Experimental Analysis of the Performance and Load Cycling of a Polymer Electrolyte Membrane Fuel Cell
Abstract
1. Introduction
2. Materials and Methods
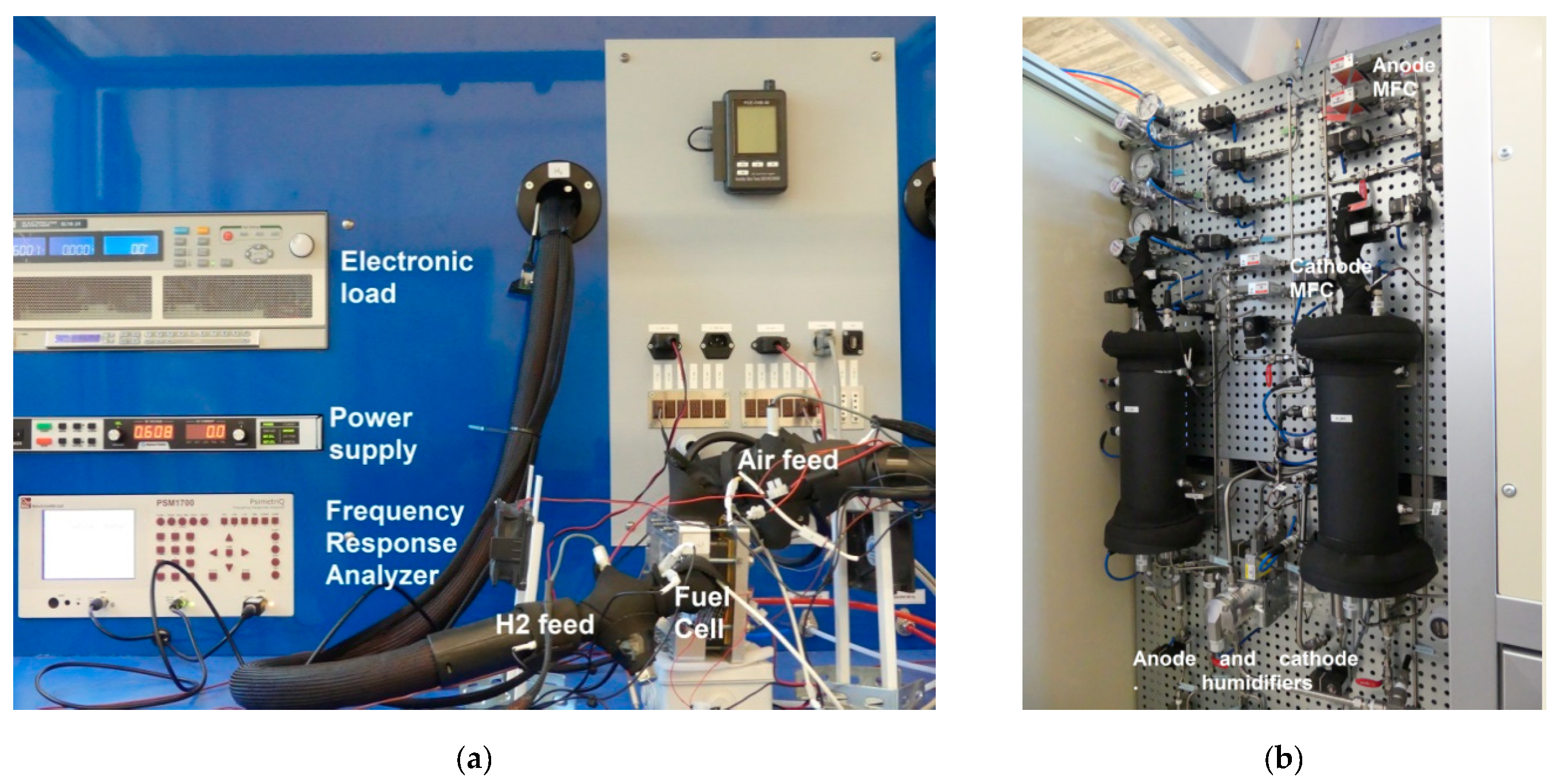
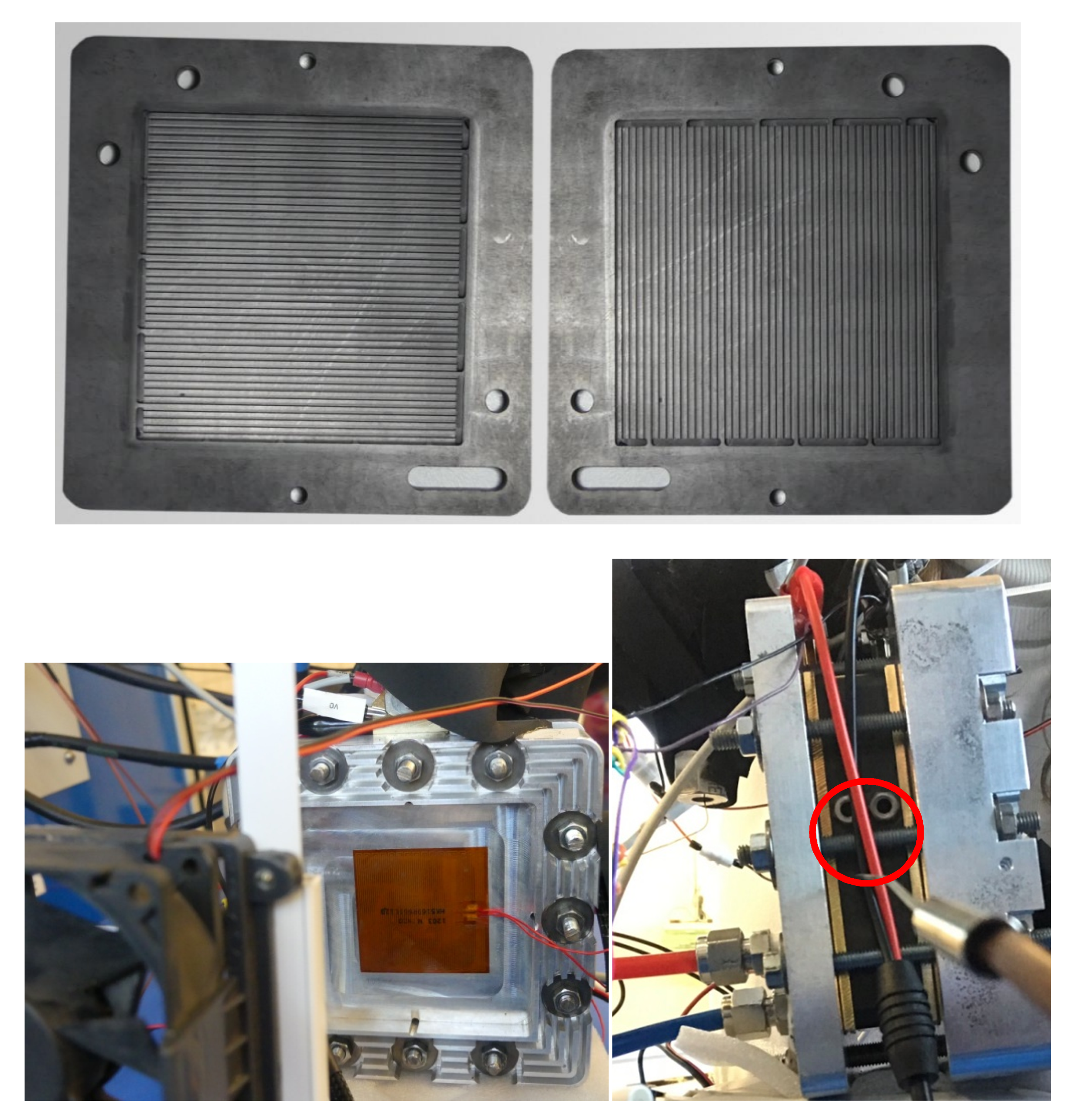
2.1. Materials
2.2. Methods
3. Results
3.1. Cell Performance under Reference Conditions
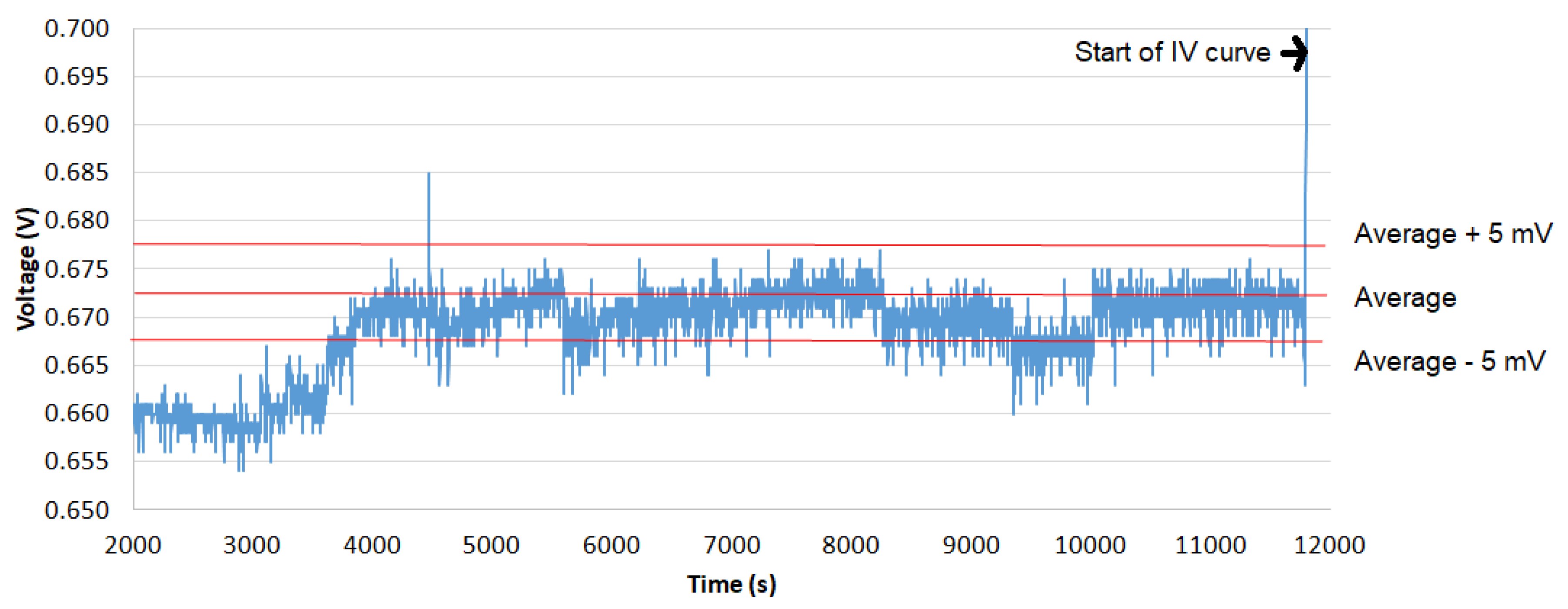
3.1.1. Cell Conditioning
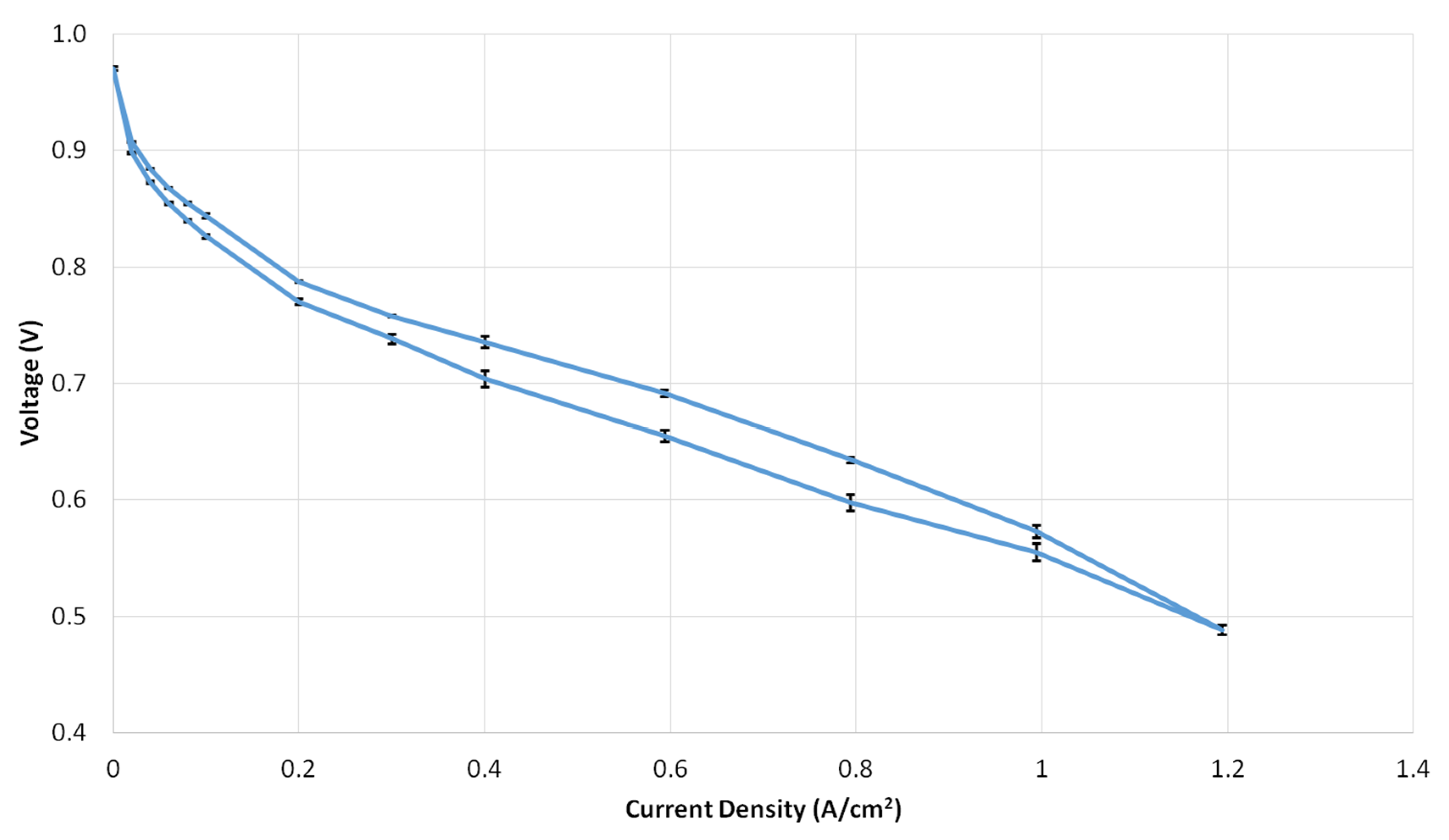
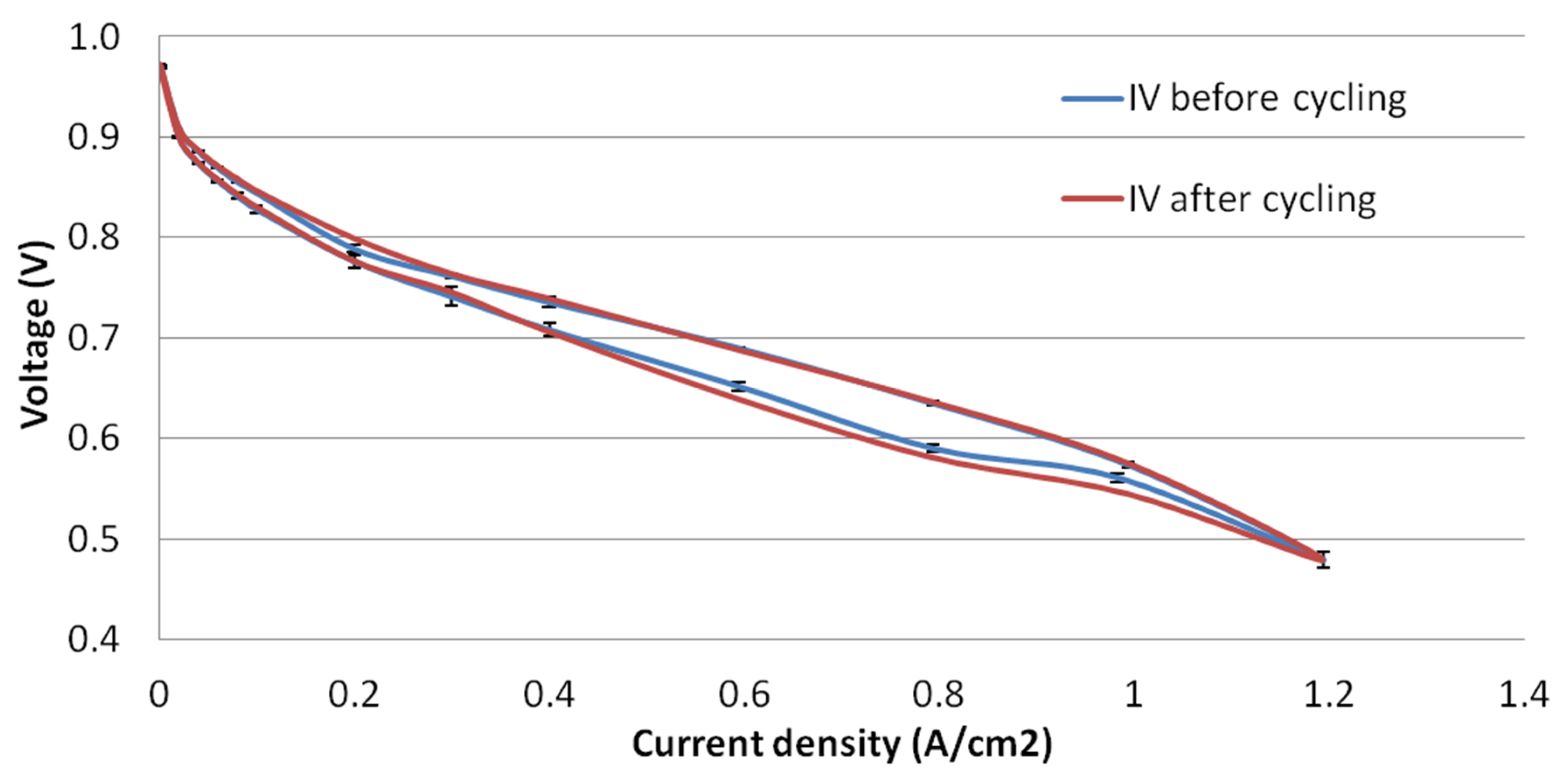
3.1.2. Polarization Curves
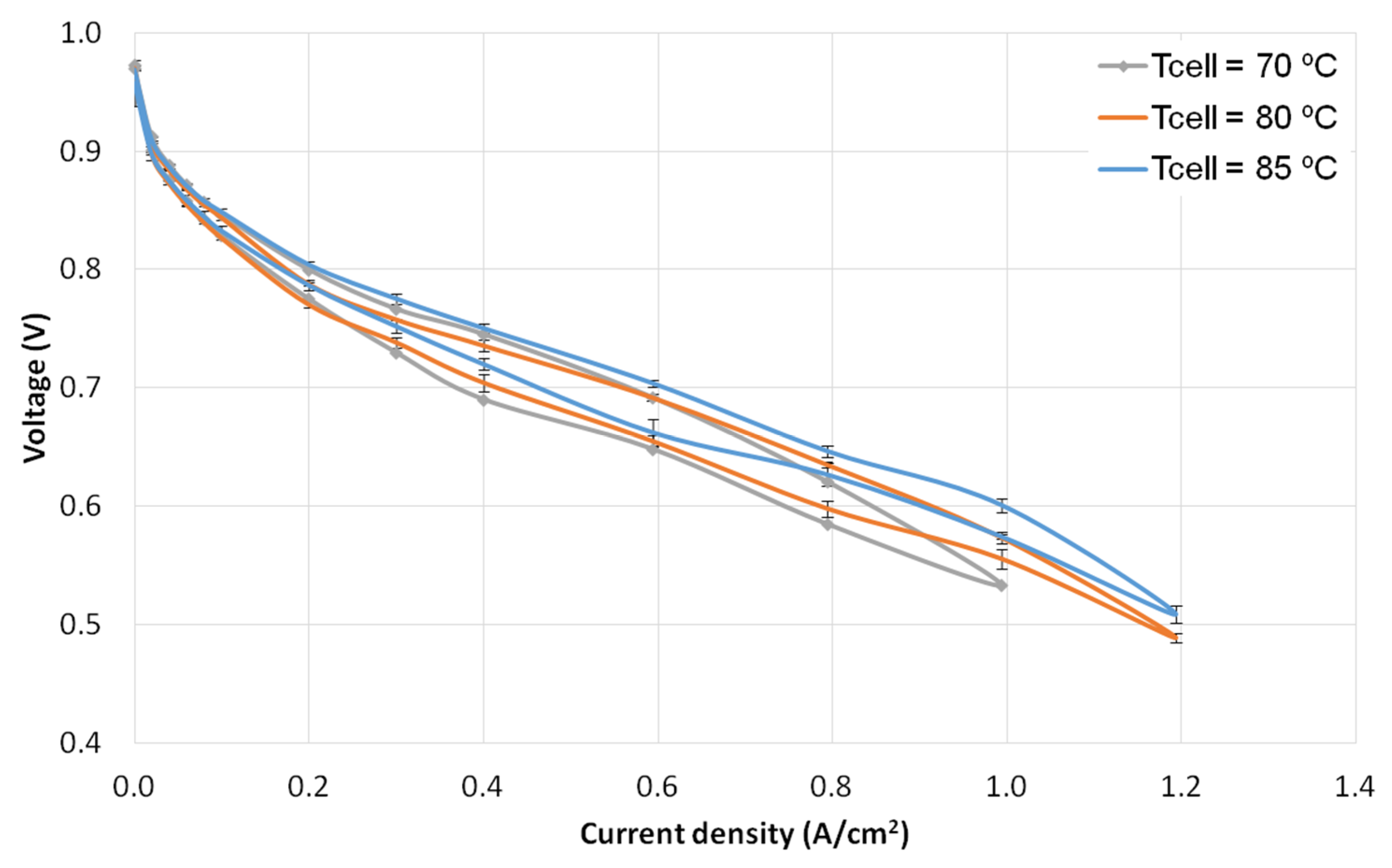
3.2. Temperature Tests
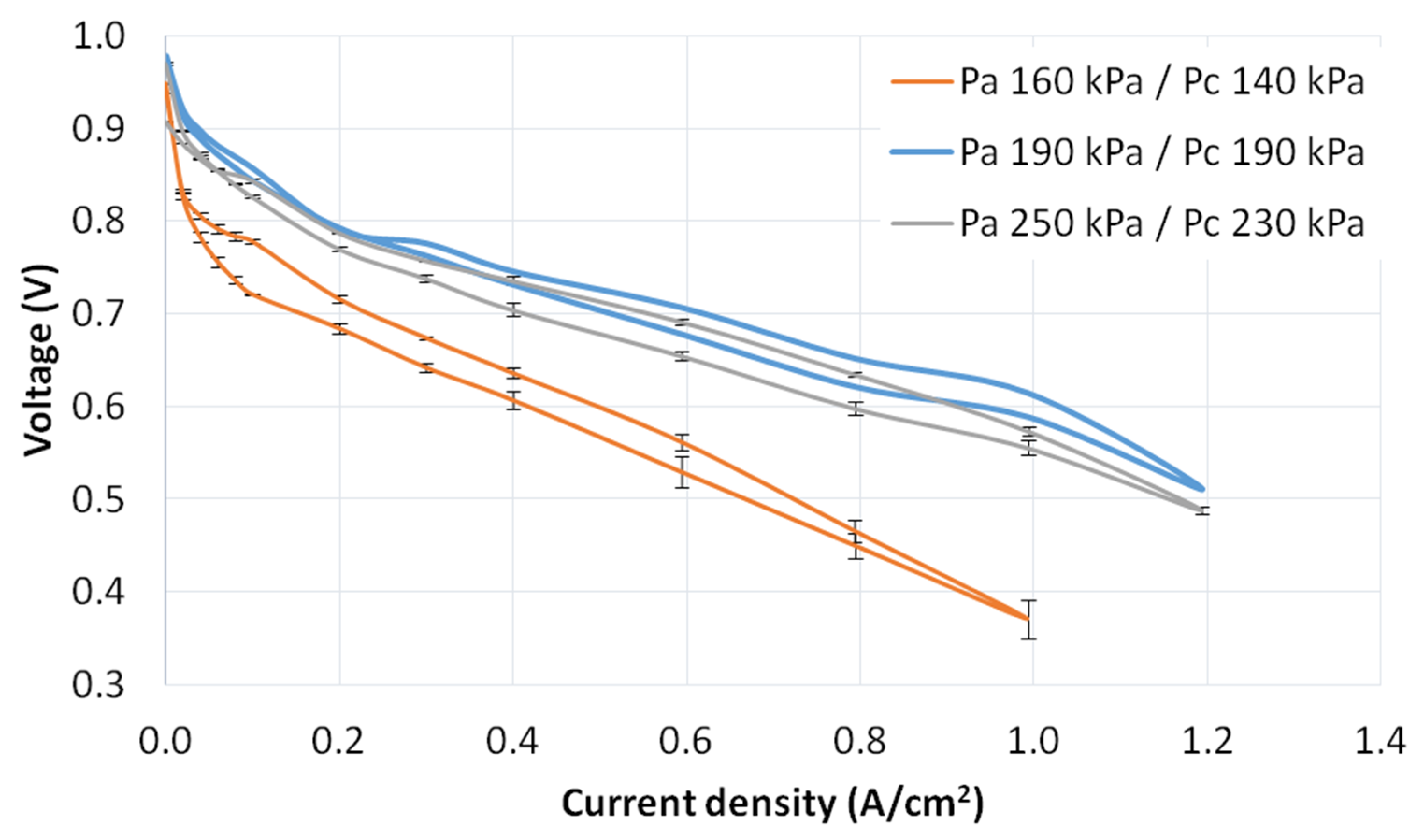
3.3. Pressure Tests
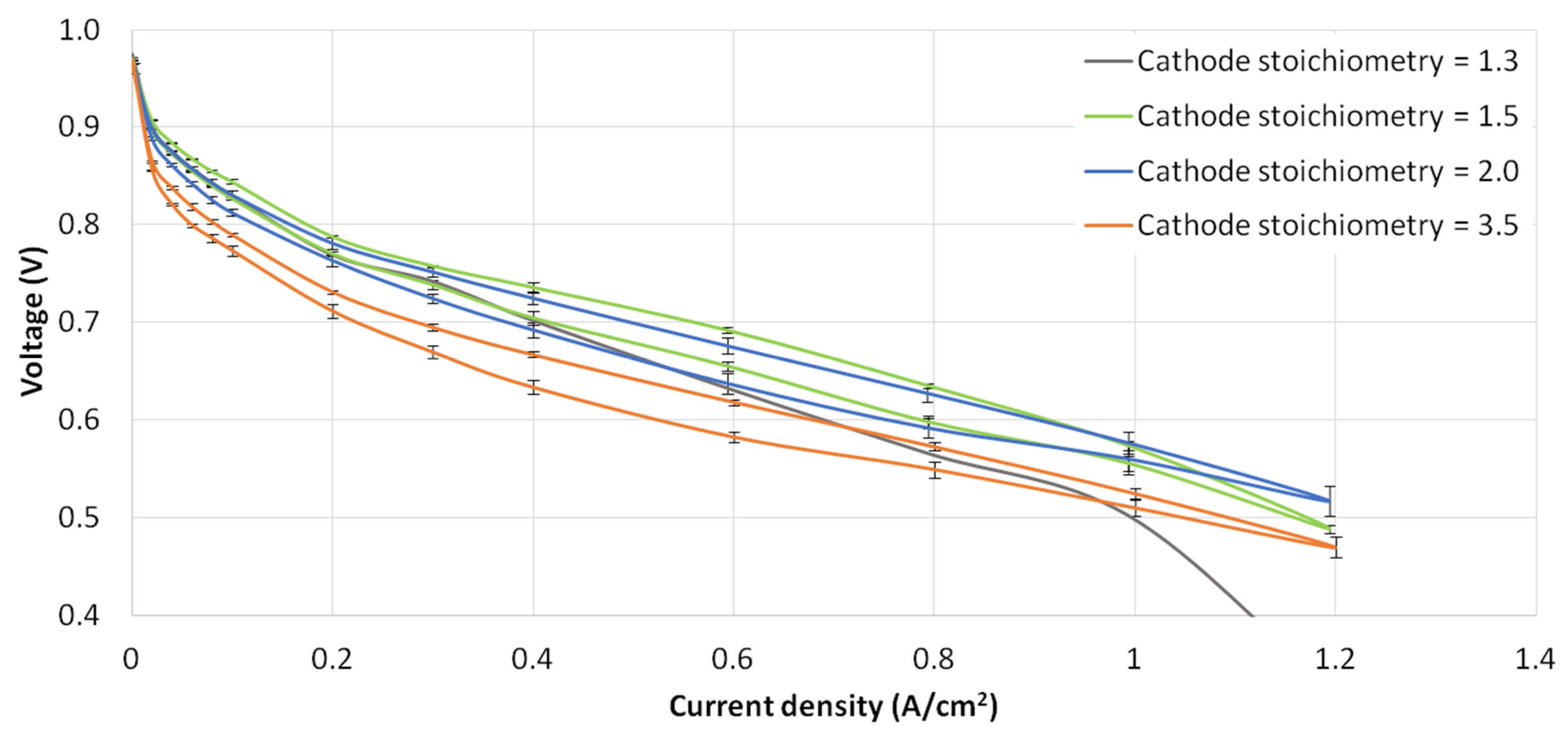
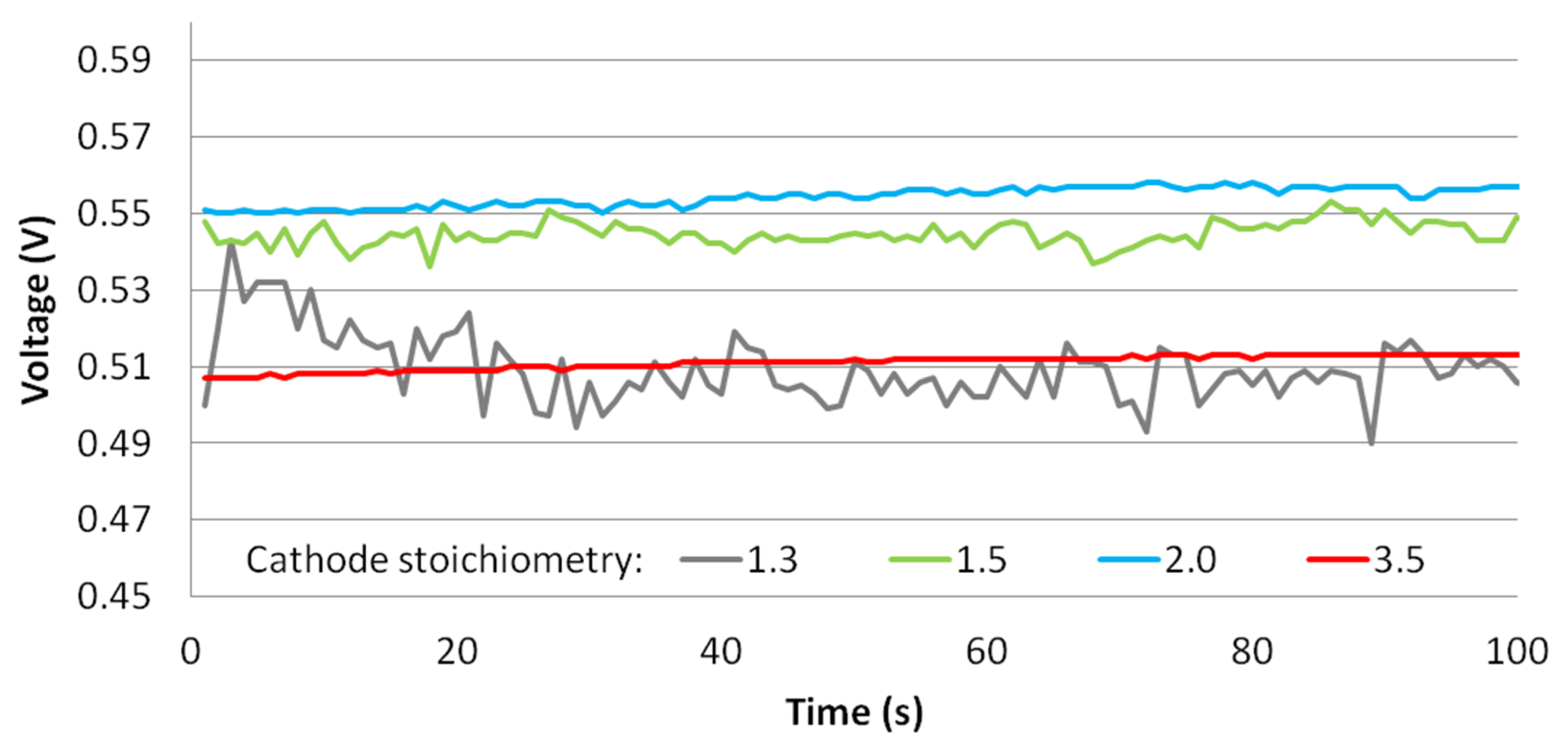
3.4. Cathode Stoichiometry Tests
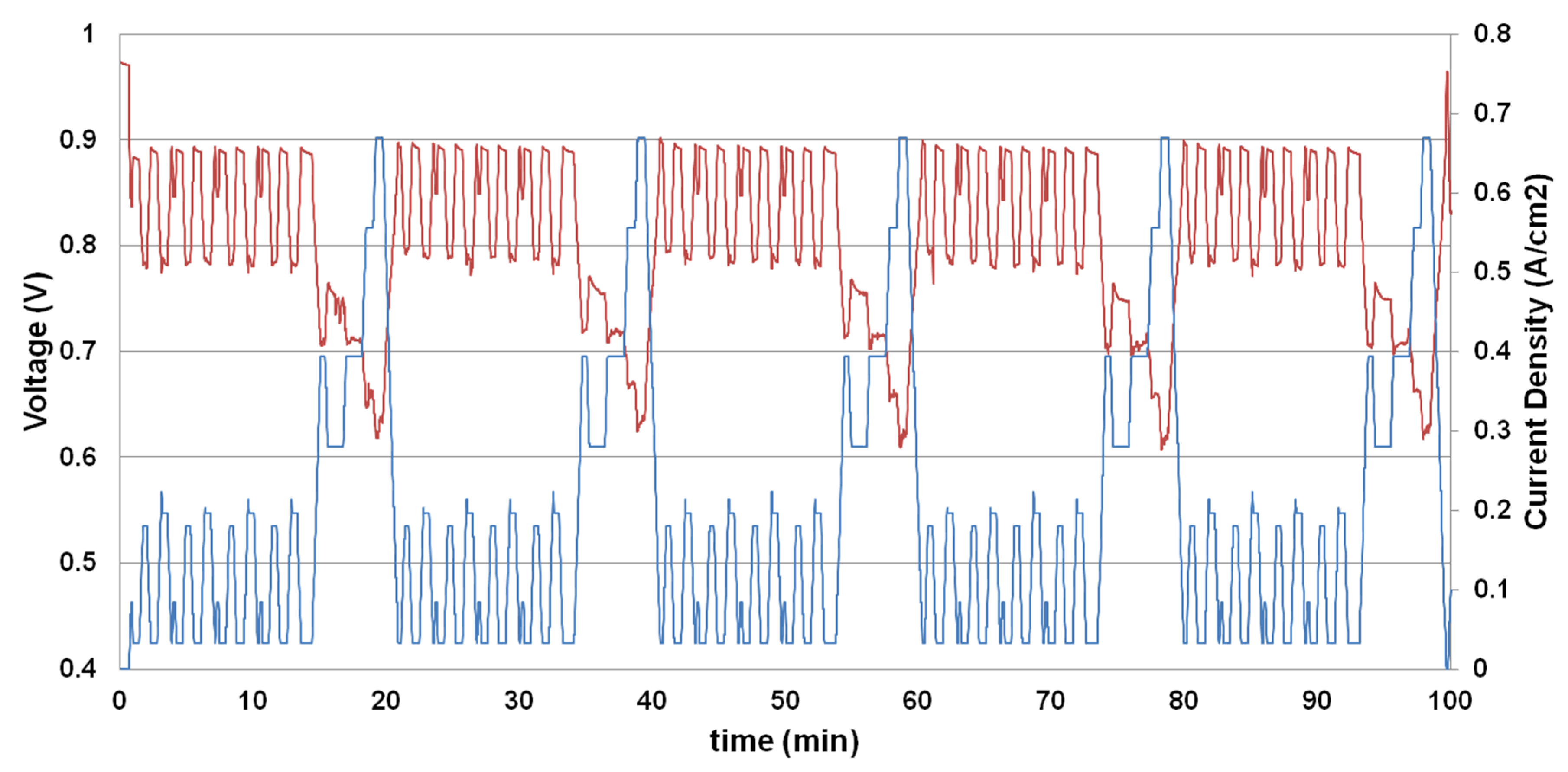
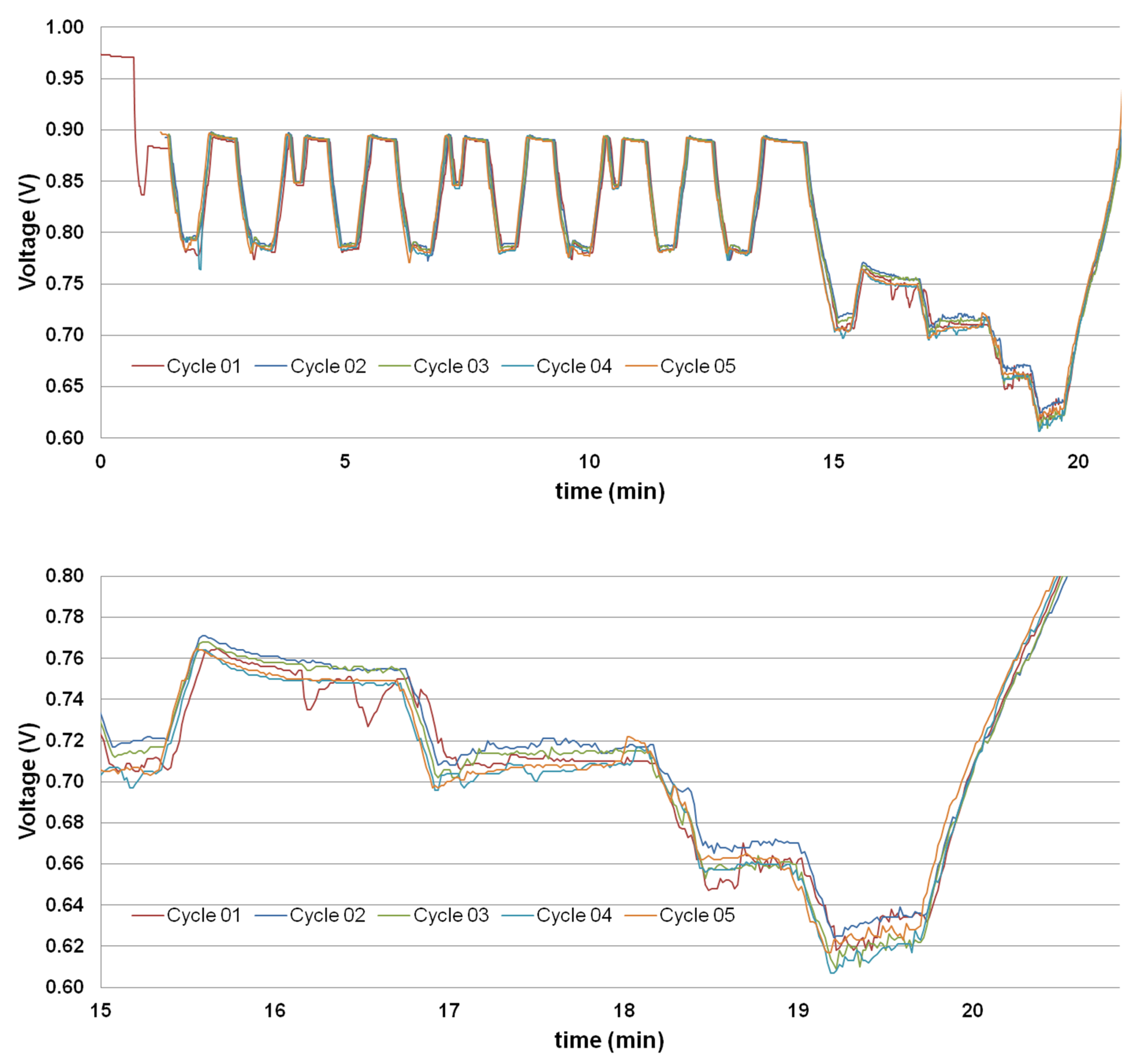
3.5. Load Cycling Tests
4. Discussion
4.1. Effect of the Operating Parameters on the Cell Performance
4.2. Load Cycling Tests
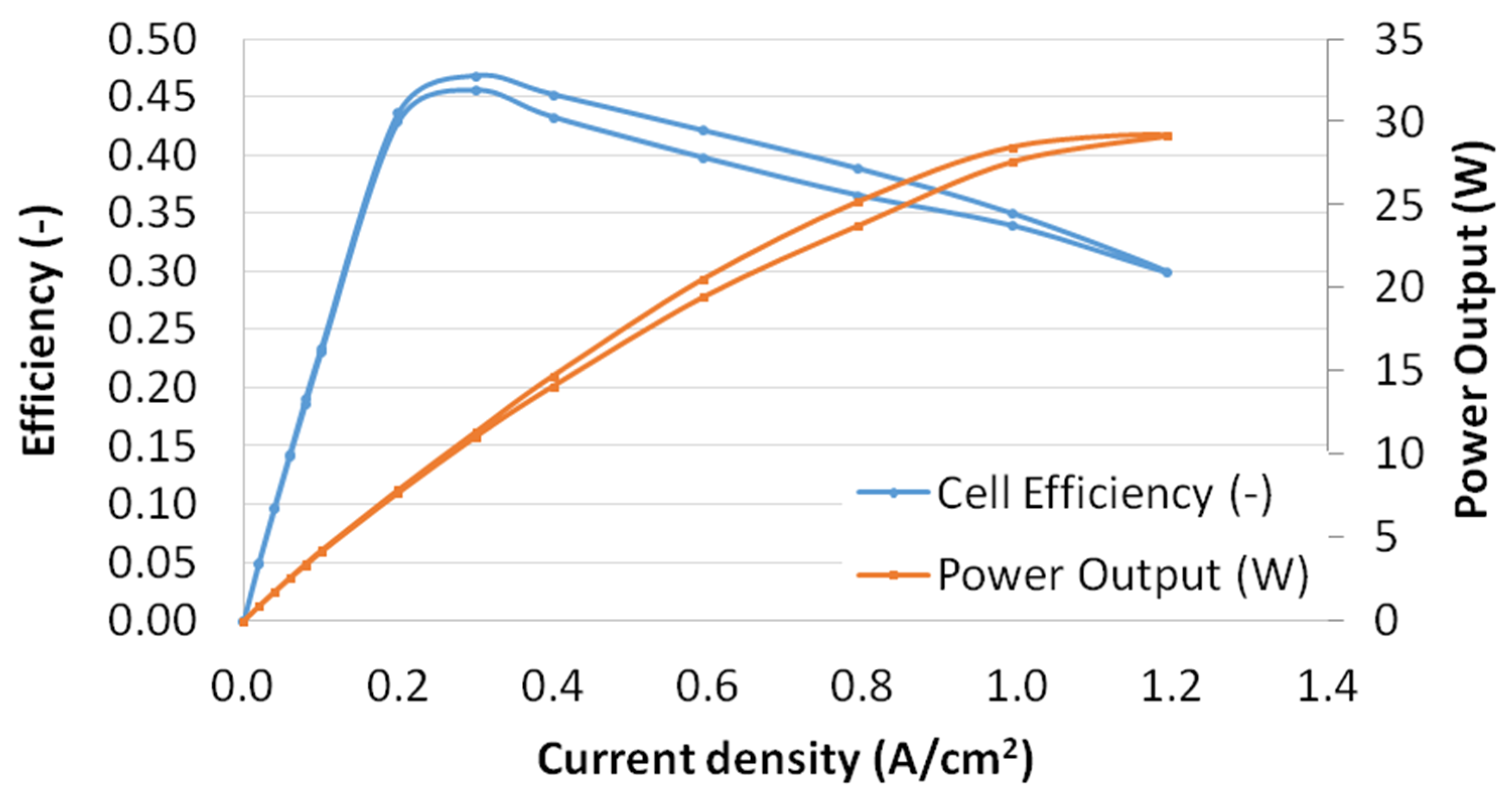
4.3. Cell Electrical Efficiency
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Set Point ID | Current Density (A/cm2) | Dwell Time (s) | Data Acquisition Time (s) |
|---|---|---|---|
| 1 | 0.00 | 60 | 30 |
| 2 | 0.02 | 60 | 30 |
| 3 | 0.04 | 60 | 30 |
| 4 | 0.06 | 60 | 30 |
| 5 | 0.08 | 60 | 30 |
| 6 | 0.10 | 60 | 30 |
| 7 | 0.20 | 200 | 30 |
| 8 | 0.30 | 200 | 30 |
| 9 | 0.40 | 200 | 30 |
| 10 | 0.60 | 200 | 30 |
| 11 | 0.80 | 200 | 30 |
| 12 | 1.00 | 200 | 30 |
| 13 | 1.20 | 200 | 30 |
| 14 | 1.00 | 200 | 30 |
| 15 | 0.80 | 200 | 30 |
| 16 | 0.60 | 200 | 30 |
| 17 | 0.40 | 200 | 30 |
| 18 | 0.30 | 200 | 30 |
| 19 | 0.20 | 200 | 30 |
| 20 | 0.10 | 60 | 30 |
| 21 | 0.08 | 60 | 30 |
| 22 | 0.06 | 60 | 30 |
| 23 | 0.04 | 60 | 30 |
| 24 | 0.02 | 60 | 30 |
| 25 | 0.00 | 60 | 30 |
Appendix B
| Step | Time (s) | Dwell (s) | Load (%) |
|---|---|---|---|
| 1 | 0 | 15 | 0.0 |
| 2 | 15 | 13 | 12.5 |
| 3 | 28 | 33 | 5.0 |
| 4 | 61 | 35 | 26.7 |
| 5 | 96 | 47 | 5.0 |
| 6 | 143 | 20 | 41.7 |
| 7 | 163 | 25 | 29.2 |
| 8 | 188 | 22 | 5.0 |
| 9 | 210 | 13 | 12.5 |
| 10 | 223 | 33 | 5.0 |
| 11 | 256 | 35 | 26.7 |
| 12 | 291 | 47 | 5.0 |
| 13 | 338 | 20 | 41.7 |
| 14 | 358 | 25 | 29.2 |
| 15 | 383 | 22 | 5.0 |
| 16 | 405 | 13 | 12.5 |
| 17 | 418 | 33 | 5.0 |
| 18 | 451 | 35 | 26.7 |
| 19 | 486 | 47 | 5.0 |
| 20 | 533 | 20 | 41.7 |
| 21 | 553 | 25 | 29.2 |
| 22 | 578 | 22 | 5.0 |
| 23 | 600 | 13 | 12.5 |
| 24 | 613 | 33 | 5.0 |
| 25 | 646 | 35 | 26.7 |
| 26 | 681 | 47 | 5.0 |
| 27 | 728 | 20 | 41.7 |
| 28 | 748 | 25 | 29.2 |
| 29 | 773 | 68 | 5.0 |
| 30 | 841 | 58 | 58.3 |
| 31 | 899 | 82 | 41.7 |
| 32 | 981 | 85 | 58.3 |
| 33 | 1066 | 50 | 83.3 |
| 34 | 1116 | 44 | 100 |
| 35 | 1160 | 21 | 0.0 |

Appendix C
Appendix D
References
- Wu, J.; Yuan, X.Z.; Martin, J.J.; Wang, H.; Zhang, J.; Shen, J.; Wu, S.; Merida, W. A review of PEM fuel cell durability: Degradation mechanisms and mitigation strategies. J. Power Sources 2008, 184, 104–119. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, X.; Wang, H.; Mérida, W.; Zhu, H.; Shen, J.; Wu, S.; Zhang, J. A review of accelerated stress tests of MEA durability in PEM fuel cells. Int. J. Hydrog. Energy 2009, 34, 388–404. [Google Scholar] [CrossRef]
- Iranzo, A.; Muñoz, M.; López, E.; Pino, J.; Rosa, F. Experimental fuel cell performance analysis under different operating conditions and bipolar plate designs. Int. J. Hydrog. Energy 2010, 35, 11437–11447. [Google Scholar] [CrossRef]
- Salva, J.A.; Iranzo, A.; Rosa, F.; Tapia, E.; Lopez, E.; Isorna, F. Optimization of a PEM fuel cell operating conditions: Obtaining the maximum performance polarization curve. Int. J. Hydrog. Energy 2016, 41, 19713–19723. [Google Scholar] [CrossRef]
- Wu, J.; Yuan, X.Z.; Wang, H.; Blanco, M.; Martin, J.J.; Zhang, J. Diagnostic tools in PEM fuel cell research: Part I Electrochemical techniques. Int. J. Hydrog. Energy 2008, 33, 1735–1746. [Google Scholar] [CrossRef]
- Salva, J.A.; Iranzo, A.; Rosa, F.; Tapia, E. Experimental validation of the polarization curve and the temperature distribution in a PEMFC stack using a one dimensional analytical model. Int. J. Hydrog. Energy 2016, 41, 20615–20632. [Google Scholar] [CrossRef]
- Zhang, G.; Jiao, K. Multi-phase models for water and thermal management of proton exchange membrane fuel cell: A review. J. Power Sources 2018, 39, 120–133. [Google Scholar] [CrossRef]
- Iranzo, A.; Muñoz, M.; Rosa, F.; Pino, J. Numerical model for the performance prediction of a PEM fuel cell. Model results and experimental validation. Int. J. Hydrog. Energy 2010, 35, 11533–11550. [Google Scholar] [CrossRef]
- Iranzo, A.; Boillat, P.; Biesdorf, J.; Salva, J. Investigation of the liquid water distributions in a 50 cm2 PEM fuel cell: Effects of reactants relative humidity, current density, and cathode stoichiometry. Energy 2015, 82, 914–921. [Google Scholar] [CrossRef]
- Bloom, I.; Walker, L.K.; Basco, J.K.; Malkow, T.; Saturnio, A.; De Marco, G.; Tsotridis, G. A comparison of Fuel Cell Testing protocols—A case study: Protocols used by the U.S. Department of Energy, European Union, International Electrotechnical Commission/Fuel Cell Testing and Standardization Network, and Fuel Cell Technical Team. J. Power Sources 2013, 243, 451–457. [Google Scholar] [CrossRef]
- Tsotridis, G.; Pilenga, A.; De Marco, G.; Malkow, T. EU Harmonised Test Protocols for PEMFC MEA Testing in Single Cell Configuration for Automotive Applications; Publications Office of the European Union: Luxembourg, 2015; ISBN: 978-92-79-54133-9 (print), 978-92-79-54132-2 (PDF). [Google Scholar] [CrossRef]
- “Agreement Concerning the Adoption of Uniform Technical Prescriptions for Wheeled Vehicles, Equipment and Parts Which Can Be fitted and/or Be Used on Wheeled Vehicles and the Conditions for Reciprocal Recognition of Approvals Granted on the Basis of These Prescriptions”, Addendum 100: Regulation no. 101, Uniform Provisions Concerning the Approval of Passenger Cars Powered by an Internal Combustion Engine Only, or Powered by a Hybrid Electric Power Train with Regard to the Measurement of the Emission of Carbon Dioxide and Fuel Consumption and/or the Measurement of Electric Energy Consumption and Electric Range, and of Categories m1 and n1 Vehicles Powered by an Electric Power Train only with Regard to the Measurement of Electric Energy Consumption and Electric Range; E/ECE/324/Rev.2/Add.100/Rev.3 or E/ECE/TRANS/505/Rev.2/Add.100/Rev.3 (12 April 2013); United Nations: Geneva, Switzerland, 2013.
- Huicui, C.; Zhen, S.; Xin, Z.; Tong, Z.; Pucheng, P.; Chen, L. A review of durability test protocols of the proton exchange membrane fuel cells for vehicle. Appl. Energy 2018, 224, 289–299. [Google Scholar]
- Zhao, J.; Li, X. A review of polymer electrolyte membrane fuel cell durability for vehicular applications: Degradation modes and experimental techniques. Energy Convers. Manag. 2019, 199, 112022. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells: Theory and Practice, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2013; ISBN 978-0-12-387710-9. [Google Scholar] [CrossRef]
- Hou, J. A study on polarization hysteresis in PEM fuel cells by galvanostatic step sweep. Int. J. Hydrog. Energy 2011, 36, 7199–7206. [Google Scholar] [CrossRef]
- DOE Technical Targets for Polymer Electrolyte Membrane Fuel Cell Components. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-polymer-electrolyte-membrane-fuel-cell-components (accessed on 12 April 2020).
- Weng, F.B.; Su, A.; Hsu, C.Y. The study of the effect of gas stoichiometric flow rate on the channel flooding and performance in a transparent fuel cell. Int. J. Hydrog. Energyy 2007, 32, 666–676. [Google Scholar] [CrossRef]
- Iranzo, A.; Salva, A.; Boillat, P.; Biesdorf, J.; Tapia, E.; Rosa, F. Water build-up and evolution during the start-up of a PEMFC: Visualization by means of Neutron Imaging. Int. J. Hydrog. Energy 2017, 42, 13839–13849. [Google Scholar] [CrossRef]
- Lin, R.; Cao, C.; Ma, J.; Gülzow, E.; Friedrich, K.A. Optimizing the relative humidity to improve the stability of a proton exchange membrane by segmented fuel cell technology. Int. J. Hydrog. Energyy 2012, 37, 3373–3381. [Google Scholar] [CrossRef]
- Iranzo, A.; Gregorio, J.M.; Boillat, P.; Rosa, F. Bipolar plate research using Computational Fluid Dynamics and neutron radiography for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2020, 45, 12432–12442. [Google Scholar] [CrossRef]
- Mukundan, R.; Baker, A.M.; Kusoglu, A.; Beattie, P.; Knights, S.; Weber, A.Z.; Borup, R.L. Membrane Accelerated Stress Test Development for Polymer Electrolyte Fuel Cell Durability Validated Using Field and Drive Cycle Testing. J. Electrochem. Soc. 2018, 165, F3085–F3093. [Google Scholar] [CrossRef]
- Stariha, S.; Macauley, N.; Sneed, B.T.; Langlois, D.; More, K.L.; Mukundan, R.; Borup, R.L. Recent Advances in Catalyst Accelerated Stress Tests for Polymer Electrolyte Membrane Fuel Cells. J. Electrochem. Soc. 2018, 165, F492–F501. [Google Scholar] [CrossRef]
- Han, J.; Han, J.; Yu, S.; Rosa, F. Experimental analysis of performance degradation of 3-cell PEMFC stack under dynamic load cycle. Int. J. Hydrog. Energy 2020, 45, 13045–13054. [Google Scholar] [CrossRef]
- Mayur, M.; Gerard, M.; Schott, P.; Bessler, W.G. A multi-timescale modeling methodology for PEMFC performance and durability in a virtual fuel cell car. Int. J. Hydrog. Energy 2015, 40, 16466–16476. [Google Scholar] [CrossRef]
- Mayur, M.; Gerard, M.; Schott, P.; Bessler, W.G. Lifetime prediction of a polymer electrolyte membrane fuel cell under automotive load cycling using a physically-based catalyst degradation model. Energies 2018, 11, 2054. [Google Scholar] [CrossRef]
- Ramírez-Cruzado, A.; Ramírez-Peña, B.; Vélez-García, R.; Iranzo, A.; Guerra, J. Data for Experimental analysis of the performance and load cycling of a Polymer Electrolyte Membrane Fuel Cell. Data 2020, 5, 47. [Google Scholar] [CrossRef]
| Operating Condition | Reference Conditions | Test Temperature | Test Pressure | Test Cathode Stoichiometry |
|---|---|---|---|---|
| Cell Temperature (°C) | 80 | 70, 85 | 80 | 80 |
| Anode Pressure (kPa) 1 | 250 | 250 | 160; 190 | 250 |
| Cathode Pressure (kPa) 1 | 230 | 230 | 140; 190 | 230 |
| Anode RH (%) | 50 | 50 | 50 | 50 |
| Cathode RH (%) | 30 | 30 | 30 | 30 |
| Anode Stoichiometry (-) | 1.3 | 1.3 | 1.3 | 1.3 |
| Cathode Stoichiometry (-) | 1.5 | 1.5 | 1.5 | 1.3, 2.0, 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Cruzado, A.; Ramírez-Peña, B.; Vélez-García, R.; Iranzo, A.; Guerra, J. Experimental Analysis of the Performance and Load Cycling of a Polymer Electrolyte Membrane Fuel Cell. Processes 2020, 8, 608. https://doi.org/10.3390/pr8050608
Ramírez-Cruzado A, Ramírez-Peña B, Vélez-García R, Iranzo A, Guerra J. Experimental Analysis of the Performance and Load Cycling of a Polymer Electrolyte Membrane Fuel Cell. Processes. 2020; 8(5):608. https://doi.org/10.3390/pr8050608
Chicago/Turabian StyleRamírez-Cruzado, Andrea, Blanca Ramírez-Peña, Rosario Vélez-García, Alfredo Iranzo, and José Guerra. 2020. "Experimental Analysis of the Performance and Load Cycling of a Polymer Electrolyte Membrane Fuel Cell" Processes 8, no. 5: 608. https://doi.org/10.3390/pr8050608
APA StyleRamírez-Cruzado, A., Ramírez-Peña, B., Vélez-García, R., Iranzo, A., & Guerra, J. (2020). Experimental Analysis of the Performance and Load Cycling of a Polymer Electrolyte Membrane Fuel Cell. Processes, 8(5), 608. https://doi.org/10.3390/pr8050608






