhood- versus Adult-onset asthma. Am. J. Respir. Crit. Care Med. 1995, 151, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Irvin, C.; Zafar, I.; Good, J.; Rollins, D.; Christianson, C.; Gorska, M.M.; Martin, R.J.; Alam, R. Increased frequency of dual-positive Th2/Th17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J. Allergy Clin. Immunol. 2014, 134, 1175–1186. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T.R.; Coffiman, R.L. Th1 and Th2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Lazarevic, V.; Glimcher, L.H. T-bet in disease. Nat. Immunol. 2011, 12, 597–606. [Google Scholar] [CrossRef]
- Zhu, J.; Jankovic, D.; Oler, A.J.; Wei, G.; Sharma, S.; Hu, G.; Guo, L.; Yagi, R.; Yamane, H.; Punkosdy, G.; et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 2012, 37, 660–673. [Google Scholar] [CrossRef] [Green Version]
- Mattes, J.; Yang, M.; Mahalingam, S.; Kuehr, J.; Webb, D.C.; Simson, L.; Hogan, S.P.; Koskinen, A.; Mckenzie, A.N.J.; Dent, L.A.; et al. Intrinsic defect in T cell production of Interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J. Exp. Med. 2002, 195, 1433–1444. [Google Scholar] [CrossRef]
- Manetti, R.; Parronchi, P.; Giudizi, M.G.; Piccinni, M.P.; Maggi, E.; Trinchieri, G.; Romagnani, S. Natural killer cell stimulatory factor (interleukin 12 (IL-12)) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4 producing Th cells. J. Exp. Med. 1993, 177, 1199–1204. [Google Scholar] [CrossRef] [Green Version]
- Yagi, R.; Zhu, J.; Paul, W.E. An updated view on transcription factor GATA3-mediated regulation on Th1 and Th2 cell differentiation. Int. Immunol. 2011, 23, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Larche, M.; Robinson, D.S.; Kay, A.B. The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 2003, 111, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; In, K.H. Immunopathogenesis of asthma. Tuberc. Respir. Dis. 2006, 60, 379–390. [Google Scholar] [CrossRef]
- Hershey, G.K. IL-13 receptors and signaling pathways: An evolving web. J. Allergy Clin. Immunol. 2003, 111, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Rankin, J.A.; Picarella, D.E.; Geba, G.P.; Temann, U.A.; Prasad, B.; DiCosmo, B.; Tarallo, A.; Stripp, B.; Whitsett, J.; Flavell, R.A. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: Lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc. Natl. Acad. Sci. USA 1996, 93, 7821–7825. [Google Scholar] [CrossRef] [Green Version]
- Wills-Karp, M.; Luyimbazi, J.; Xu, X.; Schofield, B.; Neben, T.Y.; Karp, C.L.; Donaldson, D.D. Interleukin-13: Central mediator of allergic asthma. Science 1998, 282, 2258–2261. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Homer, R.J.; Wang, Z.; Chen, Q.; Geba, G.P.; Wang, J.; Zhang, Y.; Elias, J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999, 103, 779–788. [Google Scholar] [CrossRef] [Green Version]
- Scheurich, P.; Thoma, B.; Ucer, U.; Pfizenmaier, K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: Induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J. Immunol. 1987, 138, 1786–1790. [Google Scholar]
- Berry, M.; Brightling, C.; Pavord, I.; Wardlaw, A. TNF-alpha in asthma. Curr. Opin. Pharmacol. 2007, 7, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Rincon, M.; Irvin, C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef] [Green Version]
- Bang, M.A.; Seo, J.H.; Seo, J.W.; Jo, G.H.; Jung, S.K.; Yu, R.; Park, D.H.; Park, S.J. Bacillus subtilis KCTC 11782BP-produced alginate oligosaccharide effectively suppresses asthma via T-helper cell type 2-related cytokines. PLoS ONE 2015, 10, e0117524. [Google Scholar] [CrossRef]
- Bousquet, J.; Chanez, P.; Lacoste, J.Y.; Barneon, G.; Ghavanian, N.; Enander, I.; Venge, P.; Ahlstedt, S.; Simony-Lafontaine, J.; Godard, P. Eosinophilic inflammation in asthma. N. Engl. J. Med. 1990, 323, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Monteseirin, J. Neutrophils and asthma. J. Investig. Allergol. Clin. Immunol. 2009, 19, 340–354. [Google Scholar] [PubMed]
- Burrows, B.; Martinez, F.D.; Halonen, M.; Barbee, R.A.; Cline, M.G. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N. Engl. J. Med. 1989, 320, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Bradding, P.; Symon, F.A.; Holgate, S.T.; Wardlaw, A.J.; Pavord, I.D. Mast-cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002, 346, 1699–1705. [Google Scholar] [CrossRef]
- Bentley, J.K.; Hershenson, M.B. Airway smooth muscle growth in asthma: Proliferation, hypertrophy, and migration. Proc. Am. Thorac. Soc. 2008, 5, 89–96. [Google Scholar] [CrossRef]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Slejko, J.F.; Ghushchyan, V.H.; Sucher, B.; Globe, D.R.; Lin, S.L.; Globe, G.; Sullivan, P.W. Asthma control in the United States, 20082–010: Indicators of poor asthma control. J. Allergy Clin. Immunol. 2014, 133, 1579–1587. [Google Scholar] [CrossRef]
- Ariza, A.; Fernandez, T.D.; Dona, I.; Aranda, A.; Blanca-Lopez, N.; Melendez, L.; Canto, G.; Blanca, M.; Torres, M.J.; Mayorga, C. Basophil activation after nonsteroidal anti-inflammatory drugs stimulation in patients with immediate hypersensitivity reactions to these drugs. Cytometry A 2014, 85, 400–407. [Google Scholar] [CrossRef]
- Platts-Mills, T.A. The role of immunoglobulin E in allergy and asthma. Am. J. Respir. Crit. Care Med. 2001, 164, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Russell, T.D.; Yan, Q.; Fan, G.; Khalifah, A.P.; Bishop, D.K.; Brody, S.L.; Walter, M.J. IL-12p40 homodimer-dependent macrophages chemotaxis and respiratory viral inflammation are mediated through IL-12 receptor beta 1. J. Immunol. 2003, 171, 6866–6874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jana, M.; Dasgupata, S.; Saha, R.N.; Liu, X.; Pahan, K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J. Neurochem. 2003, 86, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Zedan, M.M.; El-Chennawi, F.A.; Fouda, A.E. Interleukin-12 and peripheral blood invariant natural killer T cells as an axis in childhood asthma pathogenesis. Iran. J. Allergy Asthma Immunol. 2010, 9, 43–48. [Google Scholar]
- Wang, J.; Wang, D.; Yu, J.; Liu, C.; Li, L.; Zhang, Y. Isolation of liquiritigenin-4’-apiosylglucoside and liquiritin form the root of Glycyrrhiza uralensis by high-performance centrifugal partition chromatography. J. Chromatogr. Sci. 2014, 52, 310–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Yang, Z.; Yang, S.; Du, J.; Wang, S. Immunoregulatory effects of Paeoniflorin exerts anti-asthmatic effects via modulation of the Th1/Th2 equilibrium. Inflammation 2015, 38, 2017–2025. [Google Scholar] [CrossRef]
- Ram, A.; Mabalirajan, U.; Das, M.; Bhattacharya, I.; Dinda, A.K.; Gangal, S.V.; Ghosh, B. Glycyrrhizin alleviates experimental allergic asthma in mice. Int. Immunopharmacol. 2006, 6, 1468–1477. [Google Scholar] [CrossRef]
- Wang, Q.S.; Gao, T.; Cui, Y.L.; Gao, L.N.; Jiang, H.L. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm. Biol. 2014, 52, 1189–1195. [Google Scholar] [CrossRef]
- Yu, J.Y.; Ha, J.Y.; Kim, K.M.; Jung, Y.S.; Jung, J.C.; Oh, S. Anti-inflammatory activities of licorice extract and its active compounds, glycyrrhizic acid, liquiritin and liquiritigenin, in BV2 cells and mice liver. Molecules 2015, 20, 13041–13054. [Google Scholar] [CrossRef]
- Shin, J.W.; Seol, I.C.; Son, C.G. Interpretation of animal dose and human equivalent dose for drug development. J. Korean Orient. Med. 2010, 31, 1–7. [Google Scholar]
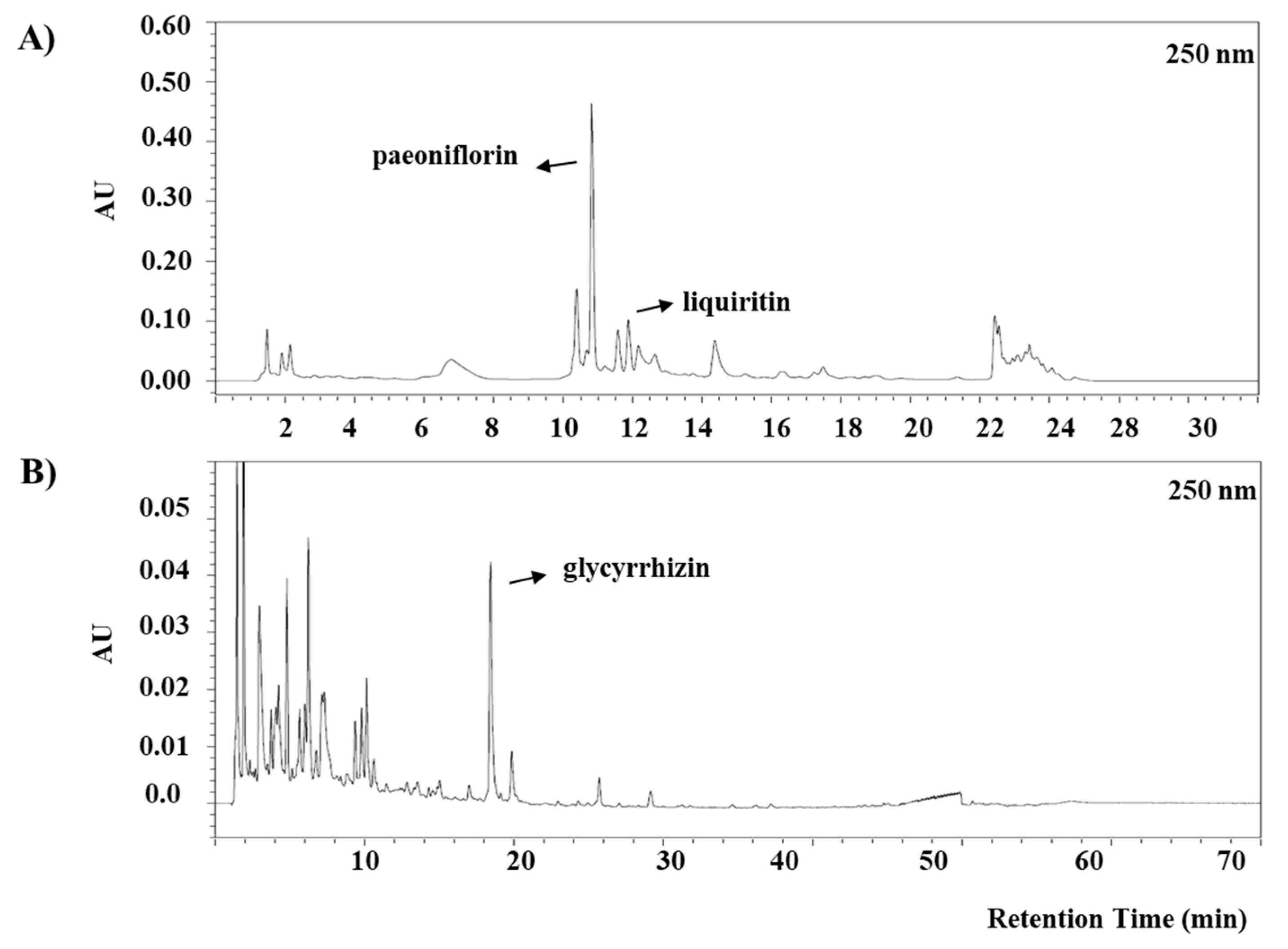



| Parameters | Conditions (Paeoniflorin, Liquiritin) | Conditions (Glycyrrhizin) | ||||
|---|---|---|---|---|---|---|
| Column | Zorbax Extended-C18 (C18, 4.6 mm × 150 mm, 5 µm) | |||||
| Flow Rate | 1 mL/min | |||||
| Injection volume | 10 μL | |||||
| Ultraviolet (UV) detection | 250 nm | |||||
| Run time | 70 min | |||||
| Gradient | Time (min) | % A | % B | Time (min) | % A | % B |
| 0 | 10 | 90 | 0 | 15 | 85 | |
| 7 | 10 | 90 | 35 | 65 | 35 | |
| 8 | 20 | 80 | 45 | 100 | 0 | |
| 20 | 25 | 75 | 50 | 100 | 0 | |
| 21 | 100 | 0 | 55 | 15 | 85 | |
| 25 | 10 | 90 | ||||
| 30 | 10 | 90 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bok, S.-H.; Cho, S.S.; Bae, C.-S.; Kang, B.; Son, H.-S.; Park, D.-H. Socheongryongtang Modulates Asthma-Related Changes via Modulation of TNF-α and T-bet as well as IFN-γ in an Asthma Murine Model. Processes 2020, 8, 1167. https://doi.org/10.3390/pr8091167
Bok S-H, Cho SS, Bae C-S, Kang B, Son H-S, Park D-H. Socheongryongtang Modulates Asthma-Related Changes via Modulation of TNF-α and T-bet as well as IFN-γ in an Asthma Murine Model. Processes. 2020; 8(9):1167. https://doi.org/10.3390/pr8091167
Chicago/Turabian StyleBok, So-Hyeon, Seung Sik Cho, Chun-Sik Bae, Bossng Kang, Hong-Seok Son, and Dae-Hun Park. 2020. "Socheongryongtang Modulates Asthma-Related Changes via Modulation of TNF-α and T-bet as well as IFN-γ in an Asthma Murine Model" Processes 8, no. 9: 1167. https://doi.org/10.3390/pr8091167
APA StyleBok, S.-H., Cho, S. S., Bae, C.-S., Kang, B., Son, H.-S., & Park, D.-H. (2020). Socheongryongtang Modulates Asthma-Related Changes via Modulation of TNF-α and T-bet as well as IFN-γ in an Asthma Murine Model. Processes, 8(9), 1167. https://doi.org/10.3390/pr8091167








