Treatment of Landfill Leachate Using Palm Oil Mill Effluent
Abstract
1. Introduction
2. Methodology
2.1. Collection of the Samples and Landfill Characteristics
2.2. Leachate and POME Characterizations
2.3. Experimental Procedure
2.4. Effect of the Leachate/POME Ratio
2.5. Optimization of Treatment Efficiencies of Targeted Parameters
Analytical Work
3. Results and Discussion
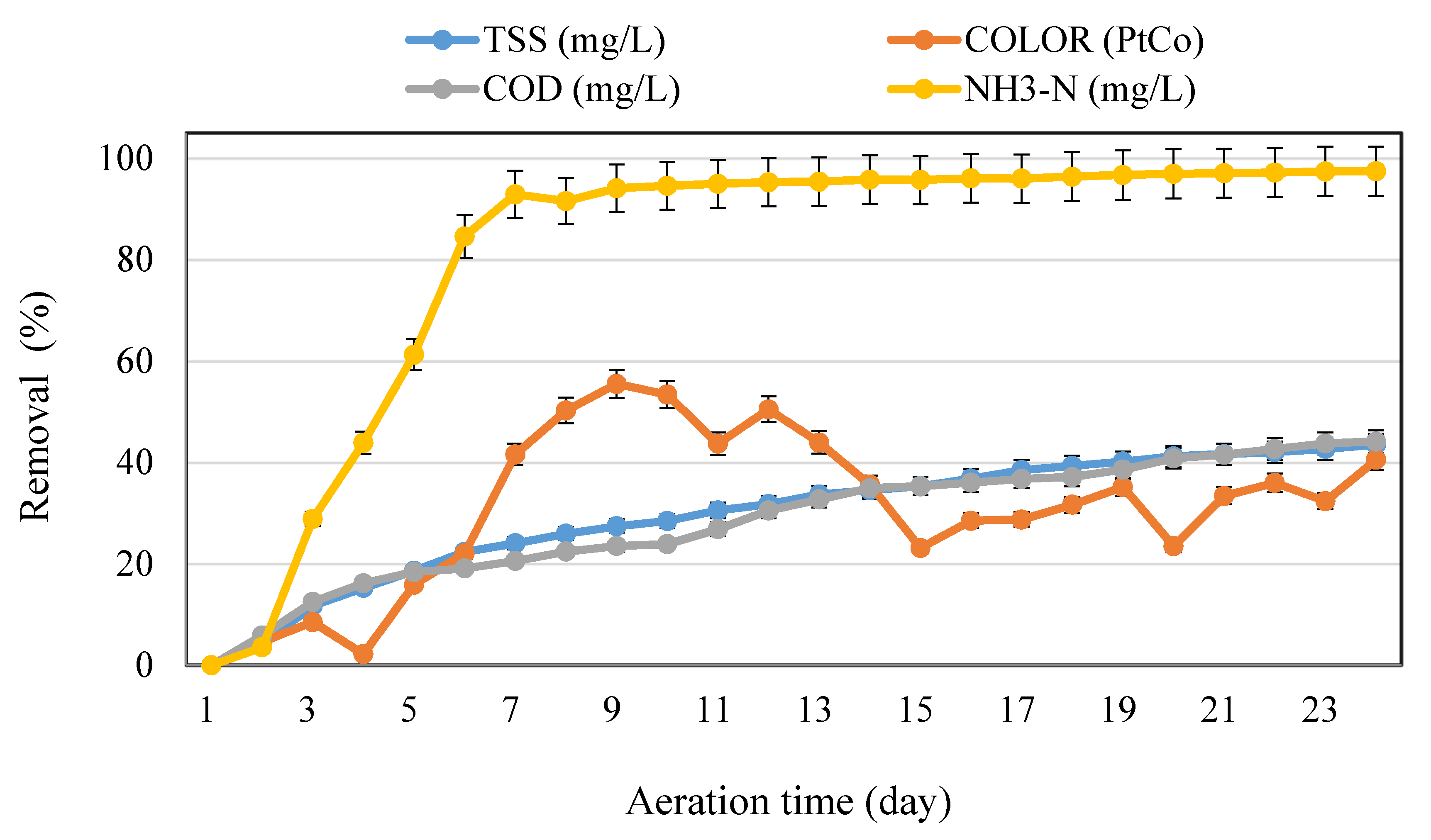
3.1. Effects of Aeration Time Variation on the Removal Efficiency during the Aeration Process of Leachate Treatment
3.2. Effects of Reaction Time Variation on the Removal Efficiency during the Aeration Time of Leachate/POME Treatment (Ratio 900 mL Leachate/100 mL POME)
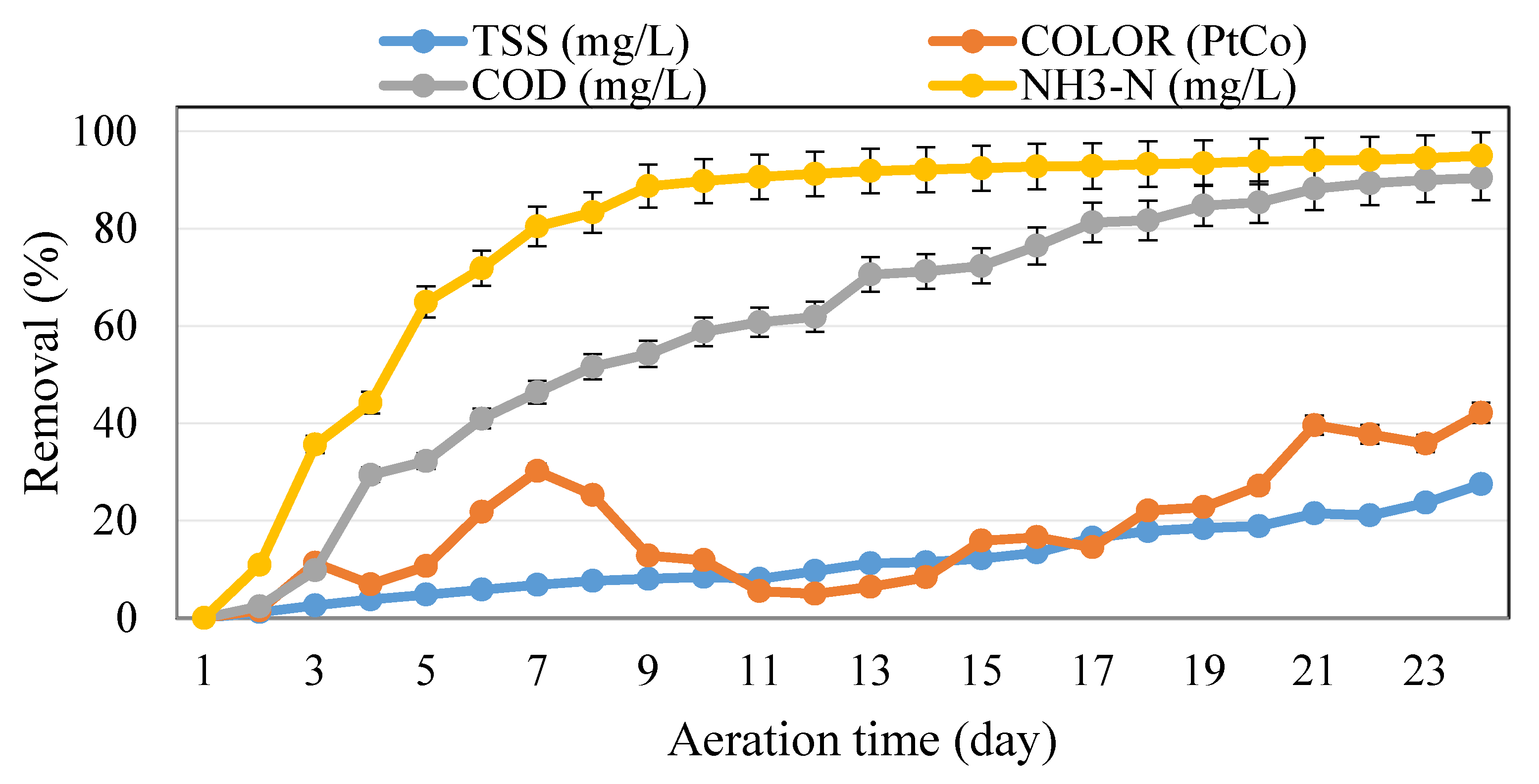
3.3. Effects of Reaction Time Variation on the Removal Efficiency during the Aeration Time of Leachate/POME Treatment (Ratio 700 mL Leachate/300 mL POME)
3.4. Effects of Reaction Time Variation on the Removal Efficiency during the Aeration Time of Leachate/POME Treatment (Ratio 500 mL Leachate/500 mL POME)
3.5. Effects of the Leachate/POME Mixing Ratio on the Leachate Aeration Process
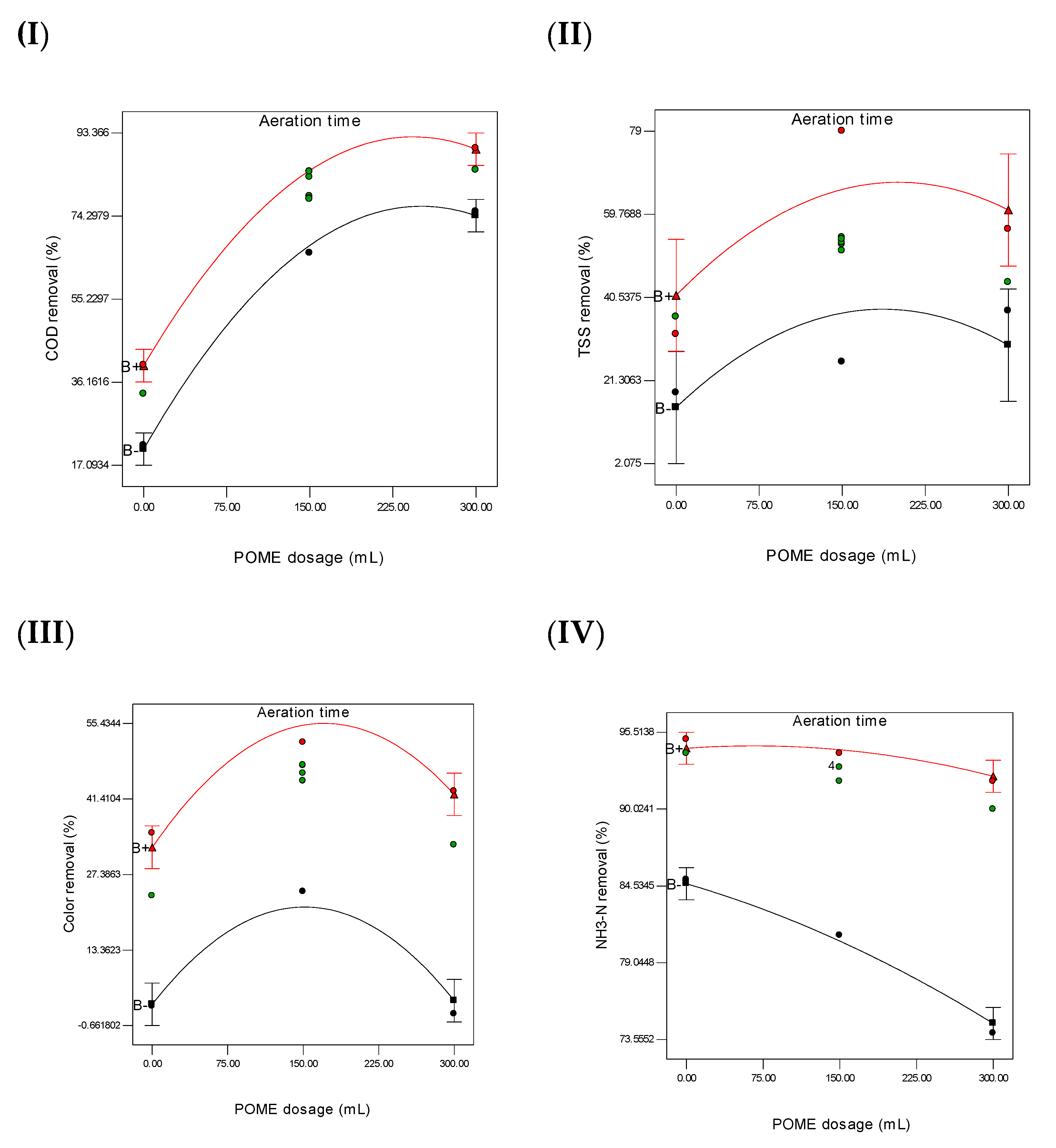
3.6. Analysis of Variance
3.7. Optimization of Leachate Treatment Using POME
3.8. Heavy Metal Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gu, Z.; Chen, W.; Li, Q.; Zhang, A. Treatment of semi-aerobic aged-refuse biofilter effluent from treating landfill leachate with the Fenton method. Process. Saf. Environ. Prot. 2020, 133, 32–40. [Google Scholar] [CrossRef]
- Guvenc, S.Y. Optimization of COD removal from leachate nanofiltration concentrate using H2O2/Fe+2/heat-activated persulfate oxidation process. Process. Saf. Environ. Prot. 2019, 126, 7–17. [Google Scholar] [CrossRef]
- Renou, S.; Givaudan, J.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.S.; Joseph, K.J. Nitrogen management in landfill leachate: Application of SHARON, ANAMMOX and combined SHARON–ANAMMOX process. Waste Manag. 2012, 32, 2385–2400. [Google Scholar] [CrossRef] [PubMed]
- Abdulhasan, M.J.; Hanafiah, M.M.; Satchet, M.S.; Abdulaali, H.S.; Toriman, M.E.; AlRaad, A.A. Combining GIS, fuzzy logic and AHP models for solid waste disposal site selection in Nasiriyah, Iraq. Appl. Ecol. Environ. Res. 2019, 17, 6701–6722. [Google Scholar] [CrossRef]
- Adhikari, B.; Dahal, K.R.; Khanal, S.N. A review of factors affecting the composition of municipal solid waste landfill leachate. Int. J. Eng. Sci. Innov. Technol. 2014, 3, 272–281. [Google Scholar]
- Hermosilla, D.; Cortijo, M.; Huang, C. Optimizing the treatment of landfill leachate by conventional Fenton and photo-Fenton processes. Sci. Total Environ. 2009, 407, 3473–3481. [Google Scholar] [CrossRef]
- Banch, T.J.; Hanafiah, M.M.; Alkarkhi, A.F.; Salem, A.M. Factorial design and optimization of landfill leachate treatment using tannin-based natural coagulant. Polymers 2019, 11, 1349. [Google Scholar] [CrossRef]
- Banch, T.J.; Hanafiah, M.M.; Alkarkhi, A.F.; Salem, A.M. Statistical evaluation of landfill leachate system and its impact on groundwater and surface water in Malaysia. Sains Malays. 2019, 48, 2391–2403. [Google Scholar] [CrossRef]
- Banch, T.J.; Hanafiah, M.M.; Alkarkhi, A.F.; Salem, A.M.; Nizam, N.U. Evaluation of different treatment processes for landfill leachate using low-cost agro-industrial materials. Processes 2020, 8, 111. [Google Scholar] [CrossRef]
- Maslahati Roudi, A.; Chelliapan, S.; Wan Mohtar, W.; Kamyab, H. Prediction and optimization of the fenton process for the treatment of landfill leachate using an artificial neural network. Water 2018, 10, 595. [Google Scholar] [CrossRef]
- Amr, S.S.A.; Aziz, H.A.; Adlan, M.N.J. Optimization of stabilized leachate treatment using ozone/persulfate in the advanced oxidation process. Waste Manag. 2013, 33, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Rada, E.; Istrate, I.; Ragazzi, M.; Andreottola, G.; Torretta, V. Analysis of electro-oxidation suitability for landfill leachate treatment through an experimental study. Sustainability 2013, 5, 3960–3975. [Google Scholar] [CrossRef]
- Bashir, M.J.; Aziz, H.A.; Amr, S.S.A.; Sethupathi, S.A.P.; Ng, C.A.; Lim, J. The competency of various applied strategies in treating tropical municipal landfill leachate. Desalin. Water Treat. 2015, 54, 2382–2395. [Google Scholar] [CrossRef]
- Mojiri, A. Review on membrane bioreactor, ion exchange and adsorption methods for landfill leachate treatment. Aust. J. Basic Appl. Sci. 2011, 5, 1365–1370. [Google Scholar]
- Mussa, Z.H.; Othman, M.R.; Abdullah, M.P. Electrochemical oxidation of landfill leachate: Investigation of operational parameters and kinetics using graphite-PVC composite electrode as anode. J. Braz. Chem. Soc. 2015, 26, 939–948. [Google Scholar] [CrossRef]
- Aziz, N.I.H.A.; Hanafiah, M.M. Life cycle analysis of biogas production from anaerobic digestion of palm oil mill effluent. Renew. Energy 2020, 145, 847–857. [Google Scholar] [CrossRef]
- Aziz, N.I.H.A.; Hanafiah, M.M.; Ali, M.Y. Sustainable biogas production from agrowaste and effluents—A promising step for small-scale industry income. Renew. Energy 2019, 132, 363–369. [Google Scholar] [CrossRef]
- Ma, W.; Han, Y.; Ma, W.; Han, H.; Zhu, H.; Xu, C.; Li, K.; Wang, D. Enhanced nitrogen removal from coal gasification wastewater by simultaneous nitrification and denitrification (SND) in an oxygen-limited aeration sequencing batch biofilm reactor. Bioresour. Technol. 2017, 244, 84–91. [Google Scholar] [CrossRef]
- Aziz, N.I.H.A.; Hanafiah, M.M.; Gheewala, S. A review on life cycle assessment of biogas production: Challenges and future perspectives in Malaysia. Biomass Bioenergy 2019, 122, 361–374. [Google Scholar] [CrossRef]
- Hao, Y.J.; Ji, M.; Chen, Y.X.; Wu, W.X.; Hao, Y.J.; Zhang, S.G.; Liu, H.Q. The pathway of in-situ ammonium removal from aerated municipal solid waste bioreactor: Nitrification/denitrification or air stripping? Waste Manag. Res. 2010, 28, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Daud, Z.; Awang, H. Integrated Leachate Treatment Technology. In Waste Management: Concepts, Methodologies, Tools, and Applications; IGI Global: Hershey, PA, USA, 2020; pp. 204–220. [Google Scholar]
- Jagaba, A.H.; Latiff, A.A.; Latiff, A.; Umaru, I.; Abubakar, S.; Lawal, I. Treatment of Palm Oil Mill Effluent (POME) by Coagulation-Flocculation using Different Natural and Chemical Coagulants: A Review. IOSR J. Mech. Civ. Eng. 2016, 13, 67–75. [Google Scholar]
- Mohamed, A.F.; Yaacob, W.W.; Taha, M.R.; Samsudin, A.R. Groundwater and soil vulnerability in the Langat Basin Malaysia. Eur. J. Sci. Res. 2009, 27, 628–635. [Google Scholar]
- Hanafiah, M.M.; Yussof, M.K.M.; Hasan, M.; AbdulHasan, M.J.; Toriman, M.E. Water quality assessment of Tekala River, Selangor, Malaysia. Appl. Ecol. Environ. Res. 2018, 16, 5157–5174. [Google Scholar] [CrossRef]
- Thaldiri, N.H.; Hanafiah, M.M.; Halim, A.A. Effect of modified micro-sand, poly-aluminium chloride and cationic polymer on coagulation-flocculation process of landfill leachate. Environ. Ecosyst. Sci. 2017, 1, 17–19. [Google Scholar] [CrossRef]
- Banch, T.J.H.; Hanafiah, M.M.; Amr, S.S.A.; Ashraf, M.A. Characterization of leachate from Ampar Tenang closed landfill site, Selangor, Malaysia. AIP Conf. Proc. 2019, 2111, 060009. [Google Scholar]
- Yusoff, I.; Alias, Y.; Yusof, M.; Ashraf, M.A. Assessment of pollutants migration at Ampar Tenang landfill site, Selangor, Malaysia. Sci. Asia 2013, 39, 392–409. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Effluent Limitations Guidelines, Pretreatment Standards and New Source Performance Standards for the Landfills Point Source Category. 2000. Available online: https://www.federalregister.gov/documents/2000/01/19/00–1037/effluent-limitations-guidelines-pretreatment-standards-and-new-sourceperformance-standards-for-the (accessed on 7 January 2020).
- Environmental Quality (Control of Pollution from Solid Waste Transfer Station and Landfill) Regulations Malaysia; FAO: Rome, Italy, 2009.
- APHA-American Public Health Association. 2540 Solids, 2540-D Total Suspended Solids Dried at 103–105 °C; Alpha Analytical, Inc.: Holmes, PA, USA, 2012. [Google Scholar]
- US Environmental Protection Agency. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005; Volume 2.
- Feron, P. (Ed.) Absorption-Based Post-Combustion Capture of Carbon Dioxide; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Bonmatı, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre-or post-treatment to mesophilic anaerobic digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Tatsi, A.A.; Zouboulis, A.I.; Matis, K.A.; Samaras, P. Coagulation–flocculation pretreatment of sanitary landfill leachates. Chemosphere 2003, 53, 737–744. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Balkhair, K.S.; Chowdhury, A.J.K.; Hanafiah, M.M. Treatment of Taman Beringin landfill leachate using the column technique. Desalin. Water Treat. 2019, 149, 370–387. [Google Scholar] [CrossRef]
- Manikam, M.K.; Halim, A.A.; Hanafiah, M.M.; Krishnamoorthy, R.R. Removal of ammonia nitrogen, nitrate, phosphorus and COD from sewage wastewater using palm oil boiler ash composite adsorbent. Desalin. Water Treat. 2019, 149, 23–30. [Google Scholar] [CrossRef]
- Hanafiah, M.M.; Zainuddin, M.F.; Nizam, N.U.M.; Rasool, A. Phytoremediation of Aluminium and Iron from Industrial Wastewater Using Ipomoea aquatica and Centella asiatica. Appl. Sci. 2020, 10, 3064. [Google Scholar] [CrossRef]
- Nizam, N.U.M.; Hanafiah, M.M.; Noor, I.M.; Karim, H.I.A. Efficiency of five selected aquatic plants in phytoremediation of aquaculture wastewater. Appl. Sci. 2020, 10, 1–11. [Google Scholar]
- Asman, N.S.; Halim, A.A.; Hanafiah, M.M.; Ariffin, F.D. Determination of rainwater quality from rainwater harvesting system at Ungku Omar College, UKM Bangi. Sains Malays. 2017, 46, 1211–1219. [Google Scholar] [CrossRef]





| Parameter | Mean and Standard Deviation | (USEPA *; DOE **) |
|---|---|---|
| pH | 7.88 ± 0.50 | 6–9 ** |
| EC (µS/cm) | 6565 ± 324 | 1400 * |
| TDS (mg/L) | 4671 ± 174 | 1000 * |
| TSS (mg/L) | 40.45 ± 8 | 50 ** |
| COD (mg/L) | 893.41 ± 202 | 400 ** |
| BOD5 (mg/L) | 59.20 ± 10 | 20 ** |
| NH3-N (mg/L) | 530.7 ± 22 | 5 ** |
| DO (mg/L) | 5.43 ± 2 | 5 * |
| Mg2+ (mg/L) | 19.72 ± 4 | 0.2 * |
| Ca2+ (mg/L) | 39.72 ± 34 | 0.1 * |
| Na+ (mg/L) | 638.80 ± 303 | 0.02 ** |
| Fe2+ (mg/L) | 0.78 ± 0.6 | 5 ** |
| Zn2+ (µg/L) | 280 ± 16 | 2 ** |
| Cu2+ (µg/L) | 41.87 ± 24 | 0.2 ** |
| Cr2+ (µg/L) | 45.11 ± 17 | 0.01 ** |
| Cd2+ (µg/L) | 0.62 ± 0.7 | 0.01 ** |
| Pb+ (µg/L) | 4.18 ± 2.7 | 0.01 ** |
| As3+ (µg/L) | 17.3 ± 7 | 0.05 ** |
| Co2+ (µg/L) | 11.05 ± 8 | 0.05 * |
| Mn2+ (µg/L) | 61.40 ± 49 | 0.02 ** |
| Parameter | Mean and Standard Deviation | (Standard of DOE *) |
|---|---|---|
| pH | 4.40 ± 0.01 | 5.0–9.0 |
| EC (µS/cm) | 8553 ± 114 | 100 |
| Salinity (ppt) | 4.9 ± 0 | NA |
| TDS (mg/L) | 5713 ± 15 | NA |
| TSS (mg/L) | 3483 ± 76 | 200 |
| Color (Pt/Co) | 5517 ± 104 | 100 |
| COD (mg/L) | 17,400 ± 100 | NA |
| BOD5 (mg/L) | 1243 ± 51 | NA |
| BOD5/COD | 0.07 ± 0 | NA |
| NH3-N (mg/L) | 308 ± 58 | NA |
| DO (mg/L) | 6.74 ± 0.05 | NA |
| Mg2+ (mg/L) | 285 ± 5 | NA |
| Ca2+ (mg/L) | 47.34 ± 0.03 | NA |
| Fe2+ (mg/L) | 45.31 ± 0.9 | 50 |
| Zn2+ (µg/L) | 2603 ± 5.77 | 10 |
| Cu2+ (µg/L) | 2130.00 ± 26.46 | 10 |
| Cr2+ (µg/L) | 910.00 ± 10 | NA |
| Cd2+ (µg/L) | 90.10 ± 0.03 | NA |
| Pb+ (µg/L) | 54.38 ± 0.31 | NA |
| As3+ (µg/L) | 100.22 ± 0.44 | NA |
| Co2+ (µg/L) | 41.08 ± 0.17 | NA |
| Mn2+ (µg/L) | 80.15 ± 0.26 | 10 |
| Run | Factor A: POME (mL) | Factor B: Aeration Time (day) | COD Removal % | Color Removal % | TSS Removal % | NH3-N Removal % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actual | Predicted | Residual | Actual | Predicted | Residual | Actual | Predicted | Residual | Actual | Predicted | Residual | |||
| 1 | 150 | 21 | 84.5 | 84.79 | −0.29 | 51.89 | 55.1 | −3.21 | 79 | 65.53 | 13.47 | 94 | 94.26 | −0.26 |
| 2 | 150 | 14 | 78.81 | 80.18 | −1.37 | 47.58 | 46.1 | 1.48 | 53.2 | 53.57 | −0.37 | 93 | 92.83 | 0.17 |
| 3 | 150 | 14 | 78.31 | 80.18 | −1.87 | 44.73 | 46.1 | −1.37 | 51.3 | 53.57 | −2.27 | 93 | 92.83 | 0.17 |
| 4 | 150 | 7 | 65.8 | 67.58 | −1.78 | 24.21 | 21.37 | 2.84 | 25.6 | 36.92 | −11.32 | 81 | 80.6 | 0.4 |
| 5 | 150 | 14 | 78.18 | 80.18 | −2 | 47.69 | 46.1 | 1.59 | 54 | 53.57 | 0.43 | 93 | 92.83 | 0.17 |
| 6 | 300 | 14 | 84.9 | 86.02 | −1.12 | 32.9 | 30.98 | 1.92 | 44 | 47.46 | −3.46 | 90 | 88.93 | 1.07 |
| 7 | 0 | 14 | 33.46 | 34.4 | −0.94 | 23.44 | 25.73 | -2.29 | 36 | 30.38 | 5.62 | 94 | 94.93 | −0.93 |
| 8 | 0 | 7 | 21.66 | 20.82 | 0.84 | 2.92 | 3.29 | -0.37 | 18.46 | 15.07 | 3.39 | 85 | 84.7 | 0.3 |
| 9 | 300 | 7 | 75.32 | 74.39 | 0.93 | 1.5 | 3.97 | −2.47 | 37.4 | 29.47 | 7.93 | 74 | 74.7 | −0.7 |
| 10 | 0 | 21 | 40.08 | 39.98 | 0.099 | 35.1 | 32.44 | 2.66 | 32 | 41 | −9 | 95 | 94.37 | 0.63 |
| 11 | 300 | 21 | 89.83 | 89.64 | 0.19 | 42.81 | 42.26 | 0.55 | 56.3 | 60.76 | −4.46 | 92 | 92.37 | −0.37 |
| 12 | 150 | 14 | 84.46 | 80.18 | 4.28 | 44.71 | 46.1 | −1.39 | 54.4 | 53.57 | 0.83 | 93 | 92.83 | 0.17 |
| 13 | 150 | 14 | 83.22 | 80.18 | 3.04 | 46.15 | 46.1 | 0.052 | 52.8 | 53.57 | −0.77 | 92 | 92.83 | −0.83 |
| COD Removal (%) | Source | Sum of Squares | DF | Mean Square | F Value | Prob > F |
| Model | 5981.97 | 5 | 1196.39 | 190.7 | <0.0001 | |
| A | 3996.42 | 1 | 3996.42 | 637 | <0.0001 | |
| B | 444.28 | 1 | 444.28 | 70.81 | <0.0001 | |
| A2 | 1101.81 | 1 | 1101.81 | 175.62 | <0.0001 | |
| B2 | 44.26 | 1 | 44.26 | 7.06 | 0.0326 | |
| AB | 3.82 | 1 | 3.82 | 0.61 | 0.4607 | |
| Residual | 43.92 | 7 | 6.27 | |||
| Lack of Fit | 7.85 | 3 | 2.62 | 0.29 | 0.8314 | |
| Pure Error | 36.07 | 4 | 9.02 | |||
| Cor Total | 6025.89 | 12 | ||||
| SD: 2.50, R2: 0.9927, mean: 69.12, CV:3.62, Pred R2: 0.9814, Adeq Precision: 40.447. | ||||||
| TSS Removal (%) | Source | Sum of Squares | DF | Mean Square | F Value | Prob > F |
| Model | 2468.26 | 5 | 493.65 | 6.46 | 0.0148 | |
| A | 437.59 | 1 | 437.59 | 5.72 | 0.048 | |
| B | 1228.08 | 1 | 1228.08 | 16.06 | 0.0051 | |
| A2 | 592.46 | 1 | 592.46 | 7.75 | 0.0272 | |
| B2 | 15.2 | 1 | 15.2 | 0.2 | 0.6691 | |
| AB | 7.18 | 1 | 7.18 | 0.094 | 0.7681 | |
| Residual | 535.16 | 7 | 76.45 | |||
| Lack of Fit | 529.33 | 3 | 176.44 | 121.02 | 0.0002 | |
| Pure Error | 5.83 | 4 | 1.46 | |||
| Cor Total | 3003.43 | 12 | ||||
| SD: 8.74, R2: 0.8218, mean: 45.73, CV:19.12, Pred R2: −0.7897, Adeq Precision: 8.495. | ||||||
| Color Removal (%) | Source | Sum of Squares | DF | Mean Square | F Value | Prob > F |
| Model | 3328.56 | 5 | 665.71 | 94.36 | <0.0001 | |
| A | 41.34 | 1 | 41.34 | 5.86 | 0.046 | |
| B | 1705.89 | 1 | 1705.89 | 241.8 | <0.0001 | |
| A2 | 869.58 | 1 | 869.58 | 123.26 | <0.0001 | |
| B2 | 170.8 | 1 | 170.8 | 24.21 | 0.0017 | |
| AB | 20.84 | 1 | 20.84 | 2.95 | 0.1294 | |
| Residual | 49.39 | 7 | 7.06 | |||
| Lack of Fit | 40.88 | 3 | 13.63 | 6.41 | 0.0523 | |
| Pure Error | 8.5 | 4 | 2.13 | |||
| Cor Total | 3377.94 | 12 | ||||
| SD: 2.66, R2: 0.9854, mean: 34.28, CV: 7.75, Pred R2: 0.8733, Adeq Precision: 28.712. | ||||||
| NH3-N Removal (%) | Source | Sum of Squares | DF | Mean Square | F Value | Prob > F |
| Model | 458.76 | 5 | 91.75 | 154.36 | <0.0001 | |
| A | 54 | 1 | 54 | 90.85 | <0.0001 | |
| B | 280.17 | 1 | 280.17 | 471.33 | <0.0001 | |
| A2 | 2.22 | 1 | 2.22 | 3.73 | 0.0446 | |
| B2 | 80.43 | 1 | 80.43 | 135.32 | <0.0001 | |
| AB | 16 | 1 | 16 | 26.92 | 0.0013 | |
| Residual | 4.16 | 7 | 0.59 | |||
| Lack of Fit | 3.36 | 3 | 1.12 | 5.6 | 0.0647 | |
| Pure Error | 0.8 | 4 | 0.2 | |||
| Cor Total | 462.92 | 12 | ||||
| SD: 0.77, R2: 0.991, mean: 89.92, CV: 0.86, Pred R2: 0.9238, Adeq Precision: 38.623. | ||||||
| POME | Aeration Time | COD Removal (%) | TSS Removal (%) | Color Removal (%) | NH3-N Removal (%) | Desirability |
|---|---|---|---|---|---|---|
| 188.38 | 21.00 | 89.83 | 67.10 | 55.19 | 93.95 | 0.935 |
| Lab experiment | 87.15 | 65.54 | 52.78 | 91.75 | ||
| Heavy Metals | Initial Concentration in Leachate | Residual after Treatment Process | Removal (%) |
|---|---|---|---|
| Fe2+ (µg/L) | 20.04 ± 7.11 | 1856 ± 0.57 | 90.73 |
| Zn2+ (µg/L) | 280.00 ± 19.63 | 10.76 ± 2.10 | 96.16 |
| Cu2+ (µg/L) | 41.91 ± 20.19 | 1.28 ± 0.64 | 96.95 |
| Cr2+ (µg/L) | 45.11 ± 12.81 | 2.80 ± 1.01 | 93.78 |
| Cd2+ (µg/L) | 22.62 ± 3.51 | 0.23 ± 0.20 | 99.00 |
| Pb+ (µg/L) | 4.18 ± 2.91 | 0.28 ± 0.12 | 93.30 |
| As3+ (µg/L) | 17.34 ± 4.29 | 16.15 ± 2.37 | 6.85 |
| Co2+ (µg/L) | 11.05 ± 5.54 | 0.53 ± 0.39 | 95.24 |
| Mn2+ (µg/L) | 48.61 ± 14.99 | 31.72 ± 7.16 | 34.75 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banch, T.J.H.; Hanafiah, M.M.; Amr, S.S.A.; Alkarkhi, A.F.M.; Hasan, M. Treatment of Landfill Leachate Using Palm Oil Mill Effluent. Processes 2020, 8, 601. https://doi.org/10.3390/pr8050601
Banch TJH, Hanafiah MM, Amr SSA, Alkarkhi AFM, Hasan M. Treatment of Landfill Leachate Using Palm Oil Mill Effluent. Processes. 2020; 8(5):601. https://doi.org/10.3390/pr8050601
Chicago/Turabian StyleBanch, Tawfiq J. H., Marlia M. Hanafiah, Salem S. Abu Amr, Abbas F. M. Alkarkhi, and Mohammed Hasan. 2020. "Treatment of Landfill Leachate Using Palm Oil Mill Effluent" Processes 8, no. 5: 601. https://doi.org/10.3390/pr8050601
APA StyleBanch, T. J. H., Hanafiah, M. M., Amr, S. S. A., Alkarkhi, A. F. M., & Hasan, M. (2020). Treatment of Landfill Leachate Using Palm Oil Mill Effluent. Processes, 8(5), 601. https://doi.org/10.3390/pr8050601








