Comprehensive Screening of Drug Encapsulation and Co-Encapsulation into Niosomes Produced Using a Microfluidic Device
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
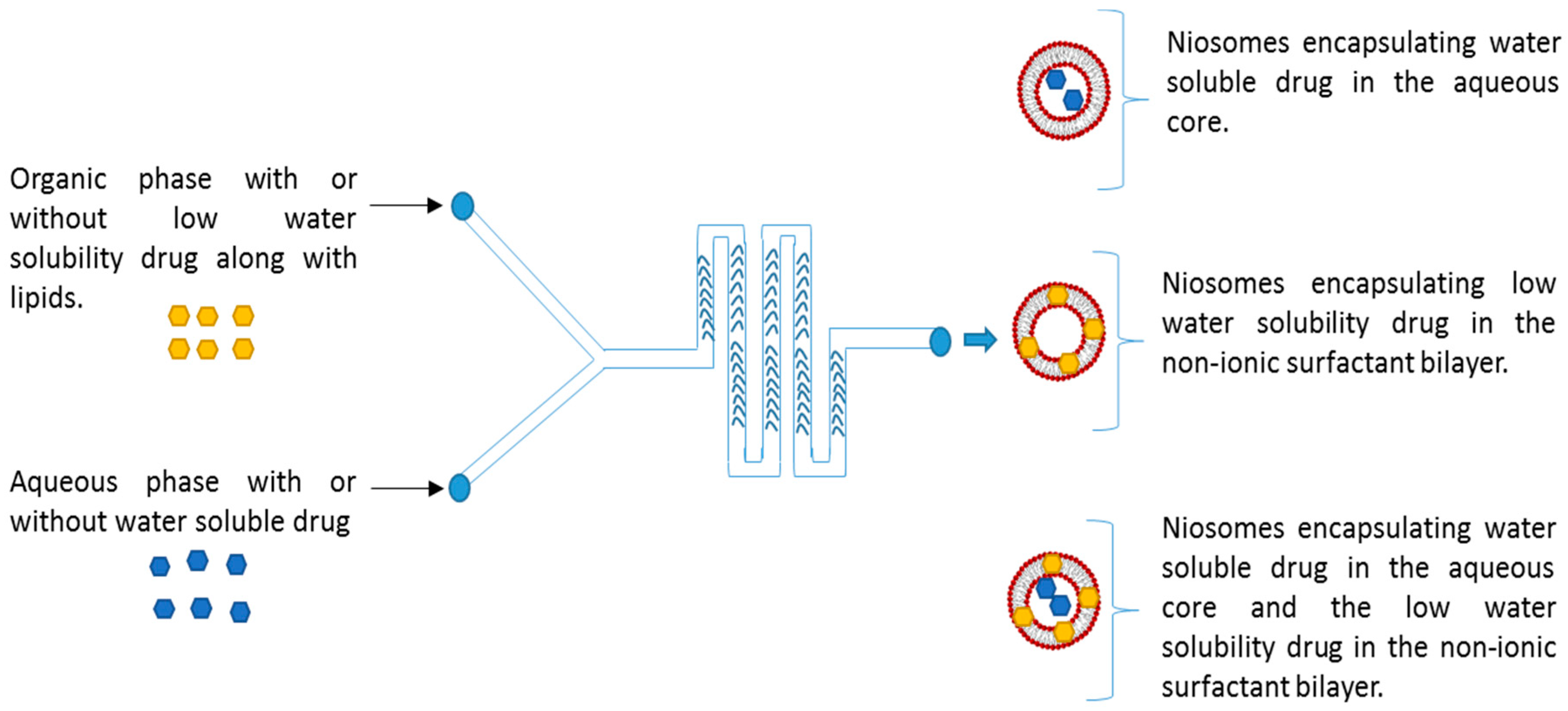
2.2. Preparation of Niosomes Using Microfluidics
2.3. Particle Characteristics
2.4. Determination of Percent Encapsulation
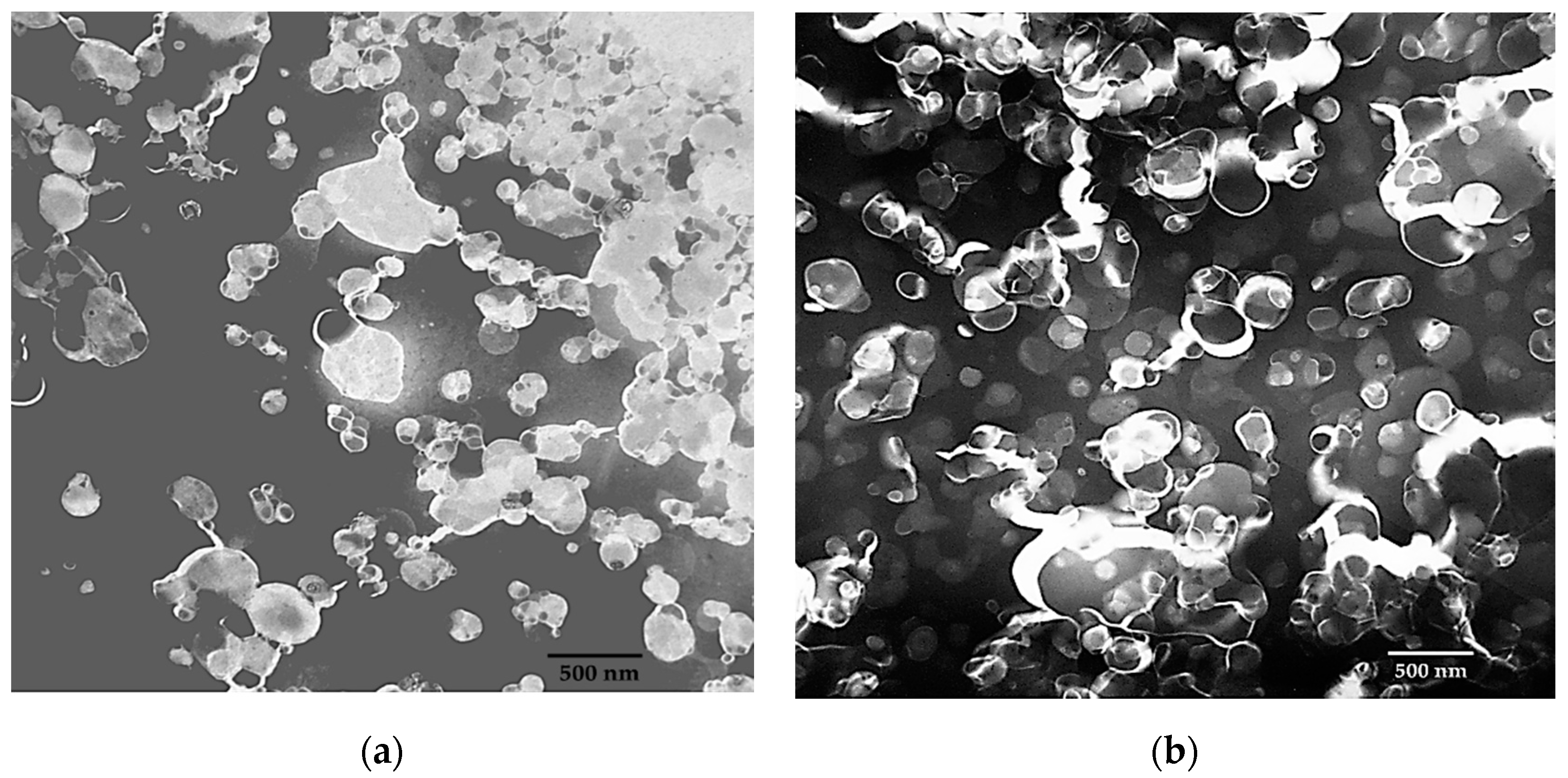
2.5. Transmission Electron Microscopy (TEM)
2.6. Statistical Analysis
3. Results
3.1. Optimization of Microfluidics Method Parameters for the Production of Niosomes
3.2. Effect of Cholesterol Concentration on Niosome Size
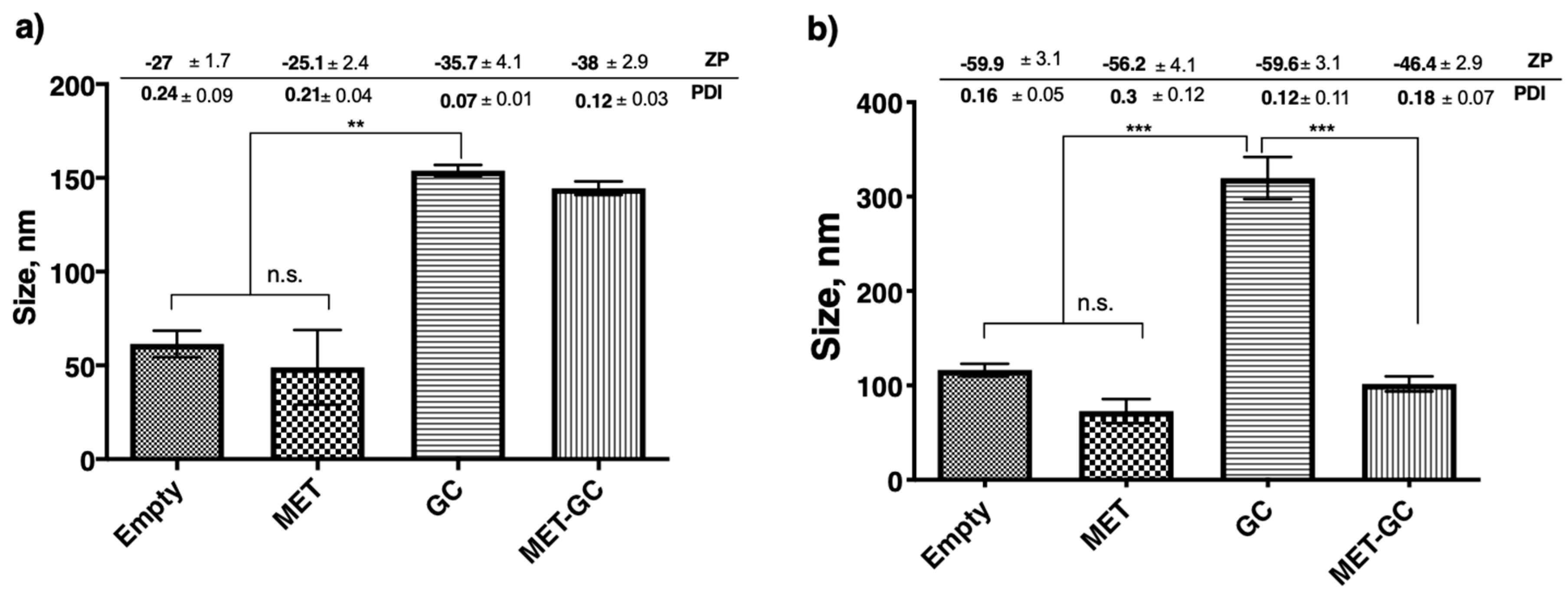
3.3. Effect of Drug Encapsulation on the Size of the Niosomes
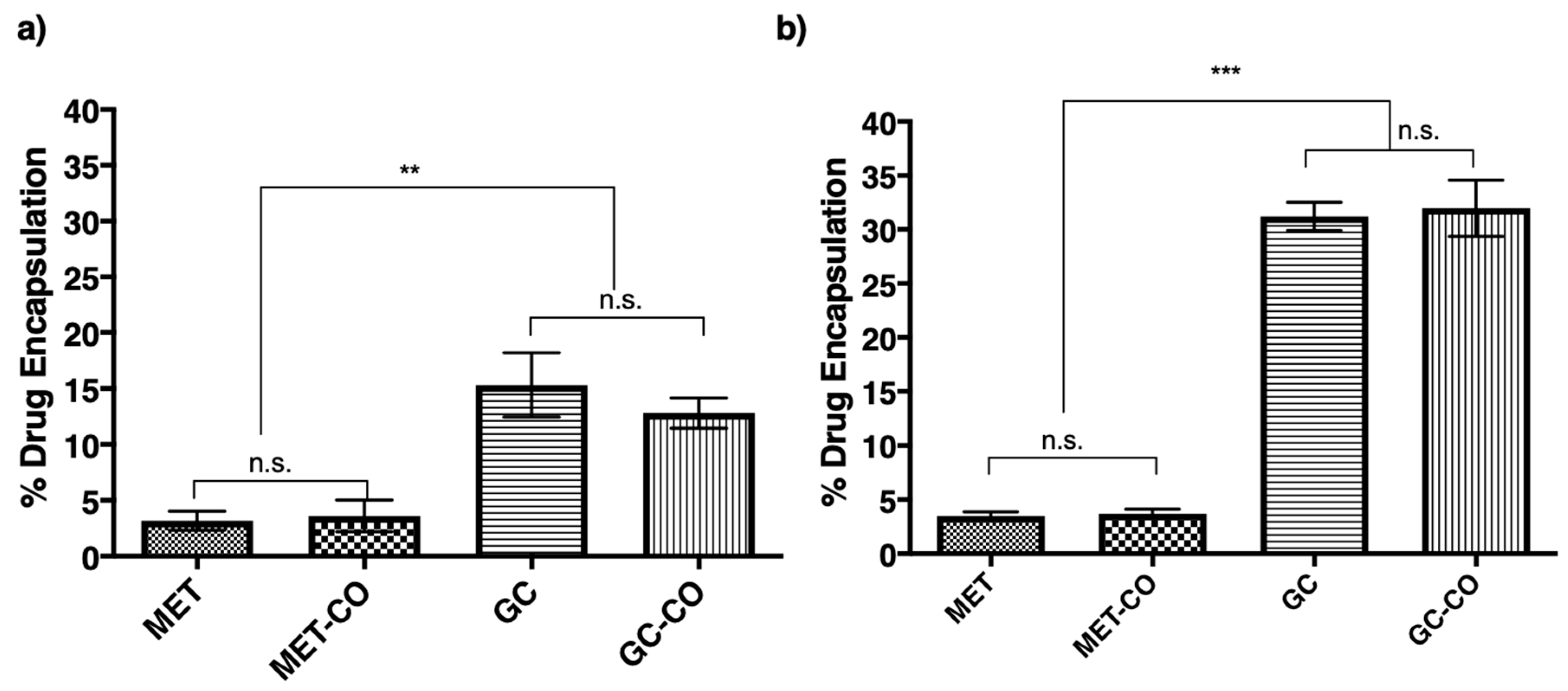
3.4. Encapsulation of Drug into the Niosomes
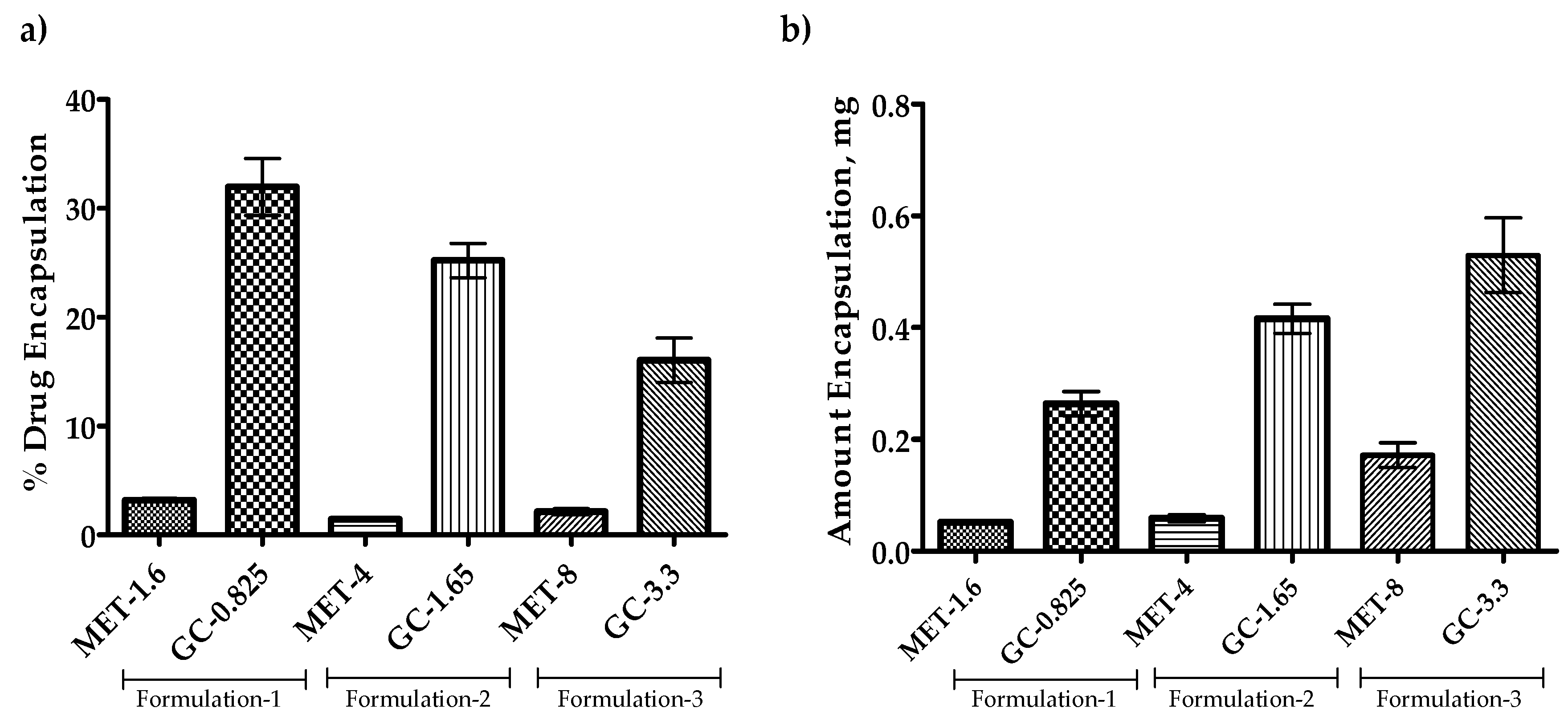
3.5. Effect of Drug Amount Escalation on Percent Encapsulation
3.6. Microscopic Elucidation Niosomal Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MET | Metformin |
| GC | Garcinol |
| HLB | Hydrophilic-lipophilic balance |
| SUV | Small unilamellar vesicles |
| MLV | Multilamellar vesicles |
| LUV | Large unilamellar vesicles |
| PDI | The polydispersity index |
| DCP | Diacetyl phosphate |
| PBS | Phosphate-buffered saline |
| TFR | Total flow rate |
| FRR | Flow rate ratio |
| TEM | Transmission electron microscopy |
References
- Kastner, E.; Kaur, R.; Lowry, D.; Moghaddam, B.; Wilkinson, A.; Perrie, Y. High-throughput manufacturing of size-tuned liposomes by a new microfluidics method using enhanced statistical tools for characterization. Int. J. Pharm. 2014, 477, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, E.; Dorati, R.; Modena, T.; Conti, B.; Genta, I. Multivariate analysis for the optimization of microfluidics-assisted nanoprecipitation method intended for the loading of small hydrophilic drugs into PLGA nanoparticles. Int. J. Pharm. 2018, 536, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Ag Seleci, D.; Maurer, V.; Stahl, F.; Scheper, T.; Garnweitner, G. Rapid Microfluidic Preparation of Niosomes for Targeted Drug Delivery. Int. J. Mol. Sci. 2019, 20, 4696. [Google Scholar] [CrossRef] [PubMed]
- Mahale, N.B.; Thakkar, P.D.; Mali, R.G.; Walunj, D.R.; Chaudhari, S.R. Niosomes: Novel sustained release nonionic stable vesicular systems—An overview. Adv. Colloid Interface Sci. 2012, 183–184, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Uchegbu, I.F.; Vyas, S.P. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int. J. Pharm. 1998, 172, 33–70. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef] [PubMed]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Huang, Y.; Chen, Z.; Ye, J.; Xu, H.; Chen, W.; Long, X. Niosomal Nanocarriers for Enhanced Skin Delivery of Quercetin with Functions of Anti-Tyrosinase and Antioxidant. Molecules 2019, 24, 2322. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Florence, A.T. Non-ionic surfactant vesicles (niosomes): Physical and pharmaceutical chemistry. Adv. Colloid Interface Sci. 1995, 58, 1–55. [Google Scholar] [CrossRef]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control Release 2014, 185, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Jadon, P.S.; Gajbhiye, V.; Jadon, R.S.; Gajbhiye, K.R.; Ganesh, N. Enhanced oral bioavailability of griseofulvin via niosomes. AAPS PharmSciTech 2009, 10, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Attia, I.A.; El-Gizawy, S.A.; Fouda, M.A.; Donia, A.M. Influence of a niosomal formulation on the oral bioavailability of acyclovir in rabbits. AAPS PharmSciTech 2007, 8, E106. [Google Scholar] [CrossRef] [PubMed]
- Bayindir, Z.S.; Yuksel, N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J. Pharm. Sci. 2010, 99, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Kirby, C.; Clarke, J.; Gregoriadis, G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem. J. 1980, 186, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Weston, N.; Coombes, A.G.; Fitzgerald, M.; Perrie, Y. Liposome formulation of poorly water soluble drugs: Optimisation of drug loading and ESEM analysis of stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef]
- Ali, M.H.; Kirby, D.J.; Mohammed, A.R.; Perrie, Y. Solubilisation of drugs within liposomal bilayers: Alternatives to cholesterol as a membrane stabilising agent. J. Pharm. Pharmacol. 2010, 62, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Bawage, S.; Tiwari, P.; Kirby, D.; Perrie, Y.; Dennis, V.; Singh, S.R. Liposomes: A promising carrier for respiratory syncytial virus therapeutics. Expert Opin. Drug Deliv. 2019, 16, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.A.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an alternative for liposomal delivery. PLoS ONE 2018, 13, e0194179. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, S.; White, R.; Sahu, R.; Dennis, V.A.; Singh, S.R. Comprehensive Screening of Drug Encapsulation and Co-Encapsulation into Niosomes Produced Using a Microfluidic Device. Processes 2020, 8, 535. https://doi.org/10.3390/pr8050535
Joshi S, White R, Sahu R, Dennis VA, Singh SR. Comprehensive Screening of Drug Encapsulation and Co-Encapsulation into Niosomes Produced Using a Microfluidic Device. Processes. 2020; 8(5):535. https://doi.org/10.3390/pr8050535
Chicago/Turabian StyleJoshi, Sameer, Roderica White, Rajnish Sahu, Vida A. Dennis, and Shree R. Singh. 2020. "Comprehensive Screening of Drug Encapsulation and Co-Encapsulation into Niosomes Produced Using a Microfluidic Device" Processes 8, no. 5: 535. https://doi.org/10.3390/pr8050535
APA StyleJoshi, S., White, R., Sahu, R., Dennis, V. A., & Singh, S. R. (2020). Comprehensive Screening of Drug Encapsulation and Co-Encapsulation into Niosomes Produced Using a Microfluidic Device. Processes, 8(5), 535. https://doi.org/10.3390/pr8050535





