Post-Polymerization Heat Effect in the Production of Polyamide 6 by Bulk Quasiliving Anionic Ring-Opening Polymerization of ε-Caprolactam with Industrial Components: A Green Processing Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymerization of ε-Caprolactam with and without Quenching
2.3. Measurements
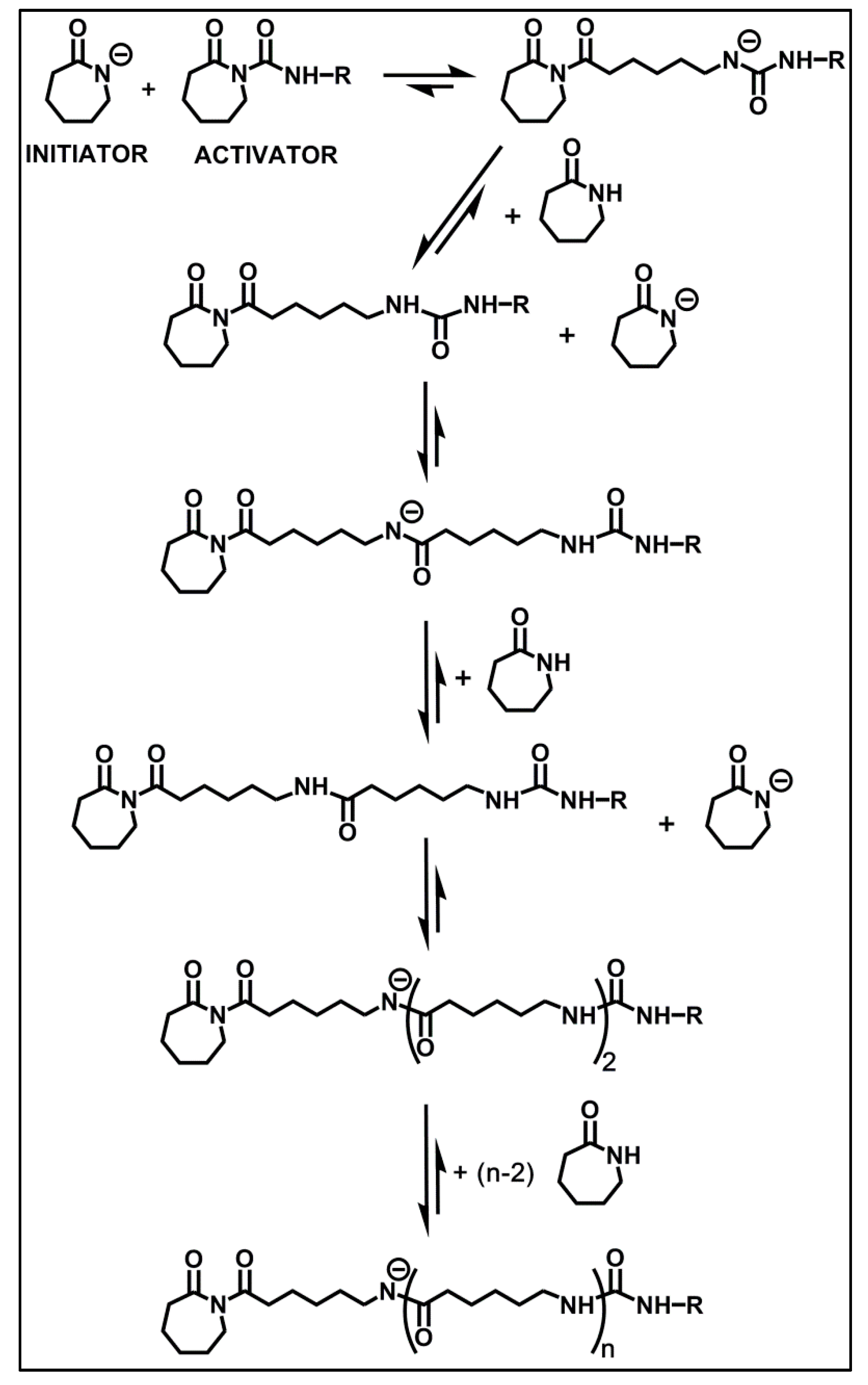
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice, 1st ed.; Oxford University Press: Oxford, UK, 1998; pp. 1–135. [Google Scholar]
- Hong, M.; Chen, E.Y.X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Bari, S.S.; Chatterjee, A.; Mishra, S. Biodegradable polymer nanocomposites: An overview. Polym. Rev. 2016, 56, 287–328. [Google Scholar] [CrossRef]
- Meier, M.A.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Bhowmick, A.K.; Kali, G. Terpene based elastomers: Synthesis, properties, and applications. Processes 2020, 8, 553. [Google Scholar] [CrossRef]
- Okan, M.; Aydin, H.M.; Barsbay, M. Current approaches to waste polymer utilization and minimization: A review. J. Chem. Technol. Biotechnol. 2019, 94, 8–21. [Google Scholar] [CrossRef]
- Osváth, Z.; Szőke, A.; Pásztor, S.; Závoczki, L.; Szarka, G.; Iván, B. Recent advances in the synthesis and analysis of Polyamide 6 and products therefrom: From polymerization chemistry of ε-caprolactam to thermoplastic resin transfer molding (T-RTM). Acad. J. Polym. Sci. 2020, 4, 555629. [Google Scholar]
- Russo, S.; Maniscalco, S.; Ricco, L. Some new perspectives of anionic polyamide 6 (APA 6) synthesis. Polym. Adv. Technol. 2015, 26, 851–854. [Google Scholar] [CrossRef]
- Wilhelm, M.; Wendel, R.; Aust, M.; Rosenberg, P.; Henning, F. Compensation of water influence on anionic polymerization of ε-caprolactam: 1. Chemistry and experiments. J. Compos. Sci. 2020, 4, 7. [Google Scholar] [CrossRef]
- Wendel, R.; Rosenberg, P.; Wilhelm, M.; Henning, F. Anionic polymerization of ε-caprolactam under the influence of water: 2. Kinetic model. J. Compos. Sci. 2020, 4, 8. [Google Scholar] [CrossRef]
- Choi, C.W.; Jin, J.W.; Lee, H.; Huh, M.; Kang, K.W. Optimal polymerization conditions in thermoplastic-resin transfer molding process for mechanical properties of carbon fiber-reinforced PA6 composites using the response surface method. Fibers Polym. 2019, 20, 1021–1028. [Google Scholar] [CrossRef]
- Andres, J.; Hoto, R.; Molle, G.; García, J.A. An efficient equipment for control and monitoring in green composites LCM manufacturing. A new proposal for anionic reactive injection molding of caprolactam. Int. Rev. Chem. Eng. 2010, 2, 904–908. [Google Scholar]
- Ageyeva, T.; Sibikin, I.; Karger-Kocsis, J. Polymers and related composites via anionic ring-opening polymerization of lactams: Recent developments and future trends. Polymers 2018, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.U.; Lee, H.S.; Kim, J.W.; Kim, S.Y.; Kim, S.H.; Hwang, I.; Kang, B.J.; Kang, M.K. Facile and cost-effective strategy for fabrication of polyamide 6 wrapped multi-walled carbon nanotube via anionic melt polymerization of ε-caprolactam. Chem. Eng. J. 2019, 373, 251–258. [Google Scholar] [CrossRef]
- Rusu, E. Nylon 612/TiO2 composites by anionic copolymerization-molding process: Comparative evaluation of thermal and mechanical performance. J. Compos. Mater. 2020, 54, 345–362. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, N.; Li, W.; Zhang, S.; He, W. Preparation of ZnO nanoparticle-reinforced polyamide 6 composite by in situ-coproduced method and their properties. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 165–170. [Google Scholar] [CrossRef]
- Fang, H.; Li, D.H.; Wu, F.J.; Peng, X.F.; Chen, A.L.; Zhang, J.; Chen, S. In situ Polymerization of Polyamide 6/boron nitride composites to enhance thermal conductivity and mechanical properties via boron nitride covalently grafted polyamide 6. Polym. Eng. Sci. 2020, 60, 710–716. [Google Scholar] [CrossRef]
- Nagel, K.; Spange, S. Polyamide/silica hybrid materials by anionic melt polymerization of lactam-substituted silane monomers with epsilon-caprolactam. Eur. Polym. J. 2019, 113, 385–394. [Google Scholar] [CrossRef]
- Ricco, L.; Russo, S.; Orefice, G.; Riva, F. Anionic poly (ε-caprolactam): Relationships among conditions of synthesis, chain regularity, reticular order, and polymorphism. Macromolecules 1999, 32, 7726–7731. [Google Scholar] [CrossRef]
- Naumann, S.; Epple, S.; Bonten, C.; Buchmeiser, M.R. Polymerization of ε-caprolactam by latent precatalysts based on protected N-heterocyclic carbenes. ACS Macro Lett. 2013, 2, 609–612. [Google Scholar] [CrossRef]
- Lee, Y.; Lin, K.Y.A.; Kwon, E.E.; Lee, J. Renewable routes to Monomeric Precursors of Nylon 66 and Nylon 6 from Food Waste: A Review. J. Cleaner Prod. 2019, 227, 624–633. [Google Scholar] [CrossRef]
- Trujillo-Vera, D.A.; Vélez-Salazar, Y. Study of different routes of renewable synthesis for caprolactam production. Dyna 2018, 85, 231–237. [Google Scholar] [CrossRef]
- Han, J. Biorenewable strategy for catalytic ε-caprolactam production using cellulose- and hemicellulose-derived γ-valerolactone. ACS Sustain. Chem. Eng. 2017, 5, 1892–1898. [Google Scholar] [CrossRef]
- Beerthuis, R.; Rothenberg, G.; Shiju, N.R. Catalytic routes towards acrylic acid, adipic acid and ε-caprolactam starting from biorenewables. Green Chem. 2015, 17, 1341–1361. [Google Scholar] [CrossRef]
- Ozmen, S.C.; Ozkoc, G.; Serhatli, E. Thermal, mechanical and physical properties of chain extended recycled polyamide 6 via reactive extrusion: Effect of chain extender types. Polym. Degrad. Stab. 2019, 162, 76–84. [Google Scholar] [CrossRef]
- Kamimura, A.; Shiramatsu, Y.; Kawamoto, T. Depolymerization of Polyamide 6 in hydrophilic ionic liquids. Green Energy Environ. 2019, 4, 166–170. [Google Scholar] [CrossRef]
- Alberti, C.; Figueira, R.; Hofmann, M.; Koschke, S.; Enthaler, S. Chemical recycling of end-of-life Polyamide 6 via ring closing depolymerization. ChemistrySelect 2019, 4, 12638–12642. [Google Scholar] [CrossRef]
- Carothers, W.H. Studies on polymerization and ring formation. I. Introduction to the general theory of condensation polymers. J. Am. Chem. Soc. 1929, 51, 2548–2559. [Google Scholar] [CrossRef]
- Yilmaz, S.; Gul, O.; Yilmaz, T. Effect of chain extender and terpolymers on tensile and fracture properties of polyamide 6. Polymer 2015, 65, 63–71. [Google Scholar] [CrossRef]
- Jeon, S.; Karkhanechi, H.; Fang, L.; Cheng, L.; Ono, T.; Nakamura, R.; Matsuyama, H.J. Novel preparation and fundamental characterization of polyamide 6 selfsupporting hollow fiber membranes via thermally induced phase separation (TIPS). Membr. Sci. 2018, 546, 1–14. [Google Scholar] [CrossRef]
- Mazur, K.; Kuciel, S.; Salasinska, K. Mechanical, fire, and smoke behaviour of hybrid composites based on polyamide 6 with basalt/carbon fibres. J. Composos. Mater. 2019, 53, 3979–3991. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, S.Y.; Chen, M.H.; Chen, H.L.; Hsiao, H.; Liu, L.; Chen, C.M. Preparation and characterization of heterocyclic Polyamide 6 (PA 6) with high transparencies and low hygroscopicities. J. Mol. Struct. 2019, 1175, 836–843. [Google Scholar] [CrossRef]
- Roda, J. Polyamides. Handbook of Ring-Opening Polymerization, 1st ed.; Dubois, P., Coulembier, O., Raquez, J., Eds.; Wiley-VCH Verlag GmbH and Co. KgaA: Weinheim, Germany, 2009; pp. 165–195. [Google Scholar]
- Ageyeva, T.; Sibikin, I.; Kovacs, J.G. A review of thermoplastic resin transfer molding: Process modeling and simulation. Polymers 2019, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Zaldua, N.; Maiz, J.; Calle, A.; Garcia-Arrieta, S.; Elizetxea, C.; Harismendy, I.; Tercjak, A.; Muller, A.J. Nucleation and crystallization of PA6 composites prepared by T-RTM: Effects of carbon and glass fiber loading. Polymers 2019, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.J.; Zhou, Y.F.; Wang, W.Q.; Wang, J.K. The reactive compatibilization of montmorillonite for immiscible anionic Polyamide 6/polystyrene blends via in situ polymerization. Polym. Plast. Technol. Mater. 2020, 59, 884–894. [Google Scholar] [CrossRef]
- Yanez-Macias, R.; Hernandez-Hernandez, E.; Gallardo-Vega, C.A.; Ledezma-Rodriguez, R.; Ziolo, R.F.; Mendoza-Tolentino, Y.; Fernandez-Tavizon, S.; Avila-Orta, C.A.; Garcia-Hernandez, Z.; Gonzalez-Morones, P. Covalent grafting of unfunctionalized pristine MWCNT with Nylon-6 by microwave assist in-situ polymerization. Polymer 2019, 185, 121946. [Google Scholar] [CrossRef]
- Min, J.H.; Huh, M.; Yun, S. Effect of interface on the properties of Polyamide 6/carbon nanotube nanocomposites prepared by in-situ anionic ring-opening polymerization. Compos. Res. 2019, 32, 375–381. [Google Scholar]
- Murray, J.J.; Robert, C.; Gleich, K.; McCarthy, E.D.; Bradaigh, C.M.O. Manufacturing of unidirectional stitched glass fabric reinforced Polyamide 6 by thermoplastic resin transfer moulding. Mater. Des. 2020, 189, 108512. [Google Scholar] [CrossRef]
- Osváth, Z.; Pásztor, S.; Szőke, A.; Szarka, G.; Kasza, G.; Závoczki, L.B.; Karger-Kocsis, J.; Iván, B. Research Centre for Natural Sciences, Budapest, Hungary. Unpublished work. 2020. [Google Scholar]
- Casazza, E.; Ricco, L.; Russo, S.; Scamporrino, E. Nature of a low molar mass peak in anionic poly (ε-caprolactam). Its identification as macrocyclic ensemble. Macromolecules 2007, 40, 739–745. [Google Scholar] [CrossRef]
- Ricco, L.; Casazza, E.; Mineo, P.; Russo, S.; Scamporrino, E. Nature of low molar mass peak in anionic poly(ε-caprolactam). Main aspects of its formation. Macromolecules 2008, 41, 3904–3911. [Google Scholar] [CrossRef]
- Hashimoto, K. Ring-opening polymerization of lactams. Living anionic polymerization and its applications. Prog. Polym. Sci. 2000, 25, 1411–1462. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Kelen, T.; Tüdős, F. Quasiliving carbocationic polymerization. I. Classification of living polymerizations in carbocationic systems. J. Macromol. Sci.: Part A-Chem. 1982, 18, 1189–1207. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Iván, B. Designed Polymers by Carbocationic Macromolecular Engineering: Theory and Practice; Hanser Publishers: Munich, Germay; New York, NY, USA, 1992. [Google Scholar]
- Iván, B. Terminology and classification of quasiliving polymerizations and ideal living polymerizations on the basis of the logic of elementary polymerization reactions, and comments on using the term “controlled”. Macromol. Chem. Phys. 2000, 201, 2621–2628. [Google Scholar]
- Russo, S.; Imperato, A.; Mariani, A.; Parodi, F. The fast activation of ε-caprolactam polymerization in quasi-adiabatic conditions. Macromol. Chem. Phys. 1995, 196, 3297–3303. [Google Scholar] [CrossRef]
- Sebenda, J. Lactam polymerization. J. Macromol. Sci. Part A-Chem. 1972, 6, 1145–1199. [Google Scholar] [CrossRef]
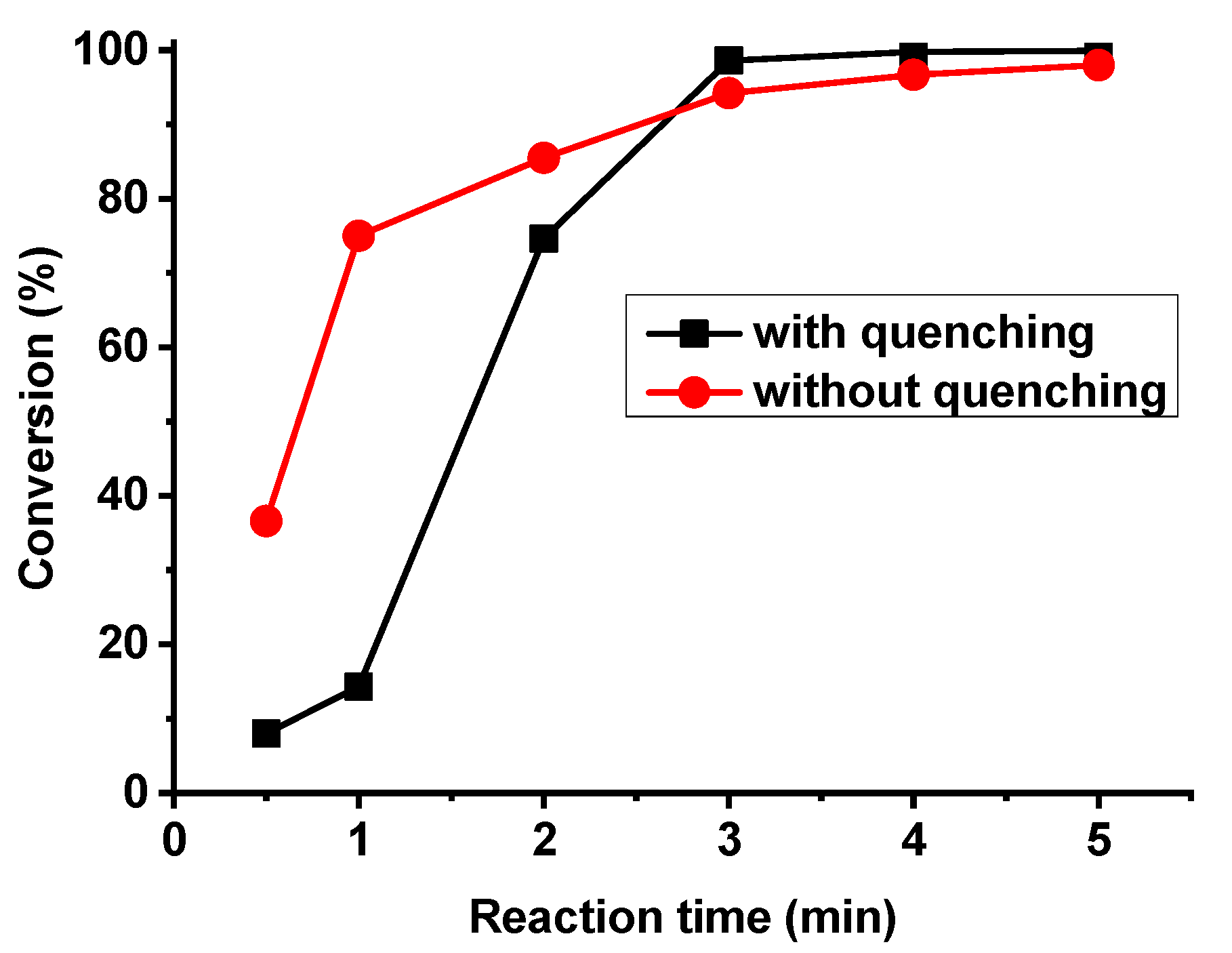
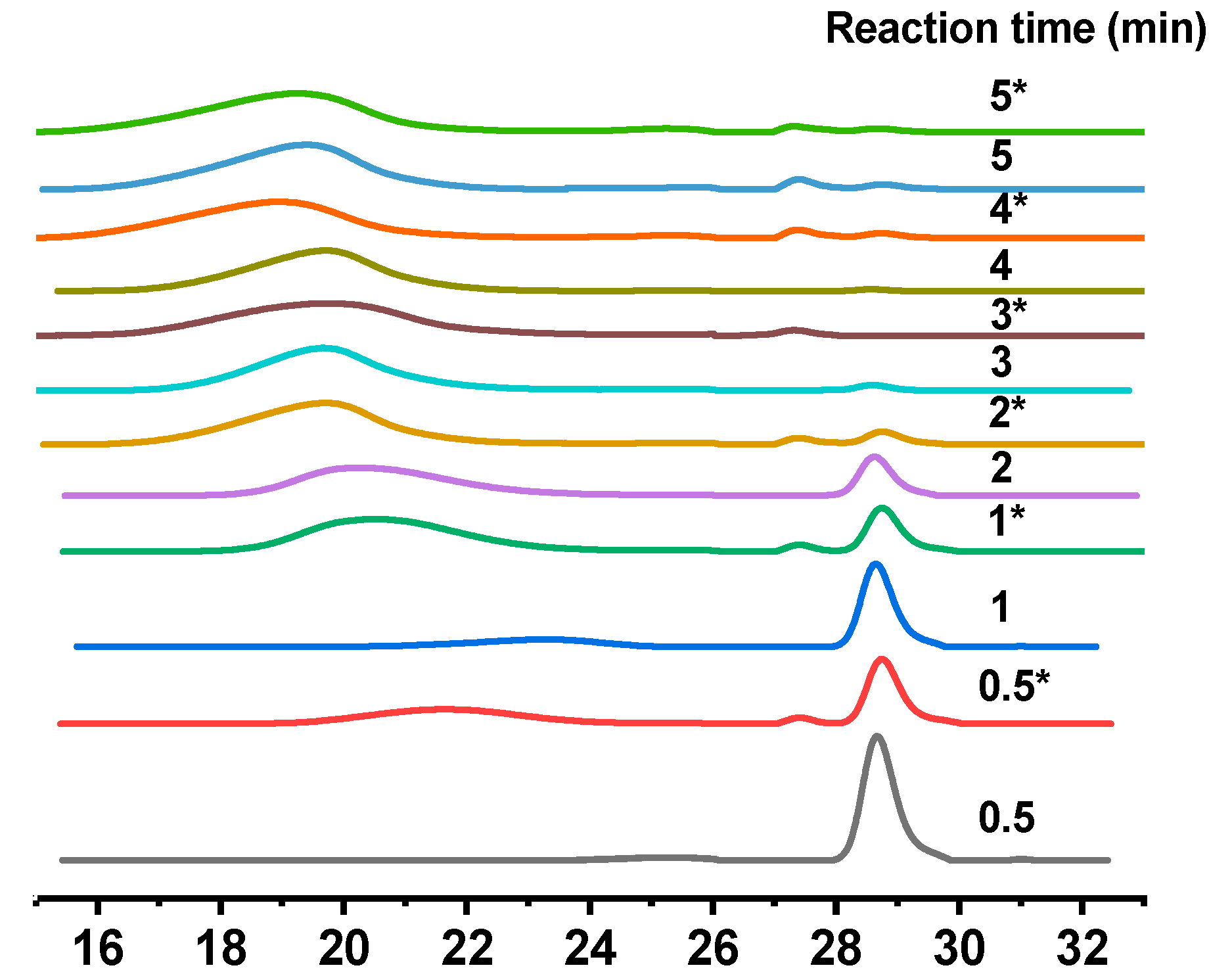
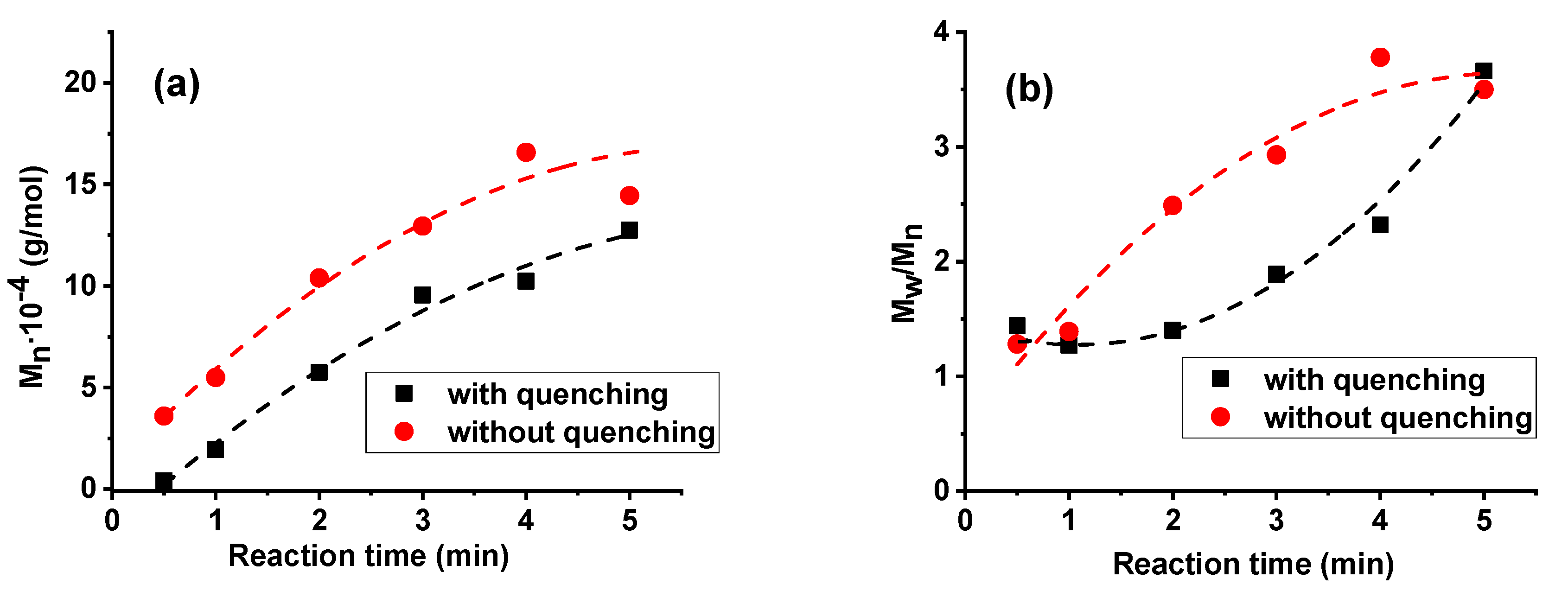
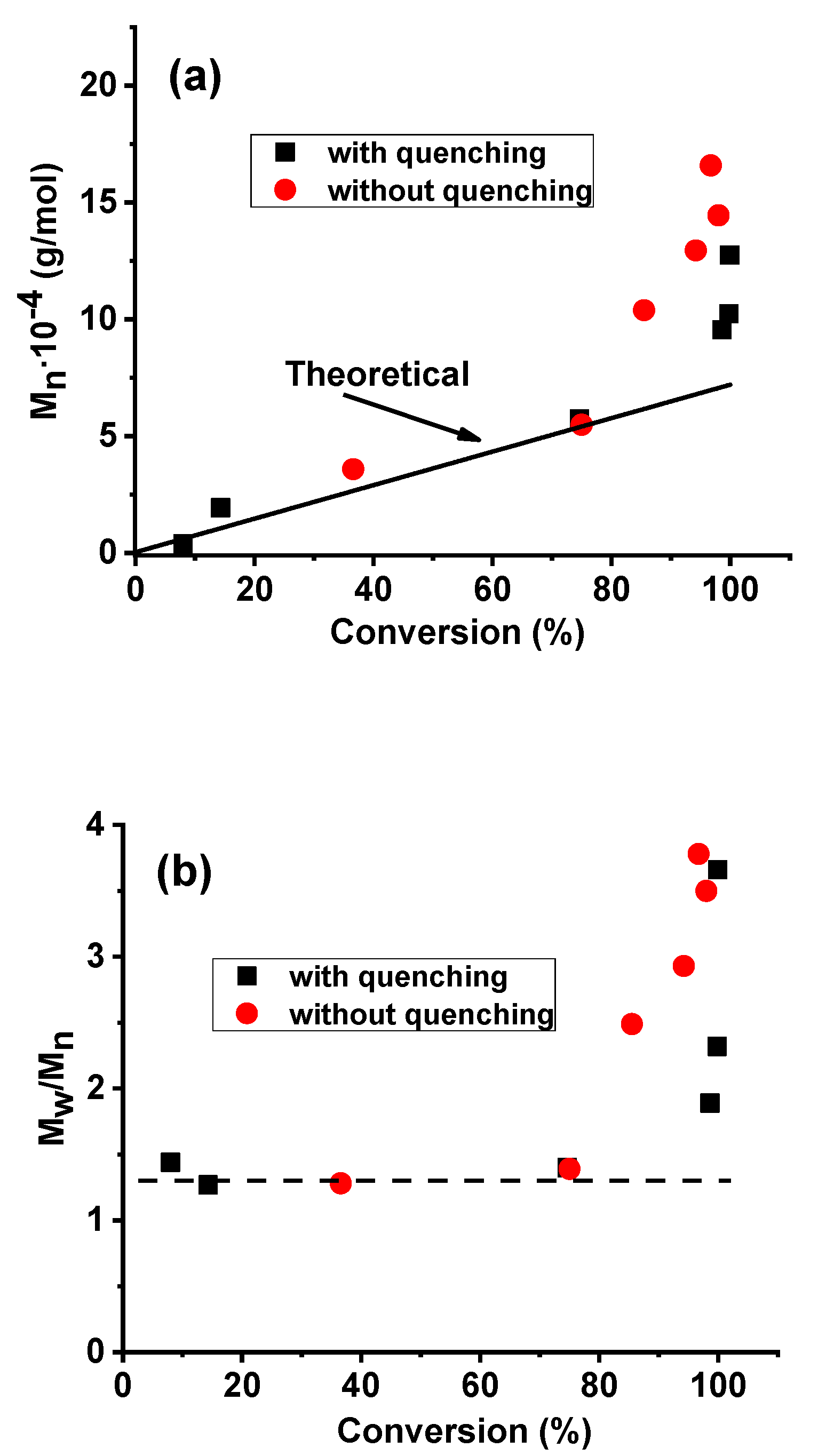
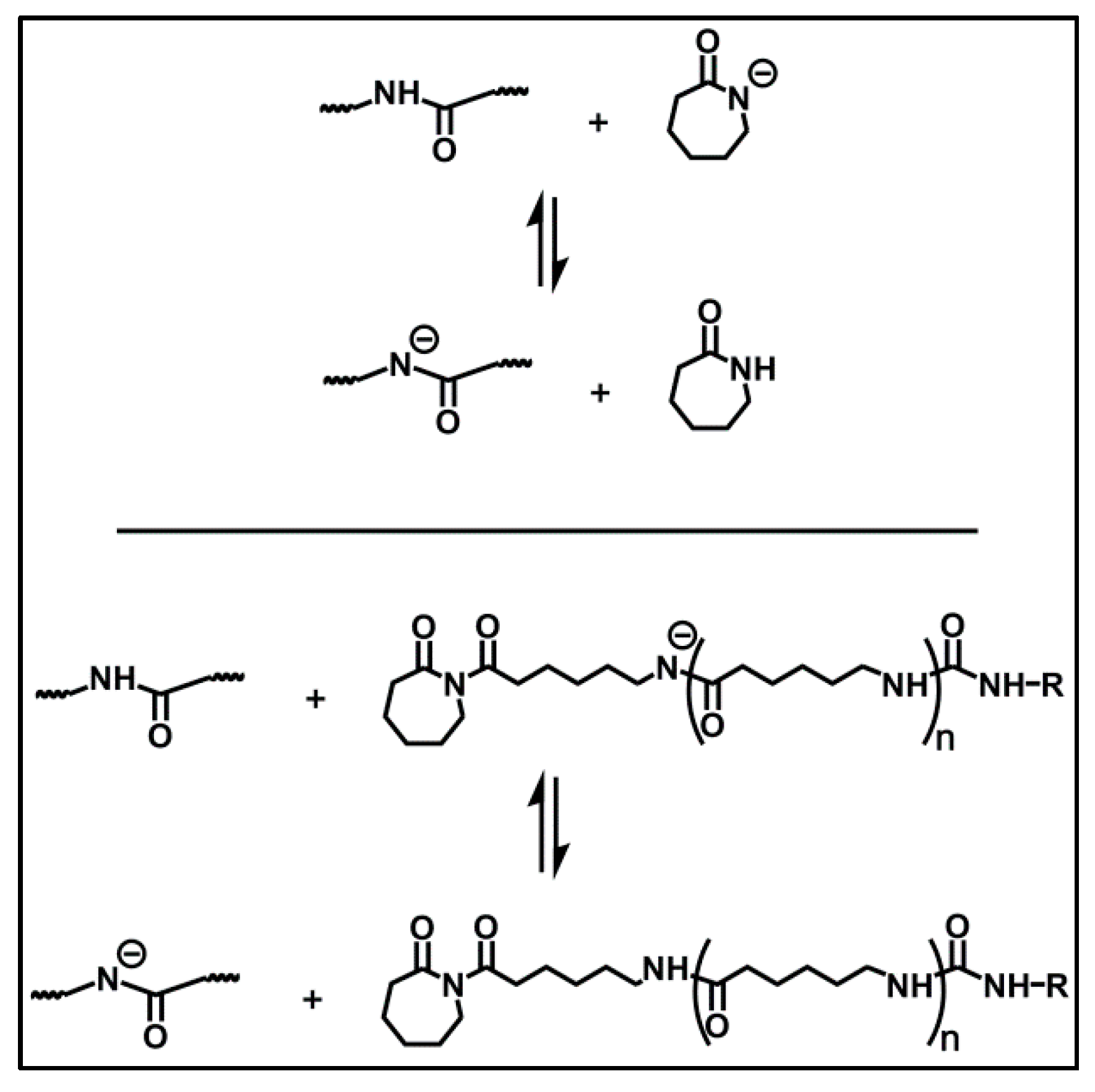
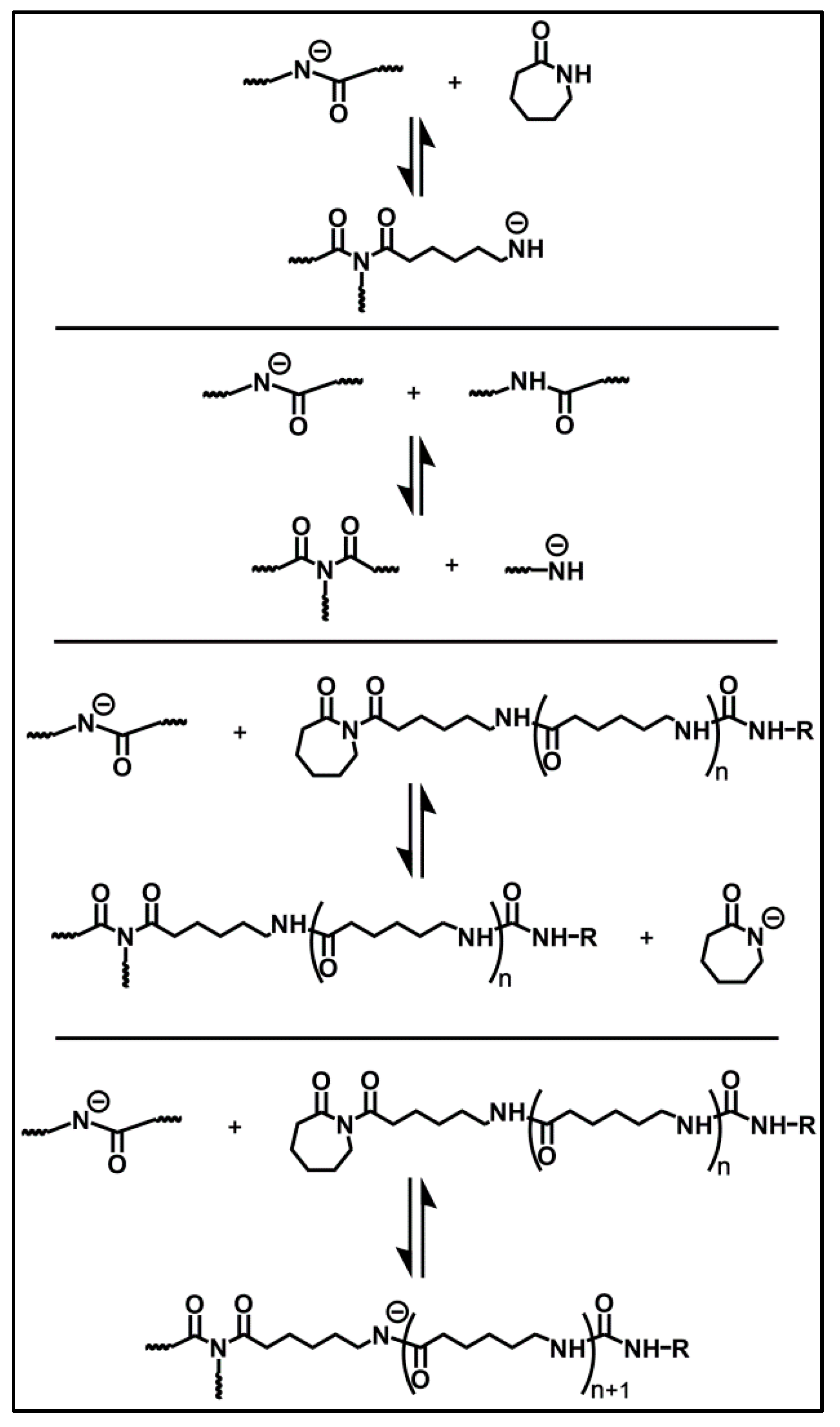
| Sample Number | Reaction Time (min) | Conversion (%) | Mn∙10−4 (g/mol) | PDI |
|---|---|---|---|---|
| 1 | 0.5 | 8.0 | 0.4 | 1.44 |
| 2 | 0.5 * | 36.6 | 3.6 | 1.28 |
| 3 | 1 | 14.3 | 1.9 | 1.27 |
| 4 | 1 * | 75.0 | 5.5 | 1.39 |
| 5 | 2 | 74.6 | 5.7 | 1.40 |
| 6 | 2 * | 85.5 | 10.4 | 2.49 |
| 7 | 3 | 98.6 | 9.6 | 1.89 |
| 8 | 3 * | 94.2 | 12.9 | 2.93 |
| 9 | 4 | 99.8 | 10.2 | 2.32 |
| 10 | 4 * | 96.7 | 16.6 | 3.78 |
| 11 | 5 | 99.9 | 12.8 | 3.66 |
| 12 | 5 * | 98.0 | 14.4 | 3.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osváth, Z.; Szőke, A.; Pásztor, S.; Szarka, G.; Závoczki, L.B.; Iván, B. Post-Polymerization Heat Effect in the Production of Polyamide 6 by Bulk Quasiliving Anionic Ring-Opening Polymerization of ε-Caprolactam with Industrial Components: A Green Processing Technique. Processes 2020, 8, 856. https://doi.org/10.3390/pr8070856
Osváth Z, Szőke A, Pásztor S, Szarka G, Závoczki LB, Iván B. Post-Polymerization Heat Effect in the Production of Polyamide 6 by Bulk Quasiliving Anionic Ring-Opening Polymerization of ε-Caprolactam with Industrial Components: A Green Processing Technique. Processes. 2020; 8(7):856. https://doi.org/10.3390/pr8070856
Chicago/Turabian StyleOsváth, Zsófia, Anita Szőke, Szabolcs Pásztor, Györgyi Szarka, László Balázs Závoczki, and Béla Iván. 2020. "Post-Polymerization Heat Effect in the Production of Polyamide 6 by Bulk Quasiliving Anionic Ring-Opening Polymerization of ε-Caprolactam with Industrial Components: A Green Processing Technique" Processes 8, no. 7: 856. https://doi.org/10.3390/pr8070856
APA StyleOsváth, Z., Szőke, A., Pásztor, S., Szarka, G., Závoczki, L. B., & Iván, B. (2020). Post-Polymerization Heat Effect in the Production of Polyamide 6 by Bulk Quasiliving Anionic Ring-Opening Polymerization of ε-Caprolactam with Industrial Components: A Green Processing Technique. Processes, 8(7), 856. https://doi.org/10.3390/pr8070856






