Abstract
The NOx reduction in the iron ore pelletizing process becomes an important environmental concern owing to its role in the formation of photochemical smog and acid rain. Thus, it is essential to develop new technologies for reducing NOx emissions in order to contribute to the cleaner production of pellets. In this paper, NOx reduction by advanced reburning ingrate-rotary kiln for oxidized pellet production was performed on a laboratory-scale gas kiln. Temperature and NH3/NOx molar ratio (NSR) were the key factors affecting the reduction of NOx. A better denitrification effect can be obtained on flus gas with higher initial NOx concentration, at temperature = 900 °C, NSR = 1.2, and reaction time exceeds one second. NOx reduction rate had reached 55–65% when the initial NOx concentration was above 400 ppm, and exceeds 70% when the initial NOx concentration was around 680 ppm. Urea solution has the best denitrification effect compared with NH3·H2O and NH4HCO3 solution. As for additives, the denitrification effect of the vanadium-titanium catalyst was better than that of ethanol and NaCl, while NaCl plays a promotive role at low NSR. Finally, a series of denitrification measures that include advanced reburning technology for achieving NOx ultra-low emission in the oxidation pellet production was proposed.
1. Introduction
Iron ore pellets are a main iron-bearing burden for the blast furnace ironmaking process [1,2]. Because of its good metallurgical properties and low energy consumption in the production process, pellets can increase production, save coke, improve technical and economic indexes of ironmaking, reduce hot metal cost and improve economic benefits when applied to the blast furnace ironmaking process, as a consequence, it has been developed rapidly. The world’s pellet production was close to 450 million tons in 2015, and China’s annual pellet production peaked 200 million tons in 2011 [3,4,5,6,7]. However, with the increasingly strict environmental protection requirements, China has formulated a series of emission standards for iron and steel enterprises, forcing pelletizing plants to do corresponding work in environmental protection. Although dust removal and desulfurization can meet the emission targets, NOx emission has become a restriction factor in the development and production of pelletizing plants because of its high removal cost and complex process [8,9,10]. Pellet production in China is mainly produced by a grate-rotary kiln process, and its output accounts for nearly 60% of the output of the total pellet [11,12]. Many studies have shown that NOx formation in rotary kilns occurs in the flame, due to either the high temperatures (thermal NOx) or the oxidation of the fuel-bound nitrogen [13,14]. When coal is combusted in iron-ore rotary kilns, the NOx formation is dominated by the conversion of char-N to NO [15,16]. At present, NOx emission reduction is mainly accomplished through reducing the injection of coal gas or pulverized coal, reducing rotary kiln temperature, and using lower NOx raw materials and fuels in China’s pelletizing plants. Although these measures can reduce the emission of NOx to a certain extent, it will cause the decline of pellet output and quality, which goes against the normal production and development of pelletizing plants. Besides, the reason for the low efficiency of primary measures is that most focus on suppressing the formation of NOx from the volatile nitrogen or the thermal NO mechanism. For the purpose of the desired reduction in NOx formation, a recent study by Edland, R. et al. suggested that more drastic measures must be implemented, such as switching fuel or designing the process so that the excess air can be reduced. Their simulations showed that replacing the reference coal with a biomass that contains 0.1% nitrogen can reduce NOx emissions by 90% [17]. Generally, the emission concentration of nitrogen oxides from pelletizing plants are generally between 150 mg/m3 and 300 mg/m3, which causes great pollution to the environment and is far from meeting the requirements of today’s environmental protection; hence, it is urgent for modern iron and steel enterprises to develop novel NOx reduction approaches in the grate-rotary kiln process.
Nitrogen oxides (NOx, x = 1,2), as major air pollutants, are resulting in a series of environmental issues, such as photochemical smog, acid rain, ozone depletion, and fine particle pollution. Many efforts have been made to reduce NOx emission by using advanced combustion technologies or by using post-combustion abatement technologies [18,19]. Among various kinds of NOx removal technologies, a simple process for reducing NOx to nitrogen and water is an ideal way. Two major post-combustion NOx control techniques are selective catalytic reduction and selective non-catalytic reduction, which are widely used in large combustion units, such as various boilers, refineries, and waste incinerators [20,21,22]. There have been many investigations on the effect of parameters on the performance of NOx catalytic reduction in the laboratory, but several investigations have been reported about NOx reduction by advanced reburning, the effects of temperature, NH3/NOx molar ratio (NSR), and other factors on NOx reduction have been studied, under the optimized conditions, the efficiency NOx reduction of advanced reburning could potentially increase to more than 85% [23,24,25,26,27]. However, these investigations are mainly focused on cement precalciner kilns and utility boilers; the application of advanced reburning in oxidized pellet production has not been reported yet. Besides, many experiments were performed in various laboratory-scale reactor, all of them which temperature was controlled by an electric heating furnace, designed to maintain the reaction temperature at the desired value, and the NOx reduction reaction process in a gas-fired environment does not simulate well. Thus, the advanced reburning of in grate-rotary kiln needs further study.
The objective of this present study was to investigate the influences of temperature, NSR, and other factors on NOx reduction and ammonia slip, by advanced reburning; as well as to optimize the operating parameters of the grate-rotary kiln for the better development of pellet production, and then proposes the mechanism or the most rational operating technology that contribute to a further reduction in the NOx content of exhaust gas in the iron ore pelletizing production.
2. Materials and Methods
The chemicals used in the laboratory-scale gas kiln test, including the ammonia liquor (NH3·H2O), ammonium bicarbonate (NH4HCO3), urea (CH4N2O), sodium chloride (NaCl), ethanol absolute (C2H6O), were analytically pure and used without further treatment. Vanadium-titanium catalyst, obtained from national engineering laboratory for multi-flue gas pollution control technology and equipment of China, was milled to produce ultrafine powders. NO (purity ≥ 99.9%) used in this work was purchased from Wuhan new radar special gas co., ltd. In the Hubei province of China.
In this paper, the advanced reburning in a grate-rotary kiln for NOx reduction was performed on a laboratory-scale gas kiln. The experimental system schematic is shown in Figure 1a, and a photograph of the grate-rotary kiln in Figure 1b–d. The inner diameter of the gas kiln is 0.4 m, and its length is 5 m. A gas burner was equipped at the head of the kiln, and an auxiliary heating device was installed on the middle side. Gas and air were pumped into the burner for combustion, and the temperature was regulated by gas flowmeters. Four temperature measuring points were arranged in the kiln body by inserting thermocouples.
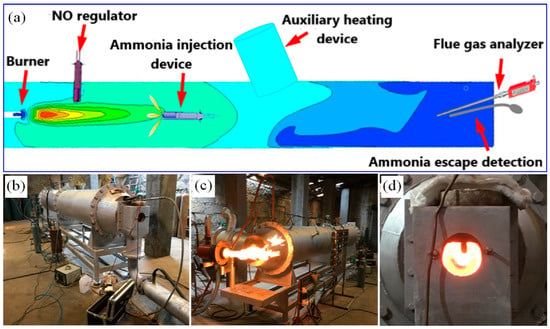
Figure 1.
Schematic of the experimental system (a) and the laboratory-scale gas kiln (b–d).
NO was injected into the front side of the kiln, and the gas concentration was controlled by a gas flowmeter. NH3·H2O injection device was inserted from the tail of the kiln, and the insertion length can be adjusted, and the amount of NH3·H2O injection was regulated by a liquid flowmeter. In addition, flue gas analyzer and ammonia slip detection device were also inserted from the tail of kiln. The experimental conditions of each group lasted for more than 6 min. The data were recorded every 2 min, and the average value was taken as the experimental results. The composition of flue gas was measured by German MRU Nova plus multi-functional flue gas analyzer through the probe, mainly including the concentration of O2, CO2, CO, NO, and NOx. The concentration of O2 and CO2 were expressed as a percentage, and the concentration of CO, NO, and NOx as a ppm. The monitoring point in the rotary kiln, as shown in Figure 1. The NOx reduction efficiency was calculated by the following Formula (1):
where ηNOx was the NOx reduction rate (%), CNOx,in (ppm) and CNOx,out (ppm) were the inlet and outlet concentrations of NOx, respectively.
ηNOx = (CNOx,in − CNOx,out)/CNOx,in × 100%
The determination of ammonia concentration in flue gas was referred to as the National Environmental Protection Standard of China “Determination of Ammonia in Ambient Air and Exhaust Gas by Nessler Reagent Spectrophotometer” (HJ 533-2009). The schematic diagram of the determination for ammonia slip, as shown in Figure 2. The principle as follows: The ammonia in flue gas was absorbed by dilute sulfuric acid firstly, and then the generated ammonium ion reacts with Nessler’s reagent to a form yellow-brown complex. The absorbance of the sampling absorption solution was measured at a wavelength of 420 nm. Since the absorbance of the above complex was proportional to the content of ammonia in solution, the ammonia content in the gas can be calculated based on the absorbance.
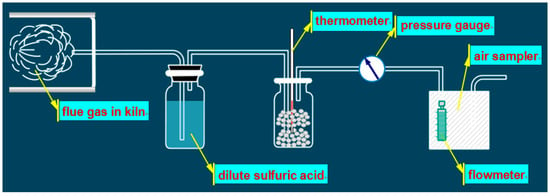
Figure 2.
Schematic diagram of the determination for ammonia slip.
The ammonia concentration in flue gas (ρNH3) was obtained using the following Formula (2):
where ρNH3 is the ammonia slip concentration (mg/m3), A is the absorbance of sample absorption solution, A0 is the absorbance of the blank absorption solution prepared in the same batch, a is the intercept of the calibration curve, b is the slope of the calibration curve, Vs is the volume of sample absorption solution (mL), V0 is the volume of absorption liquid taken during analysis (mL), Vnd is the standard volume (101.325 kPa, 273 K) of the flue gas (L), and D is Dilution factor.
ρNH3 = (A − A0 − a) × Vs × D/(b × Vnd × V0)
The standard volume of the flue gas (Vnd) was calculated by the following Formula (3):
where V is the sampling volume (L), P is the atmospheric pressure during sampling, and t is the flue gas temperature during sampling.
Vnd = V × P × 273/[101.325 × (273 + t)
3. Results and Discussion
3.1. Effect of Reaction Parameters on the NOx Reduction
Figure 3a showed the effects of temperature on the NOx reduction and ammonia slip when the NSR was around 1.1, and the initial concentration of NOx was about 390 ppm. It can be seen that excessive temperature was not conducive to reducing NOx, the NOx reduction rate slightly increased first and then decreased sharply with the rise of temperature, and the NOx reduction rate reached the highest of 71.3% at 890 °C.
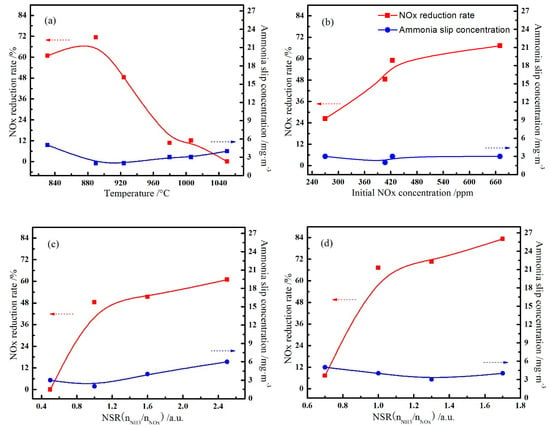
Figure 3.
Effects of reaction conditions on the NOx reduction. (Reaction temperature (a); initial NOx concentration (b); the [NH3/NOx] molar ratio with the initial NOx concentration was 390 ppm (c) and 680 ppm (d)).
Temperature determines the reaction rate of the following two reactions:
when the temperature is lower than the appropriate temperature for the abovee ractions, the generation of OH and O is limited, which makes it difficult for reactions (6) and (7) to proceed and cannot produce enough NH2 with high selectivity for NO reduction.
H + O2 → OH + O
O + H2O → OH + OH
NH3 + OH → NH2 + H2O
NH3 + O → NH2 + OH
As the temperature rises to a suitable range, reactions (4) and (5) proceed rapidly, resulting in a large number of OH and O. With its increase, a large number of NH3 is converted into NH2, which triggers the whole chain reaction, and NO is rapidly reduced as reactions (8)–(10). However, when the temperature continues to rise, the OH produced by reactions (4) and (5) continues to accumulate, and excessive OH will continue to dehydrogenate NH2 to form NH, as reaction (11) shows, which will be oxidized to NO by oxygen at high temperature through reactions (12)–(15), resulting in a decrease in the NO reduction rate [28,29].
NH2 + NO ⇌ N2 + H2O
NH2 + NO ⇌ NNH + OH
NNH + NO ⇌ N2 + HNO
NH2 + OH → NH + H2O
NH + O2 → HNO + O
NH + OH → HNO + H
NH + O2 → NO + OH
HNO + OH → H2O + NO
The same rule can be obtained by increasing the NSR, it was unfavorable to NOx reduction when the temperature goes above 1000 °C, as NOx reduction rate decreased from 66.3% to 48.0% when the temperature increased from 946 °C to 1010 °C under the condition of NSR = 2.0. Besides, excessive and low temperature will both cause increase of ammonia slip concentration, which also hit a minimum of 2 mg/m3 at 890 °C and NSR = 1.1, but overall the higher NSR value results in more ammonia slip, as the ammonia slip concentration was greater than or equal to 5 mg/m3 at NSR = 2.0.
Figure 3b showed the effects of initial NOx concentration on the NOx reduction and ammonia slip when the NSR was around 1.1 and the temperature at about 920 °C. Obviously, NOx reduction rate has a certain relationship with the initial NOx concentration, it showed that with the increase of initial NOx concentration, NOx reduction rate increased gradually, while the ammonia slip did not change much. Under the suitable NSR, the reaction rate of reactions (8)–(10) increased when the initial concentration of NOx increases, so NOx reduction rate is also increased. But at this point, the initial concentration of NOx is not the limiting factor of the whole denitrification reaction, so the increase of NOx reduction rate is not obvious. As shown in Figure 3b, it was more appropriate to complete denitrification in an atmosphere with a higher initial NOx concentration, as a consequence, grate PH zone is a suitable place to install denitrification devices ingrate-rotary kiln process for its high NOx concentration.
Figure 3 also compared the effects of NSR on the NOx reduction and ammonia slip at different initial NOx concentrations near 910 °C. As shown in Figure 3c, when the initial NOx concentration of flue gas was about 390 ppm, the NOx reduction rate increased with the increase of NH3·H2O consumption, and it reached 48.4% at NSR = 1.0; meanwhile, the ammonia slip concentration was 2 mg/m3. Continuously increasing the NH3·H2O dosage, the NOx reduction rate does not change much, but the ammonia slip increases. When NSR increases, the content of NH3 will also increases, which promotes the reactions (6) and (7) and produces more NH2; thus, more NO will be reduced. When NSR is at a low level, NH3 becomes the limiting factor of denitrification reaction, therefore, increasing NSR at this time will accelerate reducing NO rapidly. Furthermore, when NSR reaches a certain level, OH will become the limiting link of denitrification reaction, a continuous increase of NSR will not have a significant impact on the NOx reduction rate.
It can be seen from the Figure 3d that NSR had similar rules on the NOx reduction rate and ammonia slip when the initial concentration of NOx rises to 680 ppm, NOx reduction rate firstly increased sharply as NSR increases, reaching 67.2% with an ammonia slip concentration of 4 mg/m3 at NSR = 1.0, then it goes through a gentle rise and finally reached 83.3% with an ammonia slip concentration of 4 mg/m3 at NSR = 1.7. Under the situation of other conditions being equal, it shows better denitrification effect on flus gas with higher initial NOx concentration, and NSR is one of the main factors affecting the NOx reduction rate.
3.2. Effects of Reductants on the NOx Reduction
Table 1 showed the effects of NSR on the NOx reduction rate and ammonia slip by using 20 wt% NH4HCO3 solution as NOx reducing agent. The results showed that the NH4HCO3 solution had little denitrification effect when it was used at low NSR, and NOx concentration in flus gas even increased slightly at NSR = 0.5. A higher NOx reduction rate can be obtained by increasing the NSR, and it reached 65.1% at NSR = 1.5, with an ammonia slip concentration of 5 mg/m3. But overall, the denitrification effect of NH4HCO3 solution was not as good as NH3·H2O.

Table 1.
Effects of reductants on the NOx reduction with different NH3/NOx molar ratio.
Urea solution, nevertheless, has a better denitrification effect than NH3·H2O. As shown in Table 1, NOx reduction rate can reach 66.5% with an ammonia slip concentration of 3 mg/m3 at NSR = 0.9 when 20 wt% urea solution was used as NOx reducing agent, and when NSR increased to 2.0, NOx reduction rate only rises to 72.6%, while ammonia slip concentration reached 6 mg/m3.
3.3. Effects of Additives on the NOx Reduction
Table 2 compared the effects of three different additives on NOx reduction and ammonia slip, and the three additives were NaCl, ethanol, and vanadium-titanium catalyst, respectively. Table 2 showed the results of adding 0.1 wt% NaCl to 13 vol.% NH3·H2O, it can be seen that the addition of NaCl had a certain effect at low NSR, NOx reduction rate reached 58.9%, and the ammonia slip concentration was merely 2.0 mg/m3 at NSR = 1.0. However, continuously increased in NSR does not cause significant changes in the NOx reduction rate, but leads to more ammonia slip.

Table 2.
Effects of additives on the NOx reduction with different NSR.
Table 2 also showed the effects of NSR on NOx reduction and ammonia slip when 0.1 vol% ethanol was added to 13 vol.% NH3·H2O. Contrary to NaCl, ethanol turns out to be more effective in denitrification at higher NSR, and the NOx reduction rate reached 67.7% with an ammonia slip concentration of 4.0 mg/m3 at NSR = 1.7. Compared with NaCl and ethanol, the vanadium-titanium catalyst obviously had a better denitrification effect of the overall level. NOx reduction rate reached 67.5% and the ammonia slip concentration was 4.0 mg/m3 at NSR = 1.4 after 0.05 wt% vanadium-titanium catalyst was added to 13 vol.% NH3·H2O. Although a better denitrification effect can be obtained with less dosage of vanadium-titanium catalyst, the cost of vanadium-titanium catalyst is higher than that of the other two additives. Therefore, suitable additives can be selected according to different needs.
3.4. Effects of Process Conditions on the NOx Reduction
Table 3 showed the effects of O2 concentration on NOx reduction and ammonia slip as the NSR was around 1.1, the temperature was near 910 °C, and the initial concentration of NOx was about 420 ppm. The NOx reduction rate and ammonia slip did not change much as the O2 concentration in flue gas varies from 3.8% to 11.2%. NOx reduction rate only got about 10% promotion, reaching 70.8%, when O2 concentration increased from 9.0% to 11.2%, while it remains almost unchanged when O2 concentration increased from 3.8% to 9.0%. Therefore, O2 concentration had little effect on denitrification efficiency. However, reaction time has a notable influence on the NOx reduction rate and ammonia slip, and it is detrimental to reducing NOx when reaction time cannot meet the requirement; at the same time, ammonia slip increases as well. Only when the reaction time is designed to exceed one second can reducing NOx be guaranteed enough time for its full reaction, achieving a good denitrification effect. Coincidentally, the length of the grate PH zone is usually greater than 10 m, providing sufficient reaction time for NOx reduction.

Table 3.
Effects of process conditions on the NOx reduction.
Table 3 also showed the effects of NSR on the NOx reduction rate and ammonia slip by using 6.5 vol.% NH3·H2O as NOx reducing agent. Combining with the previous analysis, it can be seen that NOx reduction rate and ammonia slip had little change when the NH3·H2O concentration was 13 vol.% or 6.5 vol.% with the same other conditions, which indicated that the influence of NH3·H2O concentration on NOx reduction was not the main factor. The pressure of NH3·H2O injection had little effect on the NOx reduction and ammonia slip either. No significant changes in the NOx reduction rate and ammonia slip have been found when the NH3·H2O injection pressure was 75 kPa or 45 kPa.
4. NOx Emission Behavior and Prospects of Denitrification Technology
4.1. NOx Emission Behavior
The grate-rotary kiln process consists of three parts: The grate, the rotary kiln, and the cooler [30]. Green pellets are dried and preheated through the four zones of the grate (i.e., updraft drying zone, downdraft drying zone, tempered preheating zone, and preheating zone), preheated pellets are then fed into the rotary kiln for roasting at a high temperature to obtain better metallurgy performance, and in the rotary kiln, most often coke oven gas is used as fuel to meet the requirement of high temperatures. The flame temperature of the burner in the rotary kiln can even reach 1600–1850 °C with the combustion of gas, a large amount of thermal NOx generates for the temperature well above 1300 °C, and it dominates NOx formation, accounting for more than 80%. The generated NOx is firstly circulated with the hot flue gas to the preheating zone, and then to the downdraft drying zone. The terminal NOx emission usually exceeds 200 mg/m3.
Although most NOx generates in the rotary kiln, the temperature required by pellets roasting is too high for the effective application of low-NOx control technologies. Compared with rotary kiln, the preheating zone has a more appropriate temperature distribution (900–1050 °C) to implement low-NOx control technologies, such as selective non-catalytic reduction (SNCR). Furthermore, all NOx generated in the rotary kiln enters into the preheating zone with the flue gas according to direction the of air flow in the grate-rotary kiln process, the NOx content in the flue gas of preheating zone consequently reaches a relatively high level, exceeding 400 mg/m3. Moreover, there will be sufficient time for denitrification reaction in the preheating zone, since the length of it usually exceeds 10 m.
The application of advanced reburning in the preheating zone is considered one of the most direct and effective ways to reduce NOx emission in grate-rotary kiln. An additional burner can be added to preheating zone, coal gas can be used as a reburning fuel when injected into the high NOx flue gas, and this creates a reducing environment to effectively promote NOx reduction and provide the temperature required for pellets preheating and advanced reburning as well. Ammonia is sprayed into the preheating zone, NOx is reduced in the following four reactions [31,32]:
8NH3 + 6NO2→ 7N2 + 12H2O
4NH3 + 6NO → 5N2 + 6H2O
4NH3 + 4NO + O2→ 4N2 + 6H2O
4NH3 + 2NO2 + O2→ 3N2 + 6H2O
At the appropriate temperature (about 1000 °C) and in the temperature window range of 150 °C, a certain number of OH generates, promoting the transformation of NH3 into NH2 in large quantities, and the NH2 is highly selective for NOx reduction, which triggers the chain reaction. An advanced reburning denitrification device was installed to the grate-rotary kiln production line of an annual output of 1.2 million tons of pellets. The process flow was shown in Figure 4. It can be seen that the whole device was composed of ammonia storage and transportation system, ammonia water mixing system, ammonia water metering system, ammonia water spraying system, and wastewater discharge system.
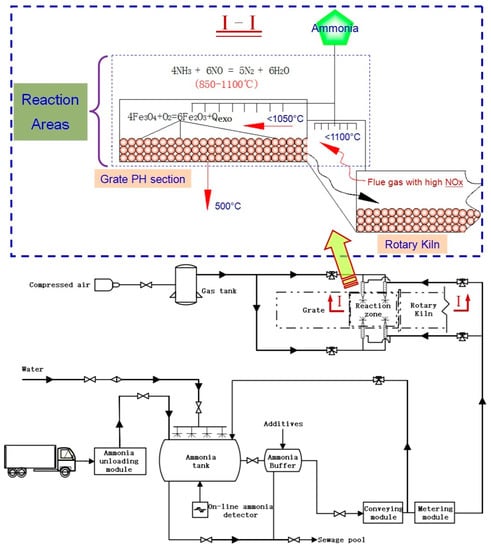
Figure 4.
Process flow of advanced reburning denitrification device and its application.
Given the high temperature (850~1100 °C), high-concentrated NOx (300~1000 mg/m3), wide reaction areas (the reaction time in the hood will more than 0.5 s) of the PH and transition section of the chain grate, An advanced reburning denitrification device can be added to the appropriate position of the chain grate PH section (In the blue box, as shown in Figure 4), which will be beneficial to lighten the burden of the NOx terminal treatment and achieve ultra-low nitrogen oxide emissions. The denitration effects before and after applying advanced reburning were shown in Table 4. As shown in Table 4, the emission concentration of NOx was 275~296 mg/Nm3 before applying advanced reburning in the pellet production process, and it reduced to 163~182 mg/Nm3 after the adoption of this technology. The amount of flue gas was 396,000~410,000 Nm3/h. The denitrification efficiency reached nearly 40%, and the escape concentration of ammonia was less than 10 ppm at the same time, which showed a remarkable denitrification effect.

Table 4.
Denitration effect of flue gas before and after advanced reburning.
4.2. Prospects of Denitrification Technology
A series of denitrification measures, such as SCR and flue gas circulation, can be combined with advanced reburning technology in order to achieve ultra-low emission standard of NOx in the oxidation pellet production of grate-rotary kiln process, as shown in Figure 5.
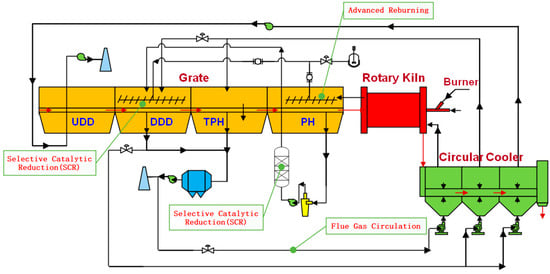
Figure 5.
Denitrification mechanism of advanced reburning technology.
In detail, the flue gas discharged from the PH section can be denitrified by SCR after dust removal, SCR can also be applied to the end treatment of flue gas in the DDD section at the same time. In addition, the flue gas discharged from the DDD and TPH sections can be circulated to each section of the cooler as required. It avoids the problems of low denitrification efficiency and excess ammonia slip caused by the high concentration of NOx in flue gas when SCR is used directly to denitrify flue gas, thus realizing the purpose of ultra-low NOx emission in pellet production.
5. Conclusions
The influences of temperature, NSR, and other factors on NOx reduction, as well as ammonia slip, was investigated; and optimum operating parameters of denitrification in the grate-rotary kiln process for pellet production was obtained. Temperature and NSR are the key factors affecting the NOx reduction rate in advanced reburning. It is conducive to reducing NOx when the temperature is 900 °C, NSR is 1.2, and reaction time is more than one second. Better denitrification effect can be obtained on flus gas with higher initial NOx concentration under the preceding conditions, for flue gas with initial NOx concentration of about 400 ppm, NOx reduction rate can reach 55–65% with an ammonia slip concentration of 2 mg/m3, and NOx reduction rate can be higher than 70% with an ammonia slip concentration of 3 mg/m3 when initial NOx concentration is around 680 ppm. Additionally, urea solution has the best denitrification effect, then NH3·H2O, and then NH4HCO3 solution. Moreover, regarding additives, the denitrification effect of the vanadium-titanium catalyst is better than that of ethanol and NaCl, while NaCl plays a promotive role at low NSR (1.0, for example). However, O2 concentration, NH3·H2O concentration, and injection pressure have little effect on reducing NOx. A series of denitrification measures that include advanced reburning technology for achieving NOx ultra-low emission in the oxidation pellet production can be implemented.
Author Contributions
Conceptualization, B.H. and P.H.; methodology, P.H.; investigation, B.L., Z.X. and L.L.; data curation, L.L. and G.C.; writing—original draft preparation, B.H. and B.L.; writing—review and editing, P.H., Z.X. and J.W.; project administration, P.H. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support that provided by Hunan Provincial Natural Science Foundation of China (2019JJ51007), National Natural Science Foundation of China (51304149), Key Project of Scientific Research Plan of Hubei Provincial Department of Education (D20191106), National Training Program of Innovation and Entrepreneurship for Undergraduates (201810488021) and Key Research Project of Science and Technology Innovation Fund for College Students of WUST (17ZRB086).
Acknowledgments
Critical comments and thoughtful suggestions by anonymous reviewers are highly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, H.J.; She, X.F.; Han, Y.H.; Wang, J.S.; Zeng, F.B.; Xue, Q.G. Softening and Melting Behavior of Ferrous Burden under Simulated Oxygen Blast Furnace Condition. J. Iron Steel Res. Int. 2015, 22, 297–303. [Google Scholar] [CrossRef]
- Bolen, J. Modern air pollution control for iron ore induration. Min. Metall. Explor. 2014, 31, 103–114. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, D.; Pan, J.; Guo, Z.; Yang, C. Effect of basicity on the structure characteristics of chromium-nickel bearing iron ore pellets. Powder Technol. 2019, 342, 409–417. [Google Scholar] [CrossRef]
- Gan, M.; Ji, Z.; Fan, X.; Chen, X.; Zheng, R.; Gao, L.; Wang, G.; Jiang, T. Value-added utilization of waste silica powder into high-quality chromite pellets preparation process. Powder Technol. 2018, 328, 122–129. [Google Scholar] [CrossRef]
- Yi, L.; Huang, Z.; Jiang, T.; Zhong, R.; Liang, Z. Iron ore pellet disintegration mechanism in simulated shaft furnace conditions. Powder Technol. 2017, 317, 89–94. [Google Scholar] [CrossRef]
- Stjernberg, J.; Ion, J.C.; Antti, M.L.; Nordin, L.O.; Lindblom, B.; Odén, M. Extended studies of degradation mechanisms in the refractory lining of a rotary kiln for iron ore pellet production. J. Eur. Ceram. Soc. 2012, 32, 1519–1528. [Google Scholar] [CrossRef]
- Stjernberg, J.; Isaksson, O.; Ion, J.C. The grate-kiln induration machine: History, advantages, and drawbacks, and outline for the future. J. S. Afr. Inst. Min. Metall. 2015, 115, 137–144. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, Y.; Lv, J.; Wu, J. Assessment of multi-air emissions: Case of particulate matter (dust), SO2, NOx and CO2 from iron and steel industry of China. J. Clean. Prod. 2019, 232, 350–358. [Google Scholar] [CrossRef]
- Lv, W.; Sun, Z.; Su, Z. Life cycle energy consumption and greenhouse gas emissions of iron pelletizing process in China, a case study. J. Clean. Prod. 2019, 233, 1314–1321. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Y.; Yan, L.; Liu, T.; Zhang, Q.; He, K. A unit-based emission inventory of SO2, NOx and PM for the Chinese iron and steel industry from 2010 to 2015. Sci. Total Environ. 2019, 676, 18–30. [Google Scholar] [CrossRef]
- Fan, X.H.; Yang, G.M.; Chen, X.L.; Gao, L.; Huang, X.X.; Li, X. Predictive models and operation guidance system for iron ore pellet induration in traveling grate–rotary kiln process. Comput. Chem. Eng. 2015, 79, 80–90. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Lu, H.; Ni, T.; Li, Y. Waste energy recovery and energy efficiency improvement in China’s iron and steel industry. Appl. Energy 2017, 191, 502–520. [Google Scholar] [CrossRef]
- Zhu, T.; Hu, Y.; Tang, C.; Wang, L.; Liu, X.; Deng, L.; Che, D. Experimental study on NOx formation and burnout characteristics of pulverized coal in oxygen enriched and deep-staging combustion. Fuel 2020, 272, 117639. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Jin, Q.; Chen, Q.; Zhou, Y. Mechanism analysis on the pulverized coal combustion flame stability and NOx emission in a swirl burner with deep air staging. J. Energy Inst. 2019, 92, 298–310. [Google Scholar] [CrossRef]
- Edland, R.; Normann, F.; Fredriksson, C.; Andersson, K. Implications of fuel choice and burner settings for combustion efficiency and NOx formation in PF-fired iron ore rotary kilns. Energy Fuels 2017, 31, 3253–3261. [Google Scholar] [CrossRef]
- Zhou, H.; Huang, Y.; Mo, G.; Liao, Z.; Cen, K. Experimental investigations of the conversion of fuel-N, volatile-N and char-N to NOx and N2O during single coal particle fluidized bed combustion. J. Energy Inst. 2017, 90, 62–72. [Google Scholar] [CrossRef]
- Edland, R.; Smith, N.; Allgurén, T.; Fredriksson, C.; Normann, F.; Haycock, D.; Johnson, C.; Frandsen, J.; Fletcher, T.H.; Andersson, K. Evaluation of NOx-Reduction Measures for Iron-Ore Rotary Kilns. Energy Fuels 2020, 34, 4934–4948. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Fan, W.; Wu, X.; Guo, H.; Zhu, J.; Liu, P.; Chen, C.; Wang, Y. Experimental study on the impact of adding NH3 on NO production in coal combustion and the effects of char, coal ash, and additives on NH3 reducing NO under high temperature. Energy 2019, 173, 109–120. [Google Scholar] [CrossRef]
- Fu, S.; Song, Q.; Yao, Q. Mechanism study on the adsorption and reactions of NH3, NO, and O2 on the CaO surface in the SNCR deNOx process. Chem. Eng. J. 2016, 285, 137–143. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Lim, Y.I.; Kim, S.J.; Eom, W.H.; Yoo, K.S. Experiment and computational fluid dynamics (CFD) simulation of urea-based selective noncatalytic reduction (SNCR) in a pilot-scale flow reactor. Energy Fuels 2008, 22, 3864–3876. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, S.; You, C.; Wang, H. Experimental Study on the Enhancement of SNCR Denitrification Process with Methane and Propane in A Circulating Fluidized Bed. Ind. Eng. Chem. Res. 2019, 58, 7825–7833. [Google Scholar] [CrossRef]
- Du, L.; Jin, B.; Zheng, X.; Niu, M. Effect of reburning zone conditions on no reduction efficiency in an online precalciner-type kiln system. Environ. Prog. Sustain. Energy 2016, 35, 439–446. [Google Scholar] [CrossRef]
- Fan, W.; Zhu, T.; Sun, Y.; Lv, D. Effects of gas compositions on NOx reduction by selective non-catalytic reduction with ammonia in a simulated cement precalciner atmosphere. Chemosphere 2014, 113, 182–187. [Google Scholar] [CrossRef]
- Li, S.; Ge, Y.; Wei, X. Experiment on NO x reduction by advanced reburning in cement precalciner. Fuel 2018, 224, 235–240. [Google Scholar] [CrossRef]
- Zhuang, H.; Niu, Y.; Gong, Y.; Zhang, Y.; Zhang, Y.; Hui, S. Influence of Biomass Reburning on NOx Reductions during Pulverized Coal Combustion. Energy Fuels 2017, 31, 5597–5602. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kajimura, S. Kinetic Study on NO Reduction Using Dimethyl Ether as a Reburning Fuel. Energy Fuels 2017, 31, 12500–12507. [Google Scholar] [CrossRef]
- Kang, Z.; Yuan, Q.; Zhao, L.; Dai, Y.; Sun, B.; Wang, T. Study of the performance, simplification and characteristics of SNCR de-NOx in large-scale cyclone separator. Appl. Therm. Eng. 2017, 123, 635–645. [Google Scholar] [CrossRef]
- Fu, S.; Song, Q.; Yao, Q. Mechanism of the Reaction between HNCO and CaO in the Urea-Selective Non-catalytic Reduction deNOx Process. Energy Fuels 2017, 31, 5318–5323. [Google Scholar] [CrossRef]
- Forsmo, S.P.E.; Forsmo, S.E.; Samskog, P.O.; Björkman, B.M.T. Mechanisms in oxidation and sintering of magnetite iron ore green pellets. Powder Technol. 2008, 183, 247–259. [Google Scholar] [CrossRef]
- Lyon, R.K. Thermal DeNOx controlling nitrogen oxides emissions by a noncatalytic process. Environ. Sci. Technol. 1987, 21, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Shon, B.H.; Yoo, J.G.; Jung, J.H.; Oh, K.J. The influence of mixing between NH3 and NO for a De-NOx reaction in the SNCR process. J. Ind. Eng. Chem. 2008, 14, 457–467. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).