Pharmacological Properties and Chemical Profiles of Passiflora foetida L. Extracts: Novel Insights for Pharmaceuticals and Nutraceuticals
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of Extracts
2.2. Profile of Bioactive Compounds
2.3. Determination of Antioxidant and Enzyme Inhibitory Effects
2.4. Brine Shrimp (Artemia salina) Lethality Test
2.5. Cell Culture
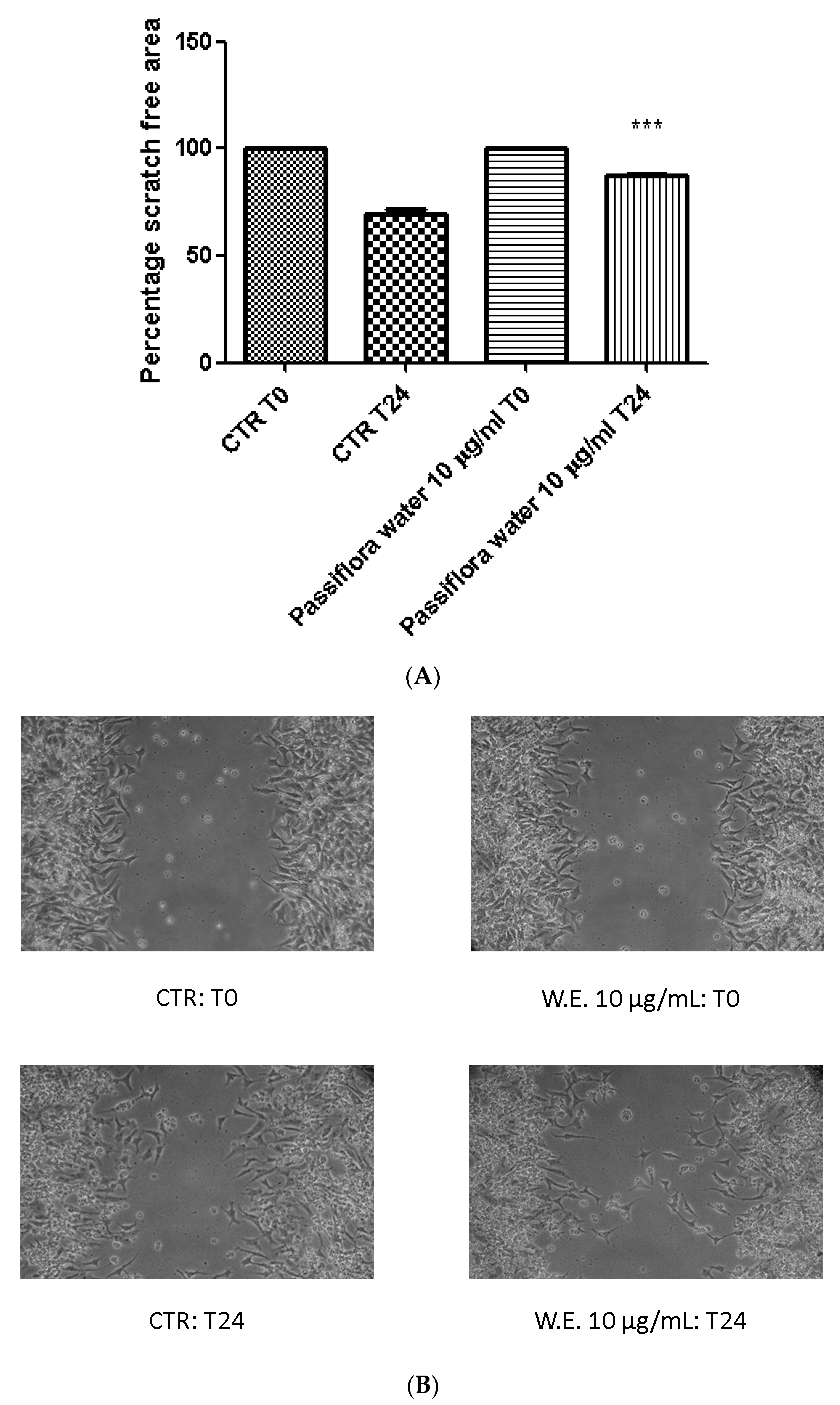
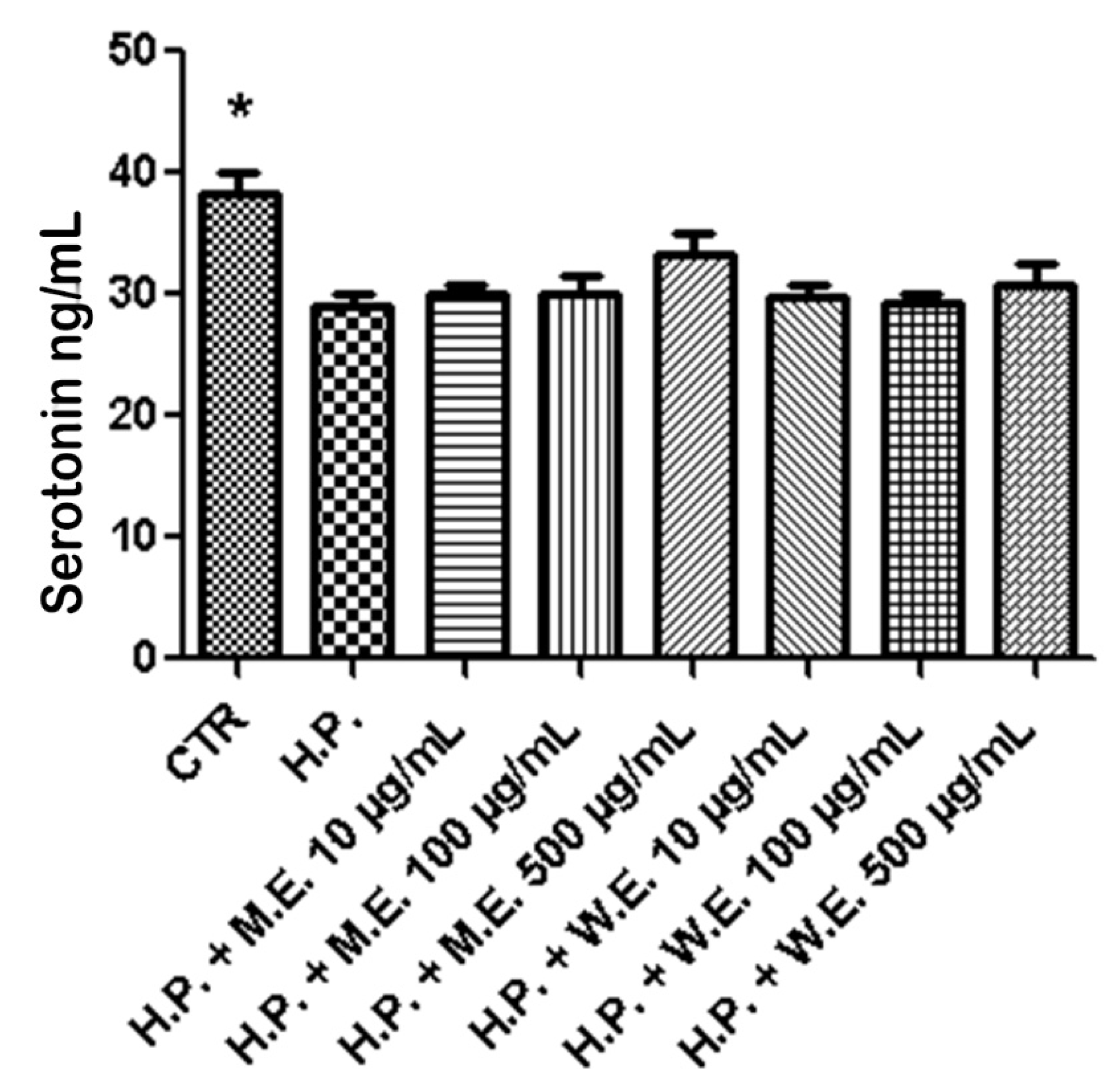
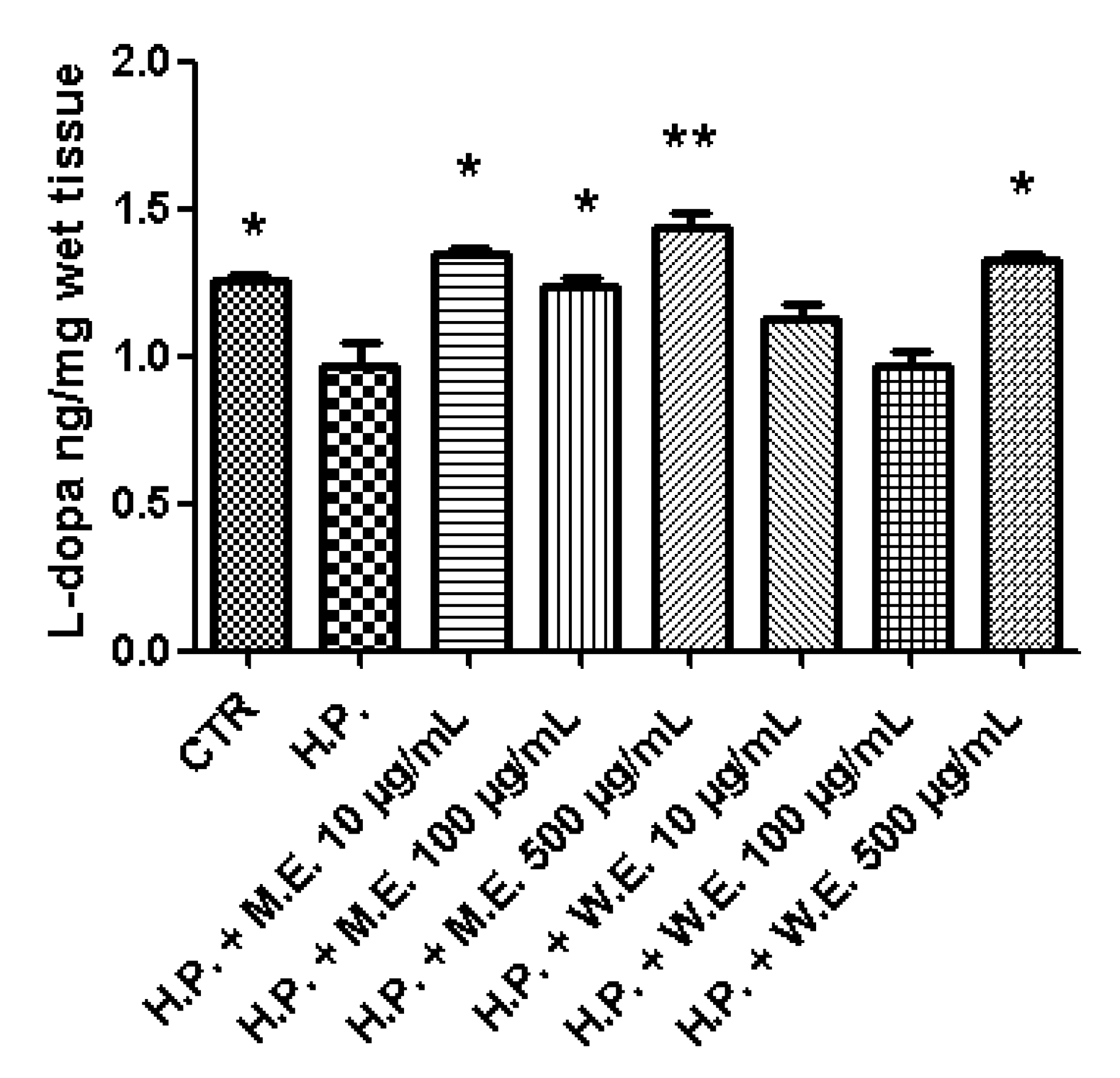
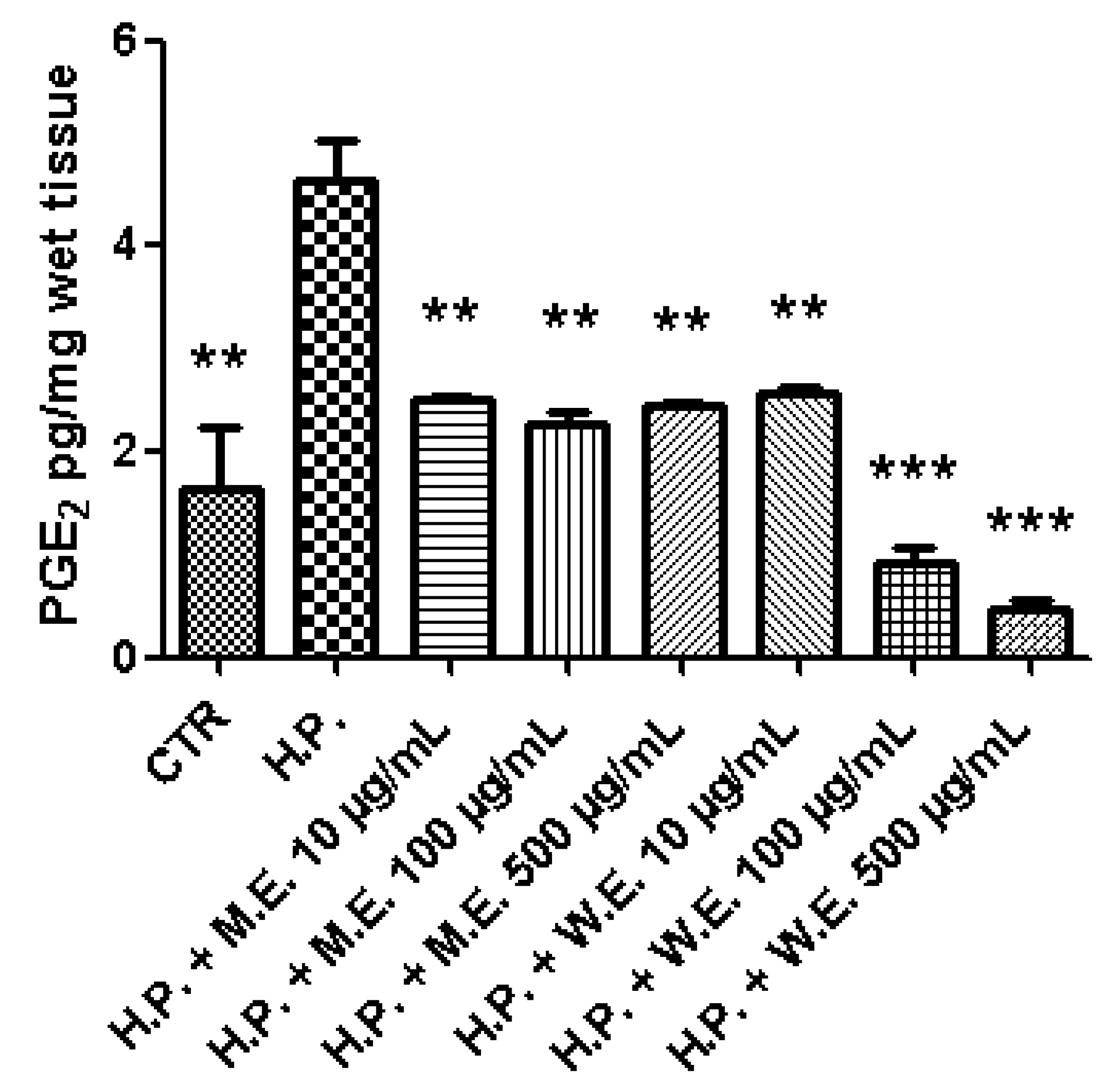
2.6. Ex Vivo Model of LPS-Induced Toxicity in Isolated Mouse Skin Tissue
2.7. HPLC Analysis
2.8. Antifungal Activity of the Extract
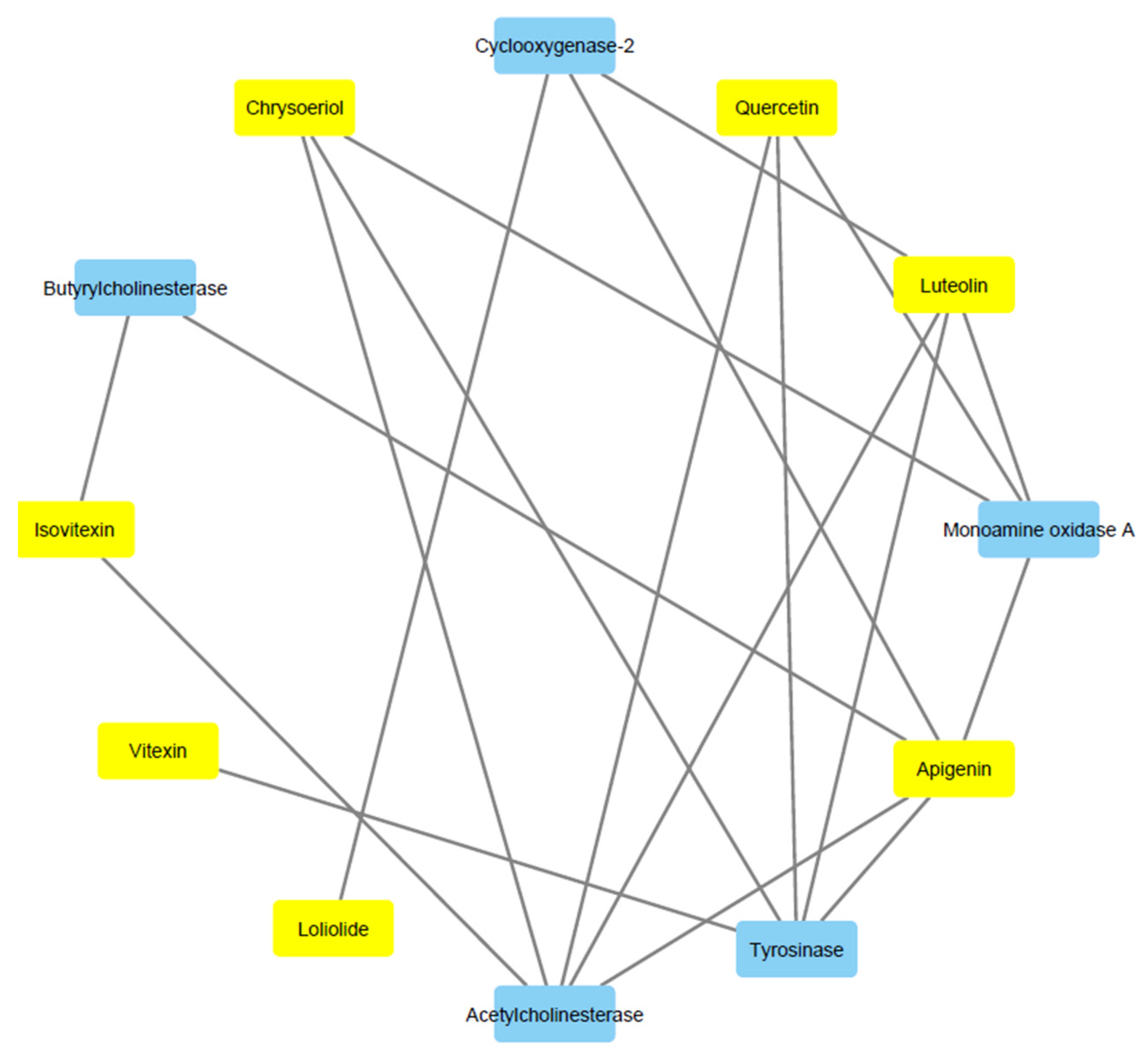
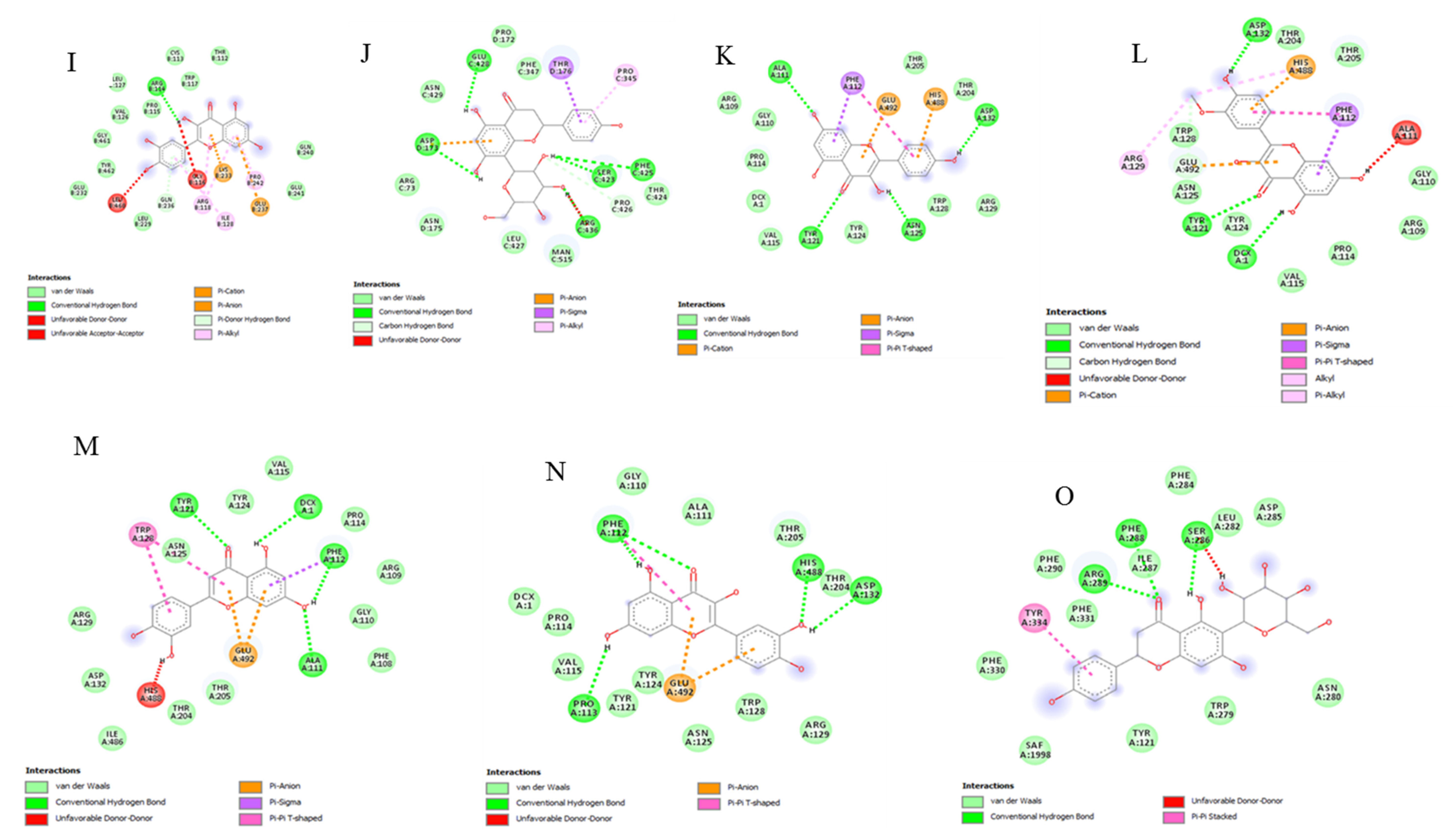
2.9. Bioinformatics and Docking Studies
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Simão, M.J.; Barboza, T.J.; Vianna, M.G.; Garcia, R.D.O.; Mansur, E.; Ignacio, A.C.P.; Pacheco, G. A comparative study of phytoconstituents and antibacterial activity of in vitro derived materials of four Passiflora species. Anais da Academia Brasileira de Ciências 2018, 90, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Casierra-Posada, F.; Jarma-Orozco, A. Nutritional Composition of Passiflora Species. In Nutritional Composition of Fruit Cultivars; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 517–534. [Google Scholar]
- Gadioli, I.L.; Cunha, M.D.S.B.D.; De Carvalho, M.V.O.; Costa, A.M.; Pineli, L.D.L.D.O. A systematic review on phenolic compounds inPassifloraplants: Exploring biodiversity for food, nutrition, and popular medicine. Crit. Rev. Food Sci. Nutr. 2017, 58, 785–807. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, K.; Dhawan, S.; Sharma, A. Passiflora: A review update. J. Ethnopharmacol. 2004, 94, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Z.; Wei, X.; Li, M.; Duan, X.; Sun, Y.-M.; Yang, R.-L.; Su, X.-D.; Huang, R.; Wang, H. Nutritional Composition and Antioxidant Properties of the Fruits of a Chinese Wild Passiflora foetida. Molecules 2018, 23, 459. [Google Scholar] [CrossRef]
- Ramaiya, S.D.; Bujang, J.S.; Zakaria, M.H.; King, W.S.; Sahrir, M.A.S. Sugars, ascorbic acid, total phenolic content and total antioxidant activity in passion fruit (Passiflora) cultivars. J. Sci. Food Agric. 2012, 93, 1198–1205. [Google Scholar] [CrossRef]
- Revathy, S.; Sunilkumar, T.C. Phytochemical and nutritional studies on the fruit pulp extract of Passiflora foetida Linn. J. Pharmacogn. Phytochem. 2019, 8, 732–734. [Google Scholar]
- Mohanasundari, C.; Natarajan, D.; Srinivasan, K.; Umamaheswari, S.; Ramachandran, A. Antibacterial properties of Passiflora foetida L. a common exotic medicinal plant. Afr. J. Biotechnol. 2007, 6, 2650–2653. [Google Scholar] [CrossRef]
- Patil, A.S.; Paikrao, H.M.; Patil, S.R. Passiflora foetida Linn: A complete morphological and phytopharmacological review. Int J Pharma. Bio. Sci. 2013, 4, 285–296. [Google Scholar]
- Sasikala, V.; Saravanan, S.; Parimelazhagan, T. Analgesic and anti–inflammatory activities of Passiflora foetida L. Asian Pac. J. Trop. Med. 2011, 4, 600–603. [Google Scholar] [CrossRef]
- Sutar, V.; Bhosale, U.P. Screening of Passiflora foetida extracts as anticancer agents on MCF 7 cell line. BIOINFOLET-A 2013, 10, 808–810. [Google Scholar]
- Anandan, R.; Jayakar, B.; Jeganathan, S.; Manavalan, R.; Kumar, S.R. Effect of ethanol extract of fruits of Passiflora foetida Linn. on CCl. J. Pharm. Res. 2009, 2, 413–415. [Google Scholar]
- Rahman, M.A.; Hossain, M.A.; Hasan, M.S.; Hossain, M.G. Antinociceptive, antidiarrhoeal and cytotoxic activities of Passiflora foetida linn. Pharmacologyonline 2011, 1, 228–236. [Google Scholar]
- Santosh, P.; Venugopl, R.; Nilakash, S.; Kunjbihari, S.; Mangala, L. Antidepressant activity of methanolic extract of Passiflora foetida leaves in mice. Int. J. Pharm. Pharm. Sci. 2011, 3, 112–115. [Google Scholar]
- Asadujjaman, M.; Mishuk, A.U.; Hossain, A.M.; Karmakar, U.K. Medicinal potential of Passiflora foetida L. plant extracts: Biological and pharmacological activities. J. Integr. Med. 2014, 12, 121–126. [Google Scholar] [CrossRef]
- Sathish, R.; Sahu, A.; Natarajan, K. Antiulcer and antioxidant activity of ethanolic extract of Passiflora foetida L. Indian J. Pharmacol. 2011, 43, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Ranganatha, N.; Kuppast, D.I.J.; Veerashekar, T. Study of Anti-Hypertension Activity of Aerial Parts of Passiflora foetida Linn. Int. Res. J. Pharm. Plant Sci. 2013, 1, 1–12. [Google Scholar]
- Nguyen, T.Y.; To, D.C.; Tran, M.H.; Lee, J.S.; Lee, J.-H.; Kim, J.A.; Woo, M.H.; Min, B.S. Anti-inflammatory Flavonoids Isolated from Passiflora foetida. Nat. Prod. Commun. 2015, 10, 929–931. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Aktumsek, A. Investigation of Antioxidant Potentials of Solvent Extracts From Different Anatomical Parts of Asphodeline Anatolica E. Tuzlaci: An Endemic Plant to Turkey. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 481–488. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Uysal, A.; Diuzheva, A.; Gunes, E.; Jekő, J.; Cziáky, Z.; Picot-Allain, C.M.N.; Mahomoodally, F.M. Characterization of phytochemical components of Ferula halophila extracts using HPLC-MS/MS and their pharmacological potentials: A multi-functional insight. J. Pharm. Biomed. Anal. 2018, 160, 374–382. [Google Scholar] [CrossRef]
- Grochowski, D.; Uysal, S.; Aktumsek, A.; Granica, S.; Dall’Acqua, S.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phytotherapy Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Iodice, P.; Ferrante, C.; Brunetti, L.; Cabib, S.; Protasi, F.; Walton, M.E.; Pezzulo, G. Fatigue modulates dopamine availability and promotes flexible choice reversals during decision making. Sci. Rep. 2017, 7, 535. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Lu, J.; Li, Q.; Wu, N.; Zhang, L.; Li, H.; Xing, W.; Zhang, X. A network-based analysis of key pharmacological pathways of Andrographis paniculata acting on Alzheimer’s disease and experimental validation. J. Ethnopharmacol. 2020, 251, 112488. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Sinan, K.I.; Zengin, G.; Mahomoodally, F.M.; Sadeer, N.B.; Etienne, O.K.; Cziáky, Z.; Jekő, J.; Glamočlija, J.; Sokovic, M.; et al. Identification of Chemical Profiles and Biological Properties of Rhizophora racemosa G. Mey. Extracts Obtained by Different Methods and Solvents. Antioxidants 2020, 9, 533. [Google Scholar] [CrossRef]
- Van Ngo, T.; Scarlett, C.J.; Bowyer, M.C.; Ngo, P.D.; Van Vuong, Q. Impact of Different Extraction Solvents on Bioactive Compounds and Antioxidant Capacity from the Root of Salacia chinensis L. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Asir, P.J.; Priyanga, S.; Hemmalakshmi, S.; Devaki, K. In Vitro Free Radical Scavenging Activity and Secondary Metabolites in Passiflora foetida L. Asian. J. Pharmaceut. Res. Health Care. 2014, 6, 3–11. [Google Scholar]
- Sisin, N.N.T.; Abdullah, H.; Sul’ain, M.D. Antiproliferative, antioxidative and compounds identification from methanolic extract of Passiflora foetida and its fractions. J. Anal. Pharm. Res. 2017, 6, 00166. [Google Scholar] [CrossRef][Green Version]
- Ajane, G.; Patil, A.S. Evaluation of Antioxidant Potential of Passiflora foetida Extract and Quantitative Evaluation of its Phytochemical Content-A Possible Natural Antioxidant. Pharm. Chem. J. 2019, 6, 14–24. [Google Scholar]
- Ulrih, N.P.; Capanoglu, E.; Jassbi, A.R.; Miron, A. Advance on the Flavonoid C-glycosides and Health Benefits. Crit. Rev. Food Sci. Nutr. 2015, 56, S29–S45. [Google Scholar] [CrossRef]
- Zucolotto, S.M.; Fagundes, C.; Reginatto, F.H.; Ramos, F.A.; Castellanos, L.; Duque, C.; Schenkel, E.P. Analysis of C -glycosyl Flavonoids from South American Passiflora Species by HPLC-DAD and HPLC-MS. Phytochem. Anal. 2011, 23, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.; Schmeda-Hirschmann, G.; Borquez, J.; Kennelly, E.J. The Passiflora tripartita (Banana Passion) Fruit: A Source of Bioactive Flavonoid C-Glycosides Isolated by HSCCC and Characterized by HPLC–DAD–ESI/MS/MS. Molecules 2013, 18, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Mahomoodally, F.M.; Paksoy, M.Y.; Picot-Allain, C.; Glamocilja, J.; Sokovic, M.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J.; et al. Phytochemical characterization and bioactivities of five Apiaceae species: Natural sources for novel ingredients. Ind. Crop. Prod. 2019, 135, 107–121. [Google Scholar] [CrossRef]
- Echeverri, F.; Cardona, G.; Torres, F.; Peláez, C.; Quiñones, W.; Renteria, E. Ermanin: An insect deterrent flavonoid from Passiflora foetida resin. Phytochem. 1991, 30, 153–155. [Google Scholar] [CrossRef]
- Shukla, R.; Pandey, V.; Vadnere, G.P.; Lodhi, S. Role of Flavonoids in Management of Inflammatory Disorders. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases, 2nd ed.; Watson, R.R., Preedy, V.R., Eds.; Elsevier: London, UK, 2019; pp. 293–322. [Google Scholar]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the Dietary Flavonoid Quercetin Upon Performance and Health. Curr. Sports Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Pizzolante, L.; Toschi, T.G.; Guzzo, F.; Ceoldo, S.; Marconi, A.M.; Andreetta, F.; Levi, M. Phenol content related to antioxidant and antimicrobial activities of Passiflora spp. extracts. Eur. Food Res. Technol. 2005, 223, 102–109. [Google Scholar] [CrossRef]
- Walter, M.; Marchesan, E. Phenolic compounds and antioxidant activity of rice. Braz. Arch. Boil. Technol. 2011, 54, 371–377. [Google Scholar] [CrossRef]
- Tandoro, Y.; Widyawati, P.S.; Budianta, T.D.W.; Sumargo, G. Phytochemical identification and antioxidant activity of passiflora foetida fruits and leaves extracts: A comparative study. Int. J. Pharm. Pharm. Sci. 2020, 12, 55–58. [Google Scholar] [CrossRef]
- Gauthier, S.; Emre, M.; Farlow, M.R.; Bullock, R.; Grossberg, G.T.; Potkin, S.G. Strategies for continued successful treatment of Alzheimer’s disease: Switching cholinesterase inhibitors. Curr. Med Res. Opin. 2003, 19, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.K.M. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Melo Filho, A.A.; Kamezaki, A.K.; Estevam Ribeiro, P.R.; Goncalves Reis De Melo, A.C.; Montero Fernandez, I.; Carvalho Dos Santos, R.; Alves Chagas, E.; Cardoso Chagas, P. Chemical Composition, Antioxidant and Biological Activity of Leaves Passiflora foetida. Chem. Eng. Trans. 2018, 64, 241–246. [Google Scholar] [CrossRef]
- Zhang, L.; Rocchetti, G.; Zengin, G.; Ak, G.; Yıldıztugay, E.; Mahomoodally, F.M.; Picot-Allain, M.C.N.; Lucini, L. Profiling of polyphenols and sesquiterpenoids using different extraction methods in Muscari turcicum, an endemic plant from Turkey. Ind. Crop. Prod. 2020, 154, 112626. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Arung, E.T.; Kusuma, I.W.; Christy, E.O.; Shimizu, K.; Kondo, R. Evaluation of medicinal plants from Central Kalimantan for antimelanogenesis. J. Nat. Med. 2009, 63, 473–480. [Google Scholar] [CrossRef]
- Cui, H.-X.; Duan, F.-F.; Jia, S.-S.; Cheng, F.-R.; Yuan, K. Antioxidant and Tyrosinase Inhibitory Activities of Seed Oils from Torreya grandis Fort. ex Lindl. BioMed Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Chatatikun, M.; Supjaroen, P.; Promlat, P.; Chantarangkul, C.; Waranuntakul, S.; Nawarat, J.; Tangpong, J.; Chiabchalard, A. Antioxidant and Tyrosinase Inhibitory Properties of an Aqueous Extract of Garcinia atroviridis Griff. ex. T. Anderson Fruit Pericarps. Pharmacogn. J. 2020, 12, 71–78. [Google Scholar] [CrossRef]
- Hullatti, K.; Telagari, M. In-vitro α-amylase and α-glucosidase inhibitory activity of Adiantum caudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar] [CrossRef]
- Islam, S.; Chipiti, T.; Ibrahim, M.; Singh, M. In vitro α-amylase and α-glucosidase inhibitory and cytotoxic activities of extracts from Cissus cornifolia planch parts. Pharmacogn. Mag. 2017, 13, 329. [Google Scholar] [CrossRef]
- Paulraj, J.A.; Subharamanian, H.; Suriyamoorthy, P.; Kanakasabapathi, D. Phytochemical screening, GC-MS analysis and enzyme inhibitory activity of Passiflora foetida L. Indo Am. J. Pharm. Res. 2014, 4, 3526–3534. [Google Scholar]
- Saravanan, S.; Parimelazhagan, T. In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Sci. Hum. Wellness 2014, 3, 56–64. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gomes, I.A.; Denadai, M.; Lima, B.D.S.; Araújo, A.A.D.S.; Narain, N.; Neta, M.T.S.L.; Serafini, M.R.; Quintans, J.S.; Thangaraj, P. UHPLC-QqQ-MS/MS identification, quantification of polyphenols from Passiflora subpeltata fruit pulp and determination of nutritional, antioxidant, α-amylase and α-glucosidase key enzymes inhibition properties. Food Res. Int. 2018, 108, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, H.; Pee, P.P.; Kee, S.H.Y.; Ow, J.T.; Yan, S.W.; Chew, L.Y.; Kong, K.-W. Malaysian brown seaweeds Sargassum siliquosum and Sargassum polycystum: Low density lipoprotein (LDL) oxidation, angiotensin converting enzyme (ACE), α-amylase, and α-glucosidase inhibition activities. Food Res. Int. 2017, 99, 950–958. [Google Scholar] [CrossRef]
- Artuğer, F.; Özkaynak, F. A Novel Method for Performance Improvement of Chaos-Based Substitution Boxes. Symmetry 2020, 12, 571. [Google Scholar] [CrossRef]
- Lee, J.J.; Chang, C.K.; Liu, I.M.; Chi, T.C.; Yu, H.-J.; Cheng, J.T. Changes in endogenous monoamines in aged rats. Clin. Exp. Pharmacol. Physiol. 2001, 28, 285–289. [Google Scholar] [CrossRef]
- Di Giacomo, V.; Ferrante, C.; Ronci, M.; Cataldi, A.; Di Valerio, V.; Rapino, M.; Recinella, L.; Chiavaroli, A.; Leone, S.; Vladimir-Knežević, S.; et al. Multiple pharmacological and toxicological investigations on Tanacetum parthenium and Salix alba extracts: Focus on potential application as anti-migraine agents. Food Chem. Toxicol. 2019, 133, 110783. [Google Scholar] [CrossRef]
- Beheshti, F.; Hashemzehi, M.; Hosseini, M.; Marefati, N.; Memarpour, S. Inducible nitric oxide synthase plays a role in depression- and anxiety-like behaviors chronically induced by lipopolysaccharide in rats: Evidence from inflammation and oxidative stress. Behav. Brain Res. 2020, 392, 112720. [Google Scholar] [CrossRef]
- Karg, E.; Odh, G.; Wittbjer, A.; Rosengren, E.; Rorsman, H. Hydrogen peroxide as an inducer of elevated tyrosinase level in melanoma cells. J. Invest. Dermatol. 1993, 100, 209–2013. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Kim, M.-J.; Kweon, D.-K.; Lim, S.-T.; Lee, S.-J. Quantification of Hypopigmentation Activity In Vitro. J. Vis. Exp. 2019, 145, e58185. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, L.; Li, F.; Xu, Y.; Lv, S.; Wang, B. Simultaneous dermatophytosis and keratomycosis caused by Trichophyton interdigitale infection: A case report and literature review. BMC Infect. Dis. 2019, 19, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Isa-Isa, R.; Arenas, R.; Isa, M. Inflammatory tinea capitis: Kerion, dermatophytic granuloma, and mycetoma. Clin. Dermatol. 2010, 28, 133–136. [Google Scholar] [CrossRef] [PubMed]
- El-Zawawy, N.A.; Ali, S.S. Pyocyanin as anti-tyrosinase and anti tinea corporis: A novel treatment study. Microb. Pathog. 2016, 100, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Carradori, S.; Di Simone, S.; Ciferri, M.C.; Zengin, G.; Ak, G.; et al. Water Extract from Inflorescences of Industrial Hemp Futura 75 Variety as a Source of Anti-Inflammatory, Anti-Proliferative and Antimycotic Agents: Results from In Silico, In Vitro and Ex Vivo Studies. Antioxidants 2020, 9, 437. [Google Scholar] [CrossRef]


| Extracts | TPC (mg GAE/g) | TFC (mg RE/g) |
|---|---|---|
| EA | 39.61 ± 0.26 a | 14.50 ± 0.54 b |
| MeOH | 24.59 ± 0.24 b | 10.52 ± 0.59 c |
| MeOH (80%) | 21.30 ± 0.20 d | 50.11 ± 0.78 a |
| Water maceration | 22.18 ± 0.16 c | 3.22 ± 0.45 d |
| Infusion | 24.24 ± 0.51 b | 4.27 ± 0.45 d |
| No. | Name | Formula | Rt | (M + H)+ | (M − H)− | Fragment 1 | Fragment 2 | Fragment 3 | Fragment 4 | Fragment 5 | Relative Ratio (%) | Literature |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pantothenic acid | C9H17NO5 | 7.24 | 220.11850 | 202.1077 | 184.0972 | 116.0346 | 90.0555 | 72.0451 | 2.16 | ||
| 2 | Kynurenic acid | C10H7NO3 | 14.18 | 190.05042 | 162.0551 | 144.0448 | 116.0501 | 89.0393 | 0.40 | [34] | ||
| 3 | Dihydroxycoumarin | C9H6O4 | 14.92 | 179.03444 | 147.0442 | 135.0443 | 133.0287 | 123.0443 | 105.0338 | 0.20 | ||
| 4 | Naringenin-6,8-di-C-glucoside | C27H32O15 | 17.38 | 595.16630 | 577.1575 | 505.1364 | 475.1267 | 385.0935 | 355.0831 | 0.08 | ||
| 5 | Luteolin-di-C-hexoside | C27H30O16 | 18.36 | 611.16121 | 593.1509 | 575.1404 | 473.1085 | 341.0658 | 311.0552 | 0.19 | ||
| 6 | Phaselic acid (2-O-Caffeoylmalic acid) | C13H12O8 | 18.73 | 295.04540 | 179.0342 | 135.0441 | 133.0131 | 115.0024 | 71.0123 | 0.37 | ||
| 7 | Isololiolide | C11H16O3 | 18.78 | 197.11777 | 179.1069 | 161.0963 | 135.1171 | 133.1015 | 107.0861 | 0.82 | ||
| 8 | Vicenin-2 (Apigenin-6,8-di-C-glucoside) | C27H30O15 | 19.36 | 595.16630 | 559.1451 | 541.1334 | 457.1133 | 325.0706 | 295.0601 | 7.26 | ||
| 9 | Apigenin-C-hexoside-C-pentoside isomer 1 | C26H28O14 | 19.78 | 565.15574 | 547.1455 | 529.1345 | 379.0815 | 325.0708 | 295.0605 | 0.19 | ||
| 10 | Luteolin-C-hexoside-C-pentoside | C26H28O15 | 20.03 | 581.15065 | 563.1394 | 545.1295 | 395.0762 | 341.0656 | 311.0549 | 0.09 | ||
| 11 | Loliolide | C11H16O3 | 20.08 | 197.11777 | 179.1069 | 161.0964 | 135.1172 | 133.1015 | 107.0860 | 4.46 | ||
| 12 | Apigenin-C-hexoside-C-pentoside isomer 2 | C26H28O14 | 20.46 | 565.15574 | 529.1345 | 511.1239 | 427.1031 | 409.0923 | 295.0605 | 0.96 | ||
| 13 | Apigenin-C-hexoside-C-pentoside isomer 3 | C26H28O14 | 20.73 | 565.15574 | 529.1350 | 511.1242 | 427.1031 | 409.0921 | 295.0603 | 1.04 | ||
| 14 | Orientin (Luteolin-8-C-glucoside) | C21H20O11 | 20.86 | 449.10839 | 431.0979 | 413.0873 | 353.0660 | 329.0658 | 299.0555 | 0.29 | [18] | |
| 15 | Apigenin-C-hexoside-C-pentoside isomer 4 | C26H28O14 | 21.11 | 565.15574 | 529.1350 | 469.1136 | 379.0816 | 325.0710 | 295.0603 | 6.32 | ||
| 16 | Isoorientin (Luteolin-6-C-glucoside) | C21H20O11 | 21.19 | 449.10839 | 431.0973 | 413.0872 | 353.0659 | 329.0659 | 299.0553 | 1.59 | ||
| 17 | Luteolin-C-hexoside | C21H20O11 | 21.56 | 449.10839 | 431.0974 | 413.0871 | 353.0658 | 329.0657 | 299.0553 | 0.10 | ||
| 18 1 | Vitexin (Apigenin-8-C-glucoside) | C21H20O10 | 21.84 | 433.11347 | 415.1031 | 397.0921 | 379.0812 | 313.0709 | 283.0605 | 11.58 | [18] | |
| 19 | Apigenin-C-hexoside-C-pentoside isomer 5 | C26H28O14 | 22.11 | 565.15574 | 529.1349 | 397.0924 | 379.0814 | 325.0710 | 295.0605 | 0.38 | ||
| 20 1 | Vitexin-2″-O-rhamnoside | C27H30O14 | 22.18 | 579.17138 | 433.1133 | 415.1025 | 397.0922 | 313.0708 | 283.0603 | 5.69 | ||
| 21 | Apigenin-C-hexoside-C-pentoside isomer 6 | C26H28O14 | 22.39 | 565.15574 | 529.1348 | 397.0922 | 379.0815 | 325.0708 | 295.0605 | 0.49 | ||
| 22 | Methoxy-tetrahydroxy(iso)flavone-O-hexoside isomer 1 | C22H22O12 | 22.47 | 477.10331 | 462.0813 | 315.0519 | 300.0281 | 299.0200 | 271.0254 | 0.06 | ||
| 23 | Apigenin-C-hexoside-C-pentoside isomer 7 | C26H28O14 | 22.61 | 565.15574 | 529.1346 | 379.0814 | 337.0710 | 325.0706 | 295.0603 | 0.36 | ||
| 24 | Isovitexin (Apigenin-6-C-glucoside) | C21H20O10 | 22.74 | 433.11347 | 415.1024 | 397.0921 | 379.0812 | 313.0708 | 283.0602 | 30.95 | ||
| 25 | Isoscoparin or Scoparin | C22H22O11 | 23.17 | 463.12404 | 445.1131 | 427.1027 | 367.0813 | 343.0813 | 313.0707 | 0.20 | ||
| 26 | Methoxy-tetrahydroxy(iso)flavone-O-hexoside isomer 2 | C22H22O12 | 23.35 | 477.10331 | 462.0819 | 315.0518 | 301.0360 | 299.0202 | 271.0253 | 0.02 | ||
| 27 1 | Isoquercitrin (Quercetin-3-O-glucoside) | C21H20O12 | 23.47 | 463.08765 | 301.0359 | 300.0280 | 271.0254 | 255.0301 | 178.9978 | 0.19 | ||
| 28 1 | Isorhamnetin-3-O-glucoside | C22H22O12 | 25.48 | 477.10330 | 315.0521 | 314.0440 | 285.0411 | 271.0252 | 243.0299 | 0.23 | ||
| 29 | Abscisic acid | C15H20O4 | 25.81 | 263.12834 | 219.1388 | 204.1151 | 201.1280 | 152.0832 | 151.0754 | 0.14 | ||
| 30 | Dihydroxy(iso)flavone-C-hexoside | C21H20O9 | 26.18 | 417.11856 | 399.1079 | 351.0865 | 321.0759 | 297.0759 | 267.0653 | 0.16 | ||
| 31 | Methoxy-pentahydroxy(iso)flavone isomer 1 | C16H12O8 | 26.34 | 331.04540 | 316.0229 | 287.0204 | 271.0254 | 270.0171 | 259.0248 | 0.24 | ||
| 32 | Methoxy-tetrahydroxy(iso)flavone-O-hexoside isomer 3 | C22H22O12 | 27.18 | 477.10331 | 462.0805 | 315.0517 | 300.0281 | 299.0202 | 271.0254 | 0.05 | ||
| 33 | Methoxy-pentahydroxy(iso)flavone isomer 2 | C16H12O8 | 27.53 | 331.04540 | 316.0229 | 287.0203 | 271.0248 | 270.0172 | 259.0243 | 0.13 | ||
| 34 1 | Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) | C15H10O7 | 27.57 | 301.03483 | 273.0402 | 178.9978 | 151.0026 | 121.0282 | 107.0125 | 0.14 | ||
| 35 1 | Naringenin (4′,5,7-Trihydroxyflavanone) | C15H12O5 | 27.75 | 271.06065 | 227.0706 | 177.0186 | 151.0026 | 119.0489 | 107.0125 | 0.15 | ||
| 36 | Methoxy-pentahydroxy(iso)flavone isomer 3 | C16H12O8 | 27.78 | 331.04540 | 316.0227 | 299.0202 | 287.0216 | 271.0253 | 259.0256 | 0.55 | ||
| 37 | Trihydroxy-trimethoxy(iso)flavone-O hexoside | C24H26O13 | 27.95 | 521.12952 | 506.1082 | 359.0779 | 358.0700 | 344.0544 | 329.0309 | 0.44 | ||
| 38 1 | Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | 28.44 | 285.03991 | 217.0500 | 199.0397 | 175.0394 | 151.0026 | 133.0283 | 1.91 | [18] | |
| 39 | Quercetin-4′-O-methyl ether | C16H12O7 | 28.79 | 315.05048 | 300.0280 | 271.0252 | 255.0301 | 243.0298 | 227.0344 | 6.71 | [18] | |
| 40 | Apigenin (4′,5,7-Trihydroxyflavone) | C15H10O5 | 30.28 | 269.04500 | 227.0352 | 225.0555 | 151.0027 | 149.0232 | 117.0332 | 1.38 | [18] | |
| 41 | Chrysoeriol (3′-Methoxy-4′,5,7-trihydroxyflavone) | C16H12O6 | 30.57 | 299.05556 | 284.0331 | 256.0379 | 227.0347 | 151.0025 | 107.0123 | 0.60 | [18] | |
| 42 | Dimethoxy-trihydroxy(iso)flavone isomer 1 | C17H14O7 | 31.11 | 329.06613 | 314.0439 | 299.0202 | 285.0413 | 271.0254 | 243.0300 | 5.51 | ||
| 43 | Dihydroxy-dimethoxy(iso)flavone isomer 1 | C17H14O6 | 32.37 | 313.07122 | 298.0484 | 297.0409 | 283.0251 | 269.0465 | 255.0298 | 0.01 | [35] | |
| 44 | Dihydroxy-dimethoxy(iso)flavone isomer 2 | C17H14O6 | 32.92 | 313.07122 | 298.0483 | 297.0409 | 283.0252 | 269.0467 | 255.0291 | 0.03 | [35] | |
| 45 | Dimethoxy-trihydroxy(iso)flavone isomer 2 | C17H14O7 | 33.29 | 329.06613 | 314.0439 | 299.0202 | 285.0402 | 271.0254 | 243.0306 | 5.12 | ||
| 46 | Dihydroxy-dimethoxy(iso)flavone isomer 3 | C17H14O6 | 34.80 | 313.07122 | 298.0487 | 283.0252 | 270.0541 | 269.0455 | 255.0300 | 0.03 | ||
| 47 | Dihydroxy-dimethoxy(iso)flavone isomer 4 | C17H14O6 | 35.48 | 313.07122 | 298.0487 | 283.0253 | 269.0460 | 255.0301 | 242.0219 | 0.06 | [35] |
| No. | Name | Formula | Rt | (M + H)+ | (M − H)− | Fragment 1 | Fragment 2 | Fragment 3 | Fragment 4 | Fragment 5 | Relative Ratio (%) | Literature |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pantothenic acid | C9H17NO5 | 7.39 | 220.11850 | 202.1077 | 184.0973 | 116.0347 | 90.0556 | 72.0452 | 1.72 | ||
| 2 | Kynurenic acid | C10H7NO3 | 14.21 | 190.05042 | 162.0551 | 144.0447 | 116.0497 | 89.0392 | 0.36 | [34] | ||
| 3 | Dihydroxycoumarin | C9H6O4 | 14.92 | 179.03444 | 147.0442 | 135.0444 | 133.0287 | 123.0444 | 105.0338 | 0.18 | ||
| 4 | Naringenin-6,8-di-C-glucoside | C27H32O15 | 17.38 | 595.16630 | 577.1563 | 505.1341 | 475.1252 | 385.0934 | 355.0828 | 0.07 | ||
| 5 | Luteolin-di-C-hexoside | C27H30O16 | 18.39 | 611.16121 | 593.1504 | 575.1391 | 473.1087 | 341.0659 | 311.0553 | 0.11 | ||
| 6 | Phaselic acid (2-O-Caffeoylmalic acid) | C13H12O8 | 18.74 | 295.04540 | 179.0342 | 135.0440 | 133.0131 | 115.0024 | 71.0123 | 0.14 | ||
| 7 | Isololiolide | C11H16O3 | 18.80 | 197.11777 | 179.1070 | 161.0962 | 135.1171 | 133.1015 | 107.0860 | 0.83 | ||
| 8 | Vicenin-2 (Apigenin-6,8-di-C-glucoside) | C27H30O15 | 19.38 | 595.16630 | 559.1458 | 541.1343 | 457.1138 | 325.0709 | 295.0604 | 4.91 | ||
| 9 | Apigenin-C-hexoside-C-pentoside isomer 1 | C26H28O14 | 19.79 | 565.15574 | 547.1466 | 529.1346 | 379.0814 | 325.0710 | 295.0606 | 0.14 | ||
| 10 | Luteolin-C-hexoside-C-pentoside | C26H28O15 | 20.05 | 581.15065 | 563.1425 | 545.1283 | 395.0771 | 341.0663 | 311.0553 | 0.05 | ||
| 11 | Loliolide | C11H16O3 | 20.09 | 197.11777 | 179.1070 | 161.0963 | 135.1172 | 133.1015 | 107.0860 | 4.84 | ||
| 12 | Apigenin-C-hexoside-C-pentoside isomer 2 | C26H28O14 | 20.48 | 565.15574 | 529.1347 | 511.1249 | 427.1035 | 409.0924 | 295.0607 | 0.73 | ||
| 13 | Apigenin-C-hexoside-C-pentoside isomer 3 | C26H28O14 | 20.75 | 565.15574 | 529.1352 | 511.1248 | 427.1032 | 409.0924 | 295.0602 | 0.73 | ||
| 14 | Orientin (Luteolin-8-C-glucoside) | C21H20O11 | 20.87 | 449.10839 | 431.0981 | 413.0876 | 353.0660 | 329.0660 | 299.0553 | 0.26 | [18] | |
| 15 | Apigenin-C-hexoside-C-pentoside isomer 4 | C26H28O14 | 21.12 | 565.15574 | 529.1350 | 469.1142 | 379.0815 | 325.0710 | 295.0605 | 4.32 | ||
| 16 | Isoorientin (Luteolin-6-C-glucoside) | C21H20O11 | 21.21 | 449.10839 | 431.0982 | 413.0872 | 353.0660 | 329.0660 | 299.0553 | 1.52 | ||
| 17 | Luteolin-C-hexoside | C21H20O11 | 21.57 | 449.10839 | 431.0982 | 413.0872 | 353.0661 | 329.0660 | 299.0554 | 0.33 | ||
| 18 1 | Vitexin (Apigenin-8-C-glucoside) | C21H20O10 | 21.84 | 433.11347 | 415.1030 | 397.0922 | 379.0818 | 313.0710 | 283.0605 | 10.27 | [18] | |
| 19 | Apigenin-C-hexoside-C-pentoside isomer 5 | C26H28O14 | 22.13 | 565.15574 | 529.1351 | 397.0923 | 379.0818 | 325.0710 | 295.0606 | 0.29 | ||
| 20 1 | Vitexin-2″-O-rhamnoside | C27H30O14 | 22.21 | 579.17138 | 433.1135 | 415.1028 | 397.0924 | 313.0709 | 283.0604 | 5.06 | ||
| 21 | Apigenin-C-hexoside-C-pentoside isomer 6 | C26H28O14 | 22.39 | 565.15574 | 529.1352 | 397.0924 | 379.0815 | 325.0710 | 295.0604 | 0.35 | ||
| 22 | Methoxy-tetrahydroxy(iso)flavone-O-hexoside isomer 1 | C22H22O12 | 22.47 | 477.10331 | 462.0812 | 315.0516 | 300.0280 | 299.0205 | 271.0252 | 0.07 | ||
| 23 | Apigenin-C-hexoside-C-pentoside isomer 7 | C26H28O14 | 22.61 | 565.15574 | 529.1351 | 379.0818 | 337.0707 | 325.0710 | 295.0603 | 0.22 | ||
| 24 | Isovitexin (Apigenin-6-C-glucoside) | C21H20O10 | 22.73 | 433.11347 | 415.1032 | 397.0920 | 379.0815 | 313.0709 | 283.0604 | 26.73 | ||
| 25 | Isoscoparin or Scoparin | C22H22O11 | 23.17 | 463.12404 | 445.1141 | 427.1030 | 367.0817 | 343.0816 | 313.0711 | 0.18 | ||
| 26 | Methoxy-tetrahydroxy(iso)flavone-O-hexoside isomer 2 | C22H22O12 | 23.36 | 477.10331 | 462.0811 | 315.0516 | 300.0280 | 299.0201 | 271.0252 | 0.42 | ||
| 27 1 | Isoquercitrin (Quercetin-3-O-glucoside) | C21H20O12 | 23.47 | 463.08765 | 301.0358 | 300.0280 | 271.0252 | 255.0300 | 178.9980 | 0.20 | ||
| 28 1 | Isorhamnetin-3-O-glucoside | C22H22O12 | 25.49 | 477.10330 | 315.0515 | 314.0437 | 285.0410 | 271.0252 | 243.0298 | 0.27 | ||
| 29 | Isorhamnetin-O-hexoside | C22H22O12 | 25.71 | 477.10330 | 315.0517 | 314.0436 | 300.0281 | 271.0253 | 243.0298 | 0.19 | ||
| 30 | Abscisic acid | C15H20O4 | 25.81 | 263.12834 | 219.1388 | 204.1152 | 201.1280 | 152.0832 | 151.0754 | 0.09 | ||
| 31 | Dihydroxy(iso)flavone-C-hexoside | C21H20O9 | 26.19 | 417.11856 | 399.1074 | 351.0864 | 321.0760 | 297.0761 | 267.0653 | 0.18 | ||
| 32 | Methoxy-pentahydroxy(iso)flavone isomer 1 | C16H12O8 | 26.35 | 331.04540 | 316.0228 | 287.0203 | 271.0253 | 270.0175 | 259.0247 | 0.74 | ||
| 33 | Methoxy-tetrahydroxy(iso)flavone-O-hexoside isomer 3 | C22H22O12 | 27.20 | 477.10331 | 462.0810 | 315.0516 | 300.0280 | 299.0205 | 271.0252 | 0.13 | ||
| 34 | Methoxy-pentahydroxy(iso)flavone isomer 2 | C16H12O8 | 27.54 | 331.04540 | 316.0228 | 287.0192 | 271.0265 | 270.0181 | 259.0237 | 0.40 | ||
| 35 1 | Quercetin (3,3′,4′,5,7-Pentahydroxyflavone) | C15H10O7 | 27.57 | 301.03483 | 273.0410 | 178.9978 | 151.0026 | 121.0282 | 107.0126 | 0.46 | ||
| 36 1 | Naringenin (4′,5,7-Trihydroxyflavanone) | C15H12O5 | 27.76 | 271.06065 | 227.0713 | 177.0184 | 151.0026 | 119.0489 | 107.0126 | 0.19 | ||
| 37 | Methoxy-pentahydroxy(iso)flavone isomer 3 | C16H12O8 | 27.77 | 331.04540 | 316.0229 | 299.0206 | 287.0214 | 271.0254 | 259.0259 | 1.26 | ||
| 38 | Trihydroxy-trimethoxy(iso)flavone-O hexoside | C24H26O13 | 27.95 | 521.12952 | 506.1118 | 359.0777 | 358.0700 | 344.0542 | 329.0309 | 0.55 | ||
| 39 1 | Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | 28.44 | 285.03991 | 217.0506 | 199.0390 | 175.0392 | 151.0027 | 133.0282 | 2.88 | [18] | |
| 40 | Quercetin-4′-O-methyl ether | C16H12O7 | 28.80 | 315.05048 | 300.0280 | 271.0252 | 255.0300 | 243.0297 | 227.0341 | 8.52 | [18] | |
| 41 | Apigenin (4′,5,7-Trihydroxyflavone) | C15H10O5 | 30.28 | 269.04500 | 227.0346 | 225.0553 | 151.0026 | 149.0234 | 117.0331 | 1.51 | [18] | |
| 42 | Chrysoeriol (3′-Methoxy-4′,5,7-trihydroxyflavone) | C16H12O6 | 30.59 | 299.05556 | 284.0330 | 256.0380 | 227.0334 | 151.0025 | 107.0125 | 1.03 | [18] | |
| 43 | Dimethoxy-trihydroxy(iso)flavone isomer 1 | C17H14O7 | 31.11 | 329.06613 | 314.0438 | 299.0201 | 285.0410 | 271.0252 | 243.0298 | 8.04 | ||
| 44 | Dihydroxy-dimethoxy(iso)flavone isomer 1 | C17H14O6 | 32.37 | 313.07122 | 298.0487 | 297.0409 | 283.0252 | 269.0457 | 255.0299 | 0.04 | [35] | |
| 45 | Dihydroxy-dimethoxy(iso)flavone isomer 2 | C17H14O6 | 32.93 | 313.07122 | 298.0487 | 297.0408 | 283.0253 | 269.0455 | 255.0301 | 0.07 | [35] | |
| 46 | Dimethoxy-trihydroxy(iso)flavone isomer 2 | C17H14O7 | 33.29 | 329.06613 | 314.0437 | 299.0201 | 285.0407 | 271.0252 | 243.0294 | 8.11 | ||
| 47 | Dihydroxy-dimethoxy(iso)flavone isomer 3 | C17H14O6 | 34.82 | 313.07122 | 298.0487 | 283.0255 | 270.0537 | 269.0461 | 255.0300 | 0.11 | [35] | |
| 48 | Dihydroxy-dimethoxy(iso)flavone isomer 4 | C17H14O6 | 35.50 | 313.07122 | 298.0488 | 283.0254 | 269.0465 | 255.0301 | 242.0224 | 0.22 | [35] |
| Extracts | DPPH | ABTS | CUPRAC | FRAP | Metal Chelating | PBD |
|---|---|---|---|---|---|---|
| (mg TE/g) | (mg EDTAE/g) | (mM TE/g) | ||||
| EA | 26.69 ± 1.85 b | 68.98 ± 2.80 a | 162.83 ± 0.24 a | 65.35 ± 0.57 a | 15.60 ± 0.26 c | 2.68 ± 0.19 a |
| MeOH | 28.57 ± 0.36 b | 50.99 ± 0.89 c | 103.00 ± 1.93 b | 35.76 ± 1.43 b | 19.21 ± 0.09 a | 2.28 ± 0.11 b |
| MeOH (80%) | 31.74 ± 0.90 a | 55.06 ± 3.32 c | 66.83 ± 0.21 c | 30.36 ± 0.48 d | 15.24 ± 0.58 c | 0.66 ± 0.10 d |
| Water maceration | 20.77 ± 0.18 c | 61.81 ± 1.44 b | 54.46 ± 1.44 e | 31.59 ± 0.28 c,d | 14.10 ± 0.40 d | 1.01 ± 0.01 c |
| Infusion | 21.81 ± 0.37 c | 62.08 ± 1.11 b | 61.33 ± 1.70 d | 32.38 ± 0.08c | 18.00 ± 0.12 b | 1.02 ± 0.02 c |
| Extracts | AChE | BChE | Tyrosinase | Amylase | Glucosidase |
|---|---|---|---|---|---|
| (mg GALAE/g) | (mg KAE/g) | (mM ACAE/g) | |||
| EA | na | 2.45 ± 0.18 | 48.48 ± 3.68 a | 0.59 ± 0.01 a | 0.70 ± 0.01 a |
| MeOH | 2.22 ± 0.04 a | na | 29.33 ± 1.71 b | 0.43 ± 0.03 b | 0.37 ± 0.01 b |
| MeOH (80%) | 0.92 ± 0.02 b | na | 35.11 ± 5.77 b | 0.35 ± 0.02 c | 0.30 ± 0.01 c |
| Water maceration | 0.77 ± 0.08 c | na | na | 0.17 ± 0.01 d | 0.11 ± 0.01 d |
| Infusion | 0.50 ± 0.08 d | na | na | 0.41 ± 0.01 b | 0.28 ± 0.01 c |
| Dermatophytes (ID strain) A3:D15 | Minimum Inhibitory Concentration (MIC) | ||
|---|---|---|---|
| Methaol Extract (µg mL−1) * | Water Extract (µg mL−1) * | Griseofulvin (µg mL−1) * | |
| Arthroderma crocatum (CCF 5300) | 12.4 (7.81–15.625) | 39.37(31.25–62.5) | >8 |
| Arthroderma curreyi (CCF 5207) | 6.19 (3.9–7.81) | 12.4(7.81–15.625) | >8 |
| Arthroderma gypseum (CCF 6261) | 157.49 (125–250) | 396(250–500) | 1.587(1–2) |
| Arthroderma insingulare (CCF 5417) | 12.4 (7.81–15.625) | 19.68(15.625–31.25) | >8 |
| Arthroderma quadrifidum (CCF 5792) | 78.74 (62.5–125) | 314.98(250–500) | >8 |
| Trichophyton mentagrophytes (CCF 4823) | 49.6 (31.25–62.5) | 99.21(62.5–125) | 2.52(2–4) |
| Trichophyton mentagrophytes (CCF 5930) | 157.49 (125–250) | 396(250–500) | 3.174(2–4) |
| Trichophyton rubrum (CCF 4933) | 39.37 (31.25–62.5) | 99.21(62.5–125) | 1.26(1–2) |
| Trichophyton rubrum (CCF 4879) | 99.21 (62.5–125) | 198,42(125–250) | 3.175(2–4) |
| Trichophyton tonsurans (CCF 4834) | 9.84 (7.81–15.625) | 9.84(7.81–15.625) | 0.198(0.125–0.25) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiavaroli, A.; Di Simone, S.C.; Sinan, K.I.; Ciferri, M.C.; Angeles Flores, G.; Zengin, G.; Etienne, O.K.; Ak, G.; Fawzi Mahomoodally, M.; Jugreet, S.; et al. Pharmacological Properties and Chemical Profiles of Passiflora foetida L. Extracts: Novel Insights for Pharmaceuticals and Nutraceuticals. Processes 2020, 8, 1034. https://doi.org/10.3390/pr8091034
Chiavaroli A, Di Simone SC, Sinan KI, Ciferri MC, Angeles Flores G, Zengin G, Etienne OK, Ak G, Fawzi Mahomoodally M, Jugreet S, et al. Pharmacological Properties and Chemical Profiles of Passiflora foetida L. Extracts: Novel Insights for Pharmaceuticals and Nutraceuticals. Processes. 2020; 8(9):1034. https://doi.org/10.3390/pr8091034
Chicago/Turabian StyleChiavaroli, Annalisa, Simonetta Cristina Di Simone, Kouadio Ibrahime Sinan, Maria Chiara Ciferri, Giancarlo Angeles Flores, Gokhan Zengin, Ouattara Katinan Etienne, Gunes Ak, Mohamad Fawzi Mahomoodally, Sharmeen Jugreet, and et al. 2020. "Pharmacological Properties and Chemical Profiles of Passiflora foetida L. Extracts: Novel Insights for Pharmaceuticals and Nutraceuticals" Processes 8, no. 9: 1034. https://doi.org/10.3390/pr8091034
APA StyleChiavaroli, A., Di Simone, S. C., Sinan, K. I., Ciferri, M. C., Angeles Flores, G., Zengin, G., Etienne, O. K., Ak, G., Fawzi Mahomoodally, M., Jugreet, S., Cziáky, Z., Jekő, J., Recinella, L., Brunetti, L., Leone, S., Angelini, P., Venanzoni, R., Menghini, L., Ferrante, C., & Orlando, G. (2020). Pharmacological Properties and Chemical Profiles of Passiflora foetida L. Extracts: Novel Insights for Pharmaceuticals and Nutraceuticals. Processes, 8(9), 1034. https://doi.org/10.3390/pr8091034















