Abstract
By-products of laying hens represent a promising raw material source with a high collagen content, which is currently not adequately used. The aim of the paper is to prepare gelatins from laying hen paws. The purified collagen raw material was processed by a biotechnological process using the food endoprotease Protamex®. After cleavage of the cross-links in the collagen structure, the gelatin was extracted by a batch process with a stirrer in two extraction steps. The influence of the extraction process on the yield of gelatins and on selected qualitative parameters of gelatins was monitored by two-level factor experiments with three selected process factors. The studied factors were: enzyme dosage (0.2–0.8%), enzyme processing time (24–72 h) and gelatin extraction time (30–120 min). After the first extraction step at 75 °C, gelatin was extracted with a yield of 8.2–21.4% and a gel strength of 275–380 Bloom. In the second extraction step at 80–100 °C, it is possible to obtain another portion (3.3–7.7%) of gelatin with a gel strength of 185–273 Bloom. Total extraction efficiency of gelatins prepared from laying hen collagen is almost 30%. The prepared gelatins are of high quality and, under proper extraction conditions, gelatins with a gel strength above 300 Bloom can be prepared, thus equaling commercial beef and pork gelatins of the highest quality. Biotechnological processing of laying hen collagen into gelatins is environmentally friendly.
Keywords:
batch process; biotechnology; by-products; crosslinking; endoprotease; extraction; gelatins; laying hens; paws; processing 1. Introduction
In 2019, worldwide poultry meat production was 128.4 million metric tons, the highest by type of meat; pork production was 115.6 million metric tons, followed by beef (71.6) and lamb (15.3). If we compare only the production of poultry meat in the last 15 years, it has increased by about 44 million metric tons [1]. Compared to 2019, by 2028 it is estimated that the production of poultry meat will increase by about 10% [2]. It is clear that as meat production increases, the share of by-products will increase. The amount of by-products produced during the processing of animals into meat may vary, depending on whether the by-products are subsequently used, e.g., in the food, feed or technical fields. Their amount represents approximately 1/3 of the live weight of the animals, but in some cases, it can be up to 55% [3,4].
According to Regulation (EC) No. 1069/2009 of the European Parliament and of the Council, animal by-products are divided into 3 categories [5]. For I category materials (animal parts and by-products specified as risk material) and II category materials (manure, digestive tract content, animal by-products collected during wastewater treatment), disposal in rendering plants by pressure sterilization is prescribed. The meal made from I category materials is burned, while that from II category is used as a fertilizer. The III category materials include the bodies of slaughtered animals and their parts; they are characterized by high content of proteins and fats and are potentially suitable, after special treatment, for human consumption. However, for commercial or customary reasons, they are rarely offered for human consumption. The only main exception is Asia, as well as some countries in North Africa, where, for example, paws (an animal’s foot having claws and pads), heads and entrails are processed for various culinary purposes. In Central and some Western European countries, on the other hand, only the entrails (liver, kidneys, stomachs, heart, lungs and intestines) are used to prepare various dishes [6,7]. The situation is different for blood, which, especially in Europe and Asia, is used (fresh, canned or dried) mainly in the food industry in the production of meat and cooked products, kitchen semi-finished products; it serves mainly to modify the sensory properties (color, taste, texture, smell) of products, to increase the ability to bind water, as an emulsifier or to increase the nutritional value. Due to its high protein content, complete content of essential amino acids and very good digestibility, blood is also a very desirable and inexpensive ingredient in compound feeds [8,9].
Keratin by-products (feathers, claws), which represent up to 10% of the live weight of poultry, are also significant. Feathers can be processed by suitable hydrolytic processes into keratin meal, which has good digestibility and is suitable, for example, as a protein additive in animal feed [10,11,12]. Keratin hydrolysates are also used as functional additives in hair and body cosmetics [13,14]. At our workplace, a combined alkaline-enzymatic hydrolysis of hen feathers to low-molecular keratin hydrolysate was designed, which was subsequently tested in cosmetic emulsions for skin care; higher hydration and improved skin barrier function were confirmed in the tested probands [15].
Currently, some solid poultry by-products from III category materials (mainly paws and heads) on the European and American continents are processed almost exclusively by rendering plants into meals, which are then used to produce compound feed for livestock [16]. On the one hand, it is a certain use of waste raw materials, which brings their producers a slight economic profit. On the other hand, the potential of these raw materials is not optimally used, as these by-products are rich in proteins, of which collagen account for the highest proportion. Thus, it is possible to obtain pure collagen by suitable purification procedures, which can be further processed into collagen products, in particular into gelatins and low-molecular hydrolysates suitable for food, pharmaceutical, cosmetic and other applications. In addition to collagen, hydrolyzed products and bioactive peptides derived from fruit (peels, pomace, seed fractions) and vegetable (e.g., cauliflower) by-products or marine organisms can be prepared and used as food supplements, nutraceuticals or in pharmaceutical industry [17,18,19,20]. In the industrial production of gelatins, collagen raw materials are most often processed from beef and pork (skin, bones, tendons and ligaments), and in limited quantities from fish [21,22,23]. The key technological operations of the process of conversion of collagen to gelatin include, in particular, conditioning of the raw material in a suitable environment and subsequent extraction with hot water [24]. Both of these technological steps take place under strictly controlled conditions (temperature, time, pH, pressure) so that it is possible to produce gelatins of the required quality (gel strength, viscosity and others) with optimal yield. At present, the acidic method (for the production of type A gelatins) or the alkaline method (for the production of type B gelatins) is used almost exclusively to condition the raw materials. During the process, which has a duration on the order of h to tens of h for the acidic treatment, or weeks to months for the alkaline treatment, the covalent cross-linked bonds are cleaved at the level of the quaternary structure of collagen. The controlled course of the process of chemical denaturation of collagen is important for the subsequent thermal denaturation of collagen (the unfolding of the tertiary structure of collagen—a triple helix), which occurs in a subsequent process step—extraction of gelatins with water, usually at 50–100 °C [25,26]. The conditioning of raw materials takes place in large vessels or in pits. Gelatin extraction takes place in tanks; different extraction process designs are used. A batch process with stirrers is one of the traditional (discontinuous) methods in which gelatin is extracted in several extraction steps (usually 3–5) at increasing extraction temperatures. In a semi-continuous process with circulation, the raw material is added to the extractor in batches and intensively extracted with circulating water; the higher temperature speeds up the extraction process. In the continuous counter-current process, the raw material is continuously fed to the extractor and counter-currently extracted with water. Optimized continuous extraction can also be performed under pressure, which leads to a shortening of the extraction process [26].
Our workplace has been engaged for a long time in the development of technologies for the processing of chicken by-products into gelatins and hydrolysates using proteolytic enzymes in the processing (conditioning) of the raw material; technologies have been successfully tested in the processing of chicken heads, paws and skins [27,28,29,30,31]. The processing of collagen by-products from the slaughter of laying hens (e.g., heads or paws) into gelatins, either by traditional technologies using acids or bases, or by using biotechnology, is not carried out in current industrial practice; nor is the contemporary literature mentioning such practices. The main goal of this work is to design and verify a biotechnological preparation process of gelatins from laying hen paws using a commercially available food proteolytic enzyme in the process of conditioning the collagen raw material. A partial goal is to monitor the influence of selected process variables on the course of the technological process of gelatin preparation (gelatin yield) and on selected qualitative parameters of gelatins (gel strength, viscosity, ash content). The specific hypothesis being tested is that by selecting a suitable proteolytic enzyme in the processing of the raw material under controlled conditions of the gelatin preparation process, hen gelatins comparable in quality to food/pharmaceutical beef and pork gelatins can be prepared, with optimal extraction process efficiency.
2. Materials and Methods
2.1. Materials, Analytical Methods and Appliances
Laying hen paws (hens aged 75 weeks) were supplied by Raciola Uherský Brod, Ltd. (Czech Republic). First, raw material analyses were performed, determining dry matter content (43.0 ± 1.0%), and in dry matter: protein content (49.5 ± 1.3%), collagen proportion in the protein content (77.2 ± 1.1%), fat content (33.4 ± 2.2%) and inorganic solids (19.0 ± 1.3%). Each analysis was repeated three times and mean values were calculated. Dry matter was determined by the indirect method of drying the sample for 18 h at 103.0 ± 2.0 °C. Ash was determined gravimetrically after burning and annealing the sample. Fat was determined by Soxhlet extraction [32]. Protein content was calculated from the nitrogen (determined by Kjeldahl method) content by multiplying by a factor of 6.25 [33]. Collagen content was calculated from the hydroxyproline content (determined colorimetrically after sample hydrolysis in 6 mol L−1 HCl) by multiplying by a factor of 8 [34]. Gelatins were analyzed according to the Official Procedure of the Gelatin Manufacturers Institute of America. The gelatin gel strength was determined from a gel formed from a 6.67% solution prepared according to prescribed conditions by measuring of force (weight) required to depress a prescribed area of the surface of the sample to a distance of 4 mm. Dynamic viscosity of a 6.67% gelatin solution was determined at 60 °C by measuring the flow time of 100 mL of the solution through a standard pipette; the viscosity was calculated from the equation [35].
Stevens LFRA Texture Analyzer for measuring gelatin gel strength (Leonard Farnell and Co Ltd., Liverpool, UK), Ubbelohde viscometer (Technisklo Ltd., Držkov, Czech Republic), meat mincer Braher P22/82 (San Sebastian, Spain), Memmert ULP 400 drying oven (Bűchenbach, Germany), Samsung fridge freezer (Seoul, South Korea), Whatman No. 1 paper (Sigma Aldrich, Gillingham, UK), filter crucible Simax P16 (Verkon, Praha, Czech Republic), Chemicals: NaCl, NaOH, HCl, acetone, ethanol and chloroform (Verkon, Praha, Czech Republic); all chemicals were of analytical grade.
For defatting the raw material, a lipolytic enzyme Lipolase 100 T (lipase with Thermomyces lanuginosus produced by submerged fermentation of a genetically modified microorganism Aspergillus oryzae) was used, with declared activity of 100 KLU/g (Novozymes, Copenhagen, Denmark). When selecting a suitable proteolytic enzyme for conditioning the raw material, we focused on the selection of the enzyme from the group of endoproteases. These enzymes cleave the peptide bonds of proteins within the protein structure and allow the production of higher molecular weight chains, which is important in the preparation of gelatins. The enzyme Protamex® from Novozymes (Copenhagen, Denmark) was selected. It is a Bacillus protease complex with declared activity 1.5 AU/g, working at mild processing conditions (pH 5.5–7.5 and temperature ˂60 °C). Enzyme complies with the recommended purity specifications for food-grade enzymes issued by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) and the Food Chemicals Codex (FCC).
2.2. General Process Layout
A general process layout of biotechnological preparation process of laying hen paw collagen into gelatins is shown in Scheme 1. In the extraction of gelatin, a discontinuous method using a batch process with stirrer was applied.
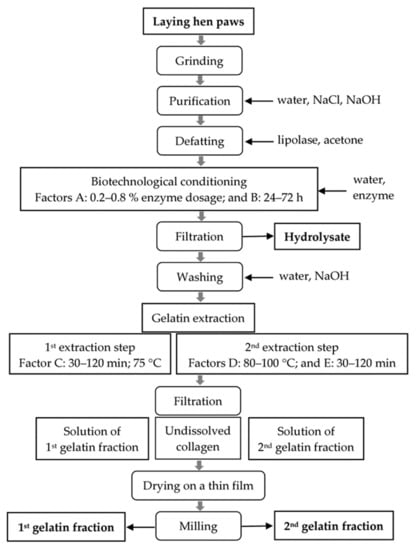
Scheme 1.
The layout of the biotechnological preparation process of laying hen paw collagen into gelatins.
The experiments were designed according to factor plans, which are used as an important tool to achieve better knowledge of processes in research and industrial practice [36]. When examining the influence of several process variables on the course of the technological process of gelatin preparation and on selected qualitative parameters of gelatins, classical experimental procedures are lengthy and expensive. A full two-level factorial design 23 with one replicate for corner points and two central points was used to examine the influence of processing conditions during the 1st extraction step on the yield and qualitative parameters of 1st fractions of gelatins, which allowed the number of experiments to be reduced to 10. The monitored process variables were as follows. Enzyme dosage, based on the raw material dry matter, was factor A: minimum 0.2%, mean 0.5%, maximum value 0.8%; enzyme processing time; factor B: minimum 24 h, mean 48 h, maximum value 72 h, 1st fraction gelatin extraction time; factor C: minimum 30 min, mean 75 min, maximum value 120 min. In addition, another two process variables were studied (independently from factors A, B and C and without using factorial schemes) in the 2nd extraction step: factor D-temperature during 2nd extraction step (80, 90, 100 °C); and factor E–gelatin extraction time during 2nd extraction step (80, 90, 100 min). The schedule of experiments is given in Table 1. To compare the results of the factor plan, a blind experiment (without enzyme dosage, 48 h of enzyme processing time and 75 min of gelatin extraction time) was performed (experiment No. 11, Table 1).

Table 1.
The experimental design of biotechnological processing of laying hen collagen into gelatins.
2.3. Workflow of Processing of Laying Hen Paw Collagen into Gelatins
Fresh lying hen paws were ground to a size of 5 mm immediately after collection from the poultry farm. Before the actual biotechnological processing into gelatins, the raw material was first purified. Blood residues were removed by washing in running cold water for 2 min. This was followed by the removal of other globular proteins. First, the globulins were washed out by shaking the raw material mixed in a ratio of 1:6 with 0.2 mol/L NaCl at room temperature for 1.5 h. After washing for 1 min with running cold water, the raw material was mixed in a ratio of 1:6 with 0.03 mol/L NaOH and shaken for 5 h at room temperature, the NaOH solution being changed at 1-hour intervals; glutellins were washed in this way. The raw material was then washed under running cold water for 2 min. This was followed by defatting in two steps. In the first step, the raw material was mixed in a ratio of 1:10 with water, the lipolytic enzyme Lipolase was added in a dose of 5% (based on the weight of the raw material) and shaken for 48 h at room temperature, changing the water after 12-h intervals and adding a new batch of enzyme. After filtration and washing with cold water for 2 min, the raw material was dried at 35 °C for 24 h. In the second defatting step, the raw material was mixed in a ratio of 1:9 with acetone and shaken for 20 h at room temperature, after 12 h the acetone was changed. In the next step, the purified collagen was subjected to biotechnological treatment with a proteolytic enzyme. Collagen was mixed with water in a ratio of 1:10 and after 20 min of gentle shaking at room temperature, the pH of the mixture was adjusted to 6.5–7.0. Then Protamex® endoprotease was added at a dose of Factor A (0.2–0.8%) and the mixture was shaken gently at room temperature for a period of Factor B (24–72 h); during the first 5 h of the enzyme treatment, the pH was checked and, if necessary, adjusted to the prescribed value (6.5–7.0). After filtration (Whatman No. 1 paper), the liquid portion (collagen hydrolysate) was dried. The biotechnologically treated collagen was first washed under running water for 2 min, then mixed in a sufficient excess with 0.03 mol/L NaOH and shaken intensively for 5 min, finally washed again under running water for 2 min. During the extraction of the 1st gelatin fraction, the enzymatically treated collagen was mixed with water in a ratio of 1:8, the mixture was heated to 75.0 ± 0.5 °C (dt/dτ = 10 °C/min), and the gelatin was extracted for a period of Factor C (30–120 min). After filtration (filter crucible Simax P16), the solution of the 1st gelatin fraction was immediately heated to a temperature of 95.0 ± 0.5 °C (dt/dτ = 15 °C/min) and maintained at this temperature for 5 min. During the extraction of the 2nd gelatin fraction, the collagen material was mixed with water in a ratio of 1:8, the mixture was heated at a rate of dt/dτ = 10 °C/min to a temperature of Factor D (80–100 °C) and the gelatin was extracted for a time of Factor E (30–120 min). After filtration (filter crucible Simax P16), a solution of the 2nd gelatin fraction was obtained. Both gelatin solutions were dried in a thin layer (4 mm) in a circulating air drier at 45.0 ± 0.5 °C; after drying for 48 h, the resulting film was scraped off, weighed and ground to a powder; the prepared gelatins were subjected to further analyzes. The undissolved collagen raw material was dried at 103.0 ± 1.0 °C to constant weight and then weighed.
Total extraction yield of gelatins (∑Y) and balance error (BE) were calculated according to the following formulas:
where YH is the yield of hydrolysate (%), YG1 is the yield of the 1st gelatin fraction (%), YG2 is the yield of the 2nd gelatin fraction (%) and UC is a undissolved collagen (%). The yield of hydrolysate is calculated as the dry matter content of the prepared hydrolysate relative to the dry matter of collagen starting material. The yields of 1st and 2nd gelatin fractions are calculated as the dry matter content of the prepared gelatins relative to the dry matter of collagen starting material.
2.4. Statistical Analysis
All analyses were performed in triplicate; arithmetic mean values were calculated using Excel software version 10 (Microsoft, Denver, CO, USA). To evaluate obtained data, regression analysis was applied to all results using Minitab® 17.2.1 statistical software for Windows (Fujitsu Ltd., Tokyo, Japan). Statistical significance of the studied processing factors in the observed limits was evaluated using the P-values at α = 0.05 confidence level (factors with a value ˂0.05 have an effect on the evaluated variables with 95% probability); the lower the P-value, the greater the influence of the processing factor. Contour plots (Figure 1, Figure 2, Figure 3 and Figure 4) were depicted using Excel software version 10 (Microsoft, Denver, CO, USA).
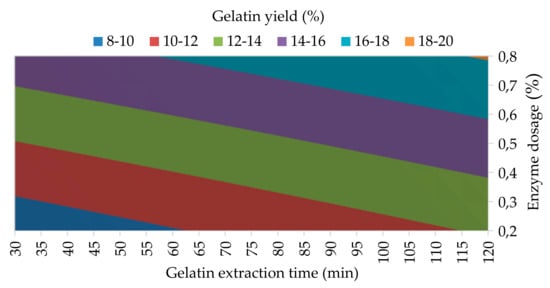
Figure 1.
The influence of enzyme dosage and gelatin extraction time (at 48 h of enzyme processing time) on the yield of the 1st gelatin fraction.
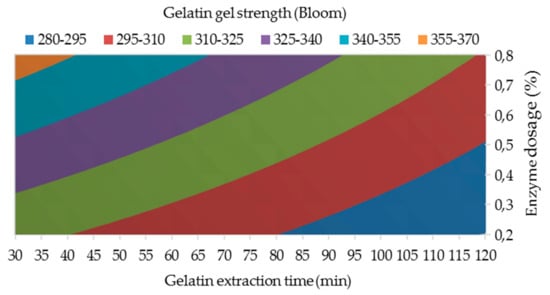
Figure 2.
The influence of enzyme dosage and gelatin extraction time (at 48 h of enzyme processing time) on the gelatin gel strength.
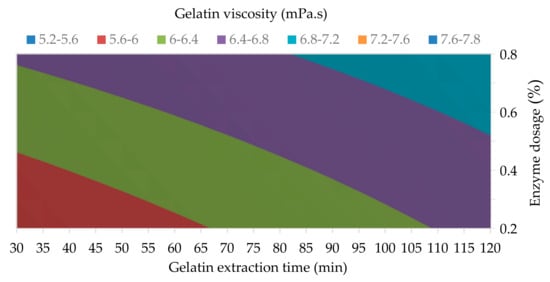
Figure 3.
The influence of enzyme dosage and gelatin extraction time (at 48 h of enzyme processing time) on the gelatin viscosity.
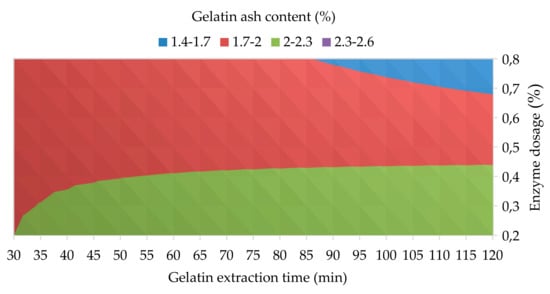
Figure 4.
The influence of enzyme dosage and gelatin extraction time (at 48 h of enzyme processing time) on the gelatin ash content.
3. Results and Discussion
The summary results of the yields of hydrolysate, the first and second gelatin fractions, including the balance error, and the results of the analysis of the gelatins of the 1st and 2nd fractions prepared by biotechnological processing of collagen of laying hen paws are shown in Table 2. Table 3 shows the results of analysis of variance for gelatin yield, gelatin gel strength, gelatin viscosity and gelatin ash content.

Table 2.
Mass balance and the results of analysis of 1st and 2nd gelatin fractions prepared by biotechnological processing of laying hen collagen.

Table 3.
Analysis of variance of the experimental design for gelatin yield, gelatin gel strength, gelatin viscosity and gelatin ash content.
3.1. Preparation of Gelatins of the 1st Fraction
3.1.1. Gelatin Yield
The results of the analysis of variance show that all three studied processing factors have statistically significant effect on the gelatin yield at 95% confidence level; α = 0.010 for Factor A, α = 0.024 for Factor B, and α = 0.020 for Factor C; see Table 3. The regression equation for the yield of 1st gelatin fraction is as follows: YG1 = 4.094 + 12.528 A + 0.02801 B + 0.05352 C − 0.0359 AB − 0.09259 AC – 0.000285 BC + 0.001775 ABC; R2 = 99.98. Figure 1 shows the influence of the two most statistically significant process factors, enzyme dosage (Factor A) and gelatin extraction time (Factor C), on the yield of the 1st gelatin fraction. It is clear that the yield of gelatin increases linearly with increasing dose of enzyme in the process of conditioning the collagen raw material and with increasing extraction time. In the area of the minimum levels of both monitored process variables (0.2% enzyme dose and 30-min extraction), it is clear from the contour that the amount of extracted gelatin is around 10%; with a 4-fold dose of enzyme and the same increase in extraction time (0.8% enzyme dose and 120-min extraction), the amount of extracted gelatin increases approximately two-fold (18–20%).
Direct comparison of results of the yields of gelatins extracted according to our biotechnological process with literature or practice is complicated; scientific and company literature does not mention the processing of laying hen paws into gelatins. However, literature data on the processing of chicken feet (the pedal extremity of vertebrates) into gelatin are available [37,38,39]. Widyasari and Rawdkuen conditioned the starting material in an acidic environment (0.2% CH3COOH), extracted gelatin with water (70 °C, 90 min) or water (70 °C, 100 min) using ultrasound (300 W) [37]. The yield of gelatin using these methods of conditioning and extraction of raw material was 12.4–12.6%, which is at the level of the minimum yield of gelatin according to our procedure; see Exp. No. 1, where the yield of the 1st gelatin fraction was 8.2% and the 2nd gelatin fraction 3.3%, which is a sum of 11.5%. Taufik et al. used a combined alkaline-acid conditioning of the starting material, the broiler collagen was first treated with 0.1% NaOH and then twice in an acidic environment (0.1% H2SO4 and 0.4% citric acid); extraction with water took place at 45–55 °C for 24 h [38]. The yield of gelatin ranged from 15.3 to 15.6%, which is approximately half that of the maximum yields of both gelatin fractions according to our procedure; see Exp. No. 8, where the yield of the 1st gelatin fraction was 21.4% and the 2nd gelatin fraction 7.7%, which is a total of 29.1%. The highest yield of gelatin (35.5%) extracted from chicken feet was in the pressure discontinuous extraction methods (120 °C, 20 min) [39]. Compared to our method, the authors reported a yield approximately 20% higher. This difference can be explained on the one hand by the age of the starting collagen raw material, which has an effect on the degree of intermolecular crosslinking of the collagen. Collagen structure, physical and mechanical properties of collagen are dependent on the age of an animal. During aging inter- and intra-molecular crosslinking of collagen occurs resulting in altering its properties [40,41]. Younger chicken collagen (34–40 days old) is more easily chemical and thermally denatured than hen collagen, as hens are usually slaughtered at 70–80 weeks of age. On the other hand, Almeida et al. [39] do not indicate important properties (especially gel strength or viscosity) of gelatin prepared from chicken feet, and therefore, it is not clear how the higher yield of gelatin extracted at high temperature (120 °C) will affect these key properties important for the gelatin applications.
3.1.2. Gelatin Gel Strength
The results of the analysis of variance showed that all three studied processing factors have statistically significant effects on the gelatin gel strength at 95% confidence level; α = 0.017 for Factor A, α = 0.033 for Factor B, and α = 0.015 for Factor C; see Table 3. The regression equation for the gelatin gel strength is as follows: F1 = 284.11 + 62.78 A + 0.4815 B – 0.2037 C + 0.579 AB − 0.0926 AC − 0.002160 BC − 0.00540 ABC; R2 = 99.97. Figure 2 shows the influence of the two most statistically significant process factors, enzyme dosage (Factor A) and gelatin extraction time (Factor C), on the gelatin gel strength. From the point of view of industrial applications, gelatins are divided into three groups according to gel strength: low gel strength (˂100 Bloom), medium gel strength (100–200 Bloom) and high gel strength (>200 Bloom) gelatins [26]. It is clear from Figure 2 that hen gelatins of the 1st fraction prepared under any studied process conditions are characterized by excellent gel strength (280–380 Bloom) and the change in process conditions does not significantly affect this parameter. The highest gel strength is generally achieved at shorter extraction times (up to about 80 min), where it can also be noted that the strength of gelatin gels with an enzyme dose of 0.2 to 0.8% does not fall below 300 Bloom. Only at long extraction times (above 90 min) does the strength of gelatin gels decrease slightly, but it is still higher than 280 Bloom.
Comparing the results of the gelatin gel strength of laying hen gelatins with gelatins obtained from other poultry by-products favors our biotechnological preparation method. For example, in the already-mentioned study on the preparation of gelatins from chicken feet after processing the raw material in acidic environment, the authors stated that the strength of gelatin gels was 79 and 185 Bloom [37], which is significantly lower than our gelatins, whose gel strength reached at least 275 Bloom (Exp. No. 2). It is similar for gelatins prepared from feet broiler collagen according to the procedure of Taufik et al., whose gelatins had a gel strength of 113–120 Bloom [38]; which is also a significantly lower value than the gelatin with the lowest gel strength prepared by us (Exp. No. 2). The gelatin gel strength was also significantly worse for gelatins prepared from skin collagen separated from chicken feet after conditioning in acidic environment (3% CH3COOH) and extraction at 50–65 °C for 5 h [42]. Although the extraction of gelatins according to the cited literature sources took place under relatively moderate conditions (at temperatures of 45–70 °C), the lower values of gelatin gel strength are most likely due to the different method chosen for conditioning collagen raw materials; all authors used an acidic or combined alkaline–acid environment, in contrast to the enzymatic treatment preferred in our procedure.
3.1.3. Gelatin Viscosity and Gelatin Ash Content
The results of the analysis of variance showed that all three studied processing factors have no statistically significant effect on the gelatin viscosity at 95% confidence level—α = 0.094 for Factor A, α = 0.082 for Factor B, and α = 0.222 for Factor C—see Table 3. The regression equation for the gelatin viscosity is as follows: ν1 = 3.75 + 4.083 A + 0.02755 B + 0.0256 C − 0.0544 AB − 0.01389 AC − 0.000316 BC + 0.000193 ABC; R2 = 99.45. Gelatin viscosity is an important parameter for gelatin processing technologies in the food industry (extrusion, casting, injection or dipping), especially in the production of confectionery; further in pharmacy in the manufacture of oral dosage forms (hard and soft gelatin capsules and tablets) by dipping, casting and compression; or in photography during the formation of emulsion layers by casting. Similar to the already-mentioned classification of gelatins according to gel strength, for the purposes of industrial practice, gelatins according to viscosity are usually also divided into three categories: low- (˂3.0 mPa.s), medium- (3.0–5.0 mPa.s) and high- (>5.0 mPa.s) viscosity gelatins [43,44]. As with the strength of gelatin gels, the prepared laying hen gelatins are in the highest category also in terms of viscosity. Regardless of the choice of process conditions, gelatins have a high viscosity (>5.3 mPa.s); see Table 3. Figure 3 shows the influence of enzyme dosage and gelatin extraction time on the gelatin viscosity. It is obvious that with the longer gelatin extraction time and the higher enzyme dosage the viscosity of hen gelatins rises from 5.3 mPa.s up to 7.3 mPa.s. Similarly high values for gelatins prepared from feet broiler collagen are also reported by Taufik et al. [38]. However, the gelatins prepared according to their procedure have significantly lower gel strength and also the yield of gelatins was lower; moreover, they used combined alkaline-acid environment for conditioning of the raw material, which is less environmentally friendly than our biotechnological process. Very high viscosity (up to 7.0 mPa.s) was also present for gelatins prepared from the skin of chicken feet, but again with lower gelatin yield and lower gel strength [42].
The results of the analysis of variance showed that all three studied processing factors have no statistically significant effect on the gelatin ash content at 95% confidence level; α = 0.079 for Factor A, α = 0.156 for Factor B, and α = 0.500 for Factor C; see Table 3. The regression equation for the gelatin ash content is as follows: Ash1 = 1.317 + 2.25 A + 0.01088 B + 0.01556 C − 0.04051 AB − 0.03889 AC − 0.000201 BC + 0.000540 ABC; R2 = 99.50. For the use of gelatins in pharmacy, a maximum of 2.0% ash content in gelatin is permitted [45]; for the food industry, this limit is more relaxed, allowing up to 3.0% ash content in gelatin [46]. Figure 4 shows the influence of enzyme dosage and gelatin extraction time on the gelatin ash content. It is clear that hen gelatins prepared from a raw material that has been conditioned with the dosages of enzyme above 0.5% safely meets the strict limit for pharmaceutical gelatins. In the food industry, laying hen gelatins prepared under any studied process conditions can be used, as the ash content of none of them exceeds 2.5%. The content of ashes in gelatins is influenced mainly by the method of conditioning the starting material (acidic, alkaline or enzymatic) and also by the type of starting material. In our case, the low ash content is due to the absence of chemicals used in conditioning the raw material. However, in industrial practice, the content of mineral substances in gelatins above the prescribed limit is solved by deionization of the gelatin solution, which is a common process step in the production of gelatins by acidic or alkaline methods [26].
3.2. Preparation of Gelatins of the 2nd Fraction
In the industrial production of gelatins, the starting material is often extracted in multiple extraction steps at increasing extraction temperature to maximize the potential of the starting material. The same approach was applied to the extraction of hen collagen. From the results of the yields of gelatins of the 2nd fraction (YG2), it is clear that by suitable choice of extraction conditions (extraction time 30–120 min at 80–100 °C), it is possible to process another 3.3–7.7% of the starting material into gelatin, which is a significant proportion, see Table 2. From the results of analyses of the gelatins of the 2nd fraction, it is clear that these gelatins are also of high quality, see Table 2. Gelatins prepared under conditions of experiments Nos. 1–4 (all with the lowest enzyme dosage of 0.2%) have a gel strength of 185–191 Bloom, which is a very good value and classifies these gelatins into the group of gelatins with medium gel strength (100–200 Bloom). For gelatins prepared according to the conditions of experiments Nos. 5–8, the value of the gel strength (205–215 Bloom) is in the category of gelatins with high gel strength; for gelatins prepared under the conditions of the central experiment, Nos. 9 and 10 (0.5% enzyme dosage, 48-h enzyme processing time, 75-min gelatin extraction time at 90 °C), the gel strength has an excellent value of approximately 260 Bloom. The viscosity of gelatins prepared under conditions combining the boundary conditions of the monitored process factors (Exp. Nos. 1–8) range from 3.4 to 4.6 mPa.s, which classifies them into the category of gelatins of medium viscosity. Similar to gel strength, gelatins prepared under the conditions of the central experiment (Nos. 9 and 10) showed an increase in viscosity to 5.5–5.6 mPa.s, which are values corresponding to high viscosity gelatins (>5.0 mPa.s). The ash content of the gelatin of the 2nd fraction is generally slightly higher than that of the gelatin of the 1st fraction, but since it does not exceed 2.8%, these gelatins meet strict food standards [46].
3.3. Proposition of Optimal Process Conditions
The optimal process conditions for the biotechnological processing of laying hen paws into gelatins can be summarized as follows. The accompanying non-collagen components and fat are first removed from the starting ground raw material by gradual treatment in water, 0.2 mol/L NaCl, 0.03 mol/L NaOH, lipolytic enzyme and acetone. Purified collagen is subjected to 72 h conditioning in water with the addition of 0.8% of the proteolytic enzyme Protamex® at room temperature and, after washing with water, the gelatins are extracted in two extraction steps by a batch process. In the first extraction step at 75 °C/120 min, the 1st fraction of gelatin is prepared in a yield of 21.4%. Gelatin has excellent gel strength (313 Bloom), high viscosity (5.6 mPa.s), very low ash content (1.7%) and meets strict standards for use in pharmaceutical applications. In the second extraction step at 100 °C/120 min, the 2nd fraction of gelatin is prepared in a yield of almost 8%. Gelatin has a very good gel strength (208 Bloom), a viscosity of 4.5 mPa.s, a low ash content (2.8%) and meets standards for use in food applications.
4. Conclusions
Laying hen paws are a highly valuable by-product of the slaughter of hens, as they contain a high proportion of collagen (approximately 77%). By removing the accompanying components, purified collagen can be obtained from hen paws, which is an excellent source of raw material for the preparation of gelatins. The aim of this study was to verify the possibilities of processing laying hen collagen into gelatin using an innovative biotechnological process of conditioning the raw material. The advantages of the biotechnological processing of laying hen collagen into gelatins include, in particular, that the yield of prepared gelatins and their properties can be modified by process control. For example, by reducing the dosage of enzyme used in the raw material conditioning process and by appropriate choice of other process conditions, gelatins with a gel strength of up to 380 Bloom can be prepared. Furthermore, a significant reduction in processing times (especially the enzyme processing time of the starting material and gelatin extraction time) and the absence of chemicals used in the conditioning process greatly reduces the financial costs of gelatin preparation, water, electricity and labor requirements. Biotechnological processing of laying hen paw collagen into gelatins is environmentally friendly. With their properties (excellent gel strength, high viscosity and low mineral content) the prepared laying hen gelatins equal the highest quality pork and beef gelatins produced by traditional technologies (using acids and alkalis), they represent a fully fledged alternative for processors and are acceptable for all religious nutrition restrictions.
This is a pioneering article and subsequent studies will have to follow to explore deeply process parameters of the biotechnological processing of laying hen collagen into gelatins and to test physico-chemical properties of prepared gelatins.
5. Patents
From the work reported in this manuscript, the following patent resulted: Patent CZ 307,665—Biotechnology-based production of food gelatine from poultry by-products (2019). At present, the international patent application for the invention under the same name (PCT/CZ2018/050054) is the subject of a research assessment.
Author Contributions
Supervision, visualization, writing—original draft preparation, writing—review and editing: P.M.; methodology, validation: P.M. and R.G.; formal analysis, resources: R.G.; funding acquisition, project administration: J.M.; software: J.P.; data curation, investigation: R.G. and N.T.H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Internal strategic project of Tomas Bata University in Zlín focused on recycling technologies for natural and synthetic polymers and use of products obtained from them.
Acknowledgments
The authors thank to David Dohnal (Přerov, the Czech Republic) for editing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Poultry Trends: The Statistical Reference for Poultry Executives. Available online: http://www.poultrytrends.com/201911/ (accessed on 15 March 2020).
- OECD-FAO Agricultural Outlook 2019–2028. Available online: http://www.fao.org/3/ca4076en/ca4076en.pdf (accessed on 16 March 2020).
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of by-products and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, A.; Abu Bakar, F.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef]
- Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 Laying Down Health Rules as Regards Animal By-Products and Derived Products Not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002 (Animal By-Products Regulation). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R1069 (accessed on 3 March 2020).
- Toldra, F.; Mora, L.; Reig, M. New insights into meat by-product utilization. Meat Sci. 2016, 120, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Seidavi, A.; Zaker-Esteghamati, H.; Scanes, C.G. Poultry byproducts. In Byproducts from Agriculture and Fisheries, 1st ed.; Simpson, B.K., Aryee, A.N., Toldrá, F., Eds.; John Wiley & Sons: Chichester, UK, 2020; pp. 123–146. [Google Scholar]
- Hsieh, Y.-H.P.; Ofori, J.A. Blood-derived products for human consumption. Revel. Sci. 2011, 1, 14–21. [Google Scholar]
- Ofori, J.A.; Hsieh, Y.H. Issues related to the use of blood in food and animal feed. Crit. Rev. Food Sci. Nutr. 2014, 54, 687–697. [Google Scholar] [CrossRef]
- McGovern, V. Recycling poultry feathers: More bang for the cluck. Environ. Health Perspect. 2000, 108, A336–A339. [Google Scholar] [CrossRef][Green Version]
- Bertsch, A.; Cello, N. A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Bioresour. Technol. 2005, 96, 1703–1708. [Google Scholar] [CrossRef]
- Grazziotin, A.; Pimentel, F.A.; de Jong, E.V.; Brandelli, A. Nutritional improvement of feather protein by treatment with microbial keratinase. Anim. Feed Sci. Technol. 2006, 126, 135–144. [Google Scholar] [CrossRef]
- Ścibisz, M.; Arct, J.; Pytkowska, K. Hydrolysed proteins in cosmetic production, part II. SOFW J. Polish Ed. 2008, 1, 12–20. [Google Scholar]
- Teglia, A.; Secchi, G. Proteins in cosmetics. In Principles of Polymer Science and Technology in Cosmetics and Personal Care, 1st ed.; Goddard, E.D., Gruber, J.V., Eds.; Marcel Dekker: New York, NY, USA, 1999; Chapter 9; pp. 404–477. [Google Scholar]
- Mokrejš, P.; Huťťa, M.; Pavlačková, J.; Egner, P.; Beníček, L. The cosmetic and dermatological potential of keratin hydrolysate. J. Cosmet. Dermatol. 2017, 16, e21–e27. [Google Scholar] [CrossRef]
- Ockerman, H.W.; Hansen, C.I. Animal By-Product Processing and Utilization, 1st ed.; CRC Press: London, UK, 2000; pp. 23–83. [Google Scholar]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Characterization of antioxidant and angiotensin-converting enzyme inhibitory peptides derived from cauliflower by-products by multidimensional liquid chromatography and bioinformatics. J. Funct. Foods 2018, 44, 40–47. [Google Scholar] [CrossRef]
- Caliceti, C.; Capriotti, A.L.; Calabria, D.; Bonvicini, F.; Zenezini Chiozzi, R.; Montone, C.M.; Piovesana, S.; Zangheri, M.; Mirasoli, M.; Simoni, P.; et al. Peptides from cauliflower by-products, obtained by an efficient, ecosustainable, and semi-industrial method, exert protective effects on endothelial function. Oxid. Med. Cell. Longev. 2019, 2019, 13. Available online: https://www.hindawi.com/journals/omcl/2019/1046504/ (accessed on 2 July 2020). [CrossRef]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2016, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Norziah, M.H.; Al-Hassan, A.; Khairulnizam, A.B.; Mordi, M.N.; Norita, M. Characterization of fish gelatin from surimi processing wastes: Thermal analysis and effect of transglutaminase on gel properties. Food Hydrocoll. 2009, 23, 1610–1616. [Google Scholar] [CrossRef]
- Kasankala, L.M.; Xue, Y.; Weilong, Y.; Hong, S.D.; He, Q. Optimization of gelatine extraction from grass carp (Catenopharyngodon idella) fish skin by response surface methodology. Bioresour. Technol. 2007, 98, 3338–3343. [Google Scholar] [CrossRef]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Peterková, P.; Lapčík, L., Jr. Kolagen—Vlastnosti, modifikace a aplikace. Chem. Listy 2000, 94, 371–379. [Google Scholar]
- Schrieber, R.; Gareis, H. Gelatine Handbook—Theory and Industrial Practice, 1st ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 45–117. [Google Scholar]
- Mokrejš, P.; Gál, R.; Janáčová, D.; Plšková, M.; Brychtová, M. Chicken paws by-products as an alternative source of proteins. Orient. J. Chem. 2017, 33, 2209–2216. [Google Scholar] [CrossRef]
- Mokrejš, P.; Mrázek, P.; Gál, R.; Pavlačková, J. Biotechnological preparation of gelatines from chicken paws. Polymers 2019, 11, 1060. [Google Scholar] [CrossRef]
- Mokrejš, P.; Gál, R.; Pavlačková, J.; Janáčová, D.; Mrázek, P.; Orsavová, J. Utilisation of chicken slaughterhouse collagen by-products for preparation of gelatines and hydrolysates. Chem. Listy 2019, 113, 121–125. [Google Scholar]
- Gál, R.; Mokrejš, P.; Mrázek, P.; Pavlačková, J.; Janáčová, D.; Orsavová, J. Chicken heads as a promising by-product for preparation of food gelatins. Molecules 2020, 25, 494. [Google Scholar] [CrossRef]
- Mrázek, P.; Mokrejš, P.; Gál, R.; Orsavová, J. Chicken skin gelatine as an alternative to pork and beef gelatins. Potravinarstvo Slovak J. Food Sci. 2019, 13, 224–233. [Google Scholar] [CrossRef]
- Nollet, L.M.L.; Toldrá, F. Handbook of Food Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 357–754. [Google Scholar]
- ISO 937:1978. Meat and Meat Products—Determination of Nitrogen Content. Available online: https://law.resource.org/pub/in/bis/S06/is.5960.1.1996.pdf (accessed on 15 February 2020).
- ISO 3496:1994. Meat and Meat Products—Determination of Hydroxyproline Content. Available online: https://cdn.standards.iteh.ai/samples/8848/908d030b1d6a4807bc2ac15fee8d51f9/SIST-ISO-3496-1995.pdf (accessed on 15 February 2020).
- Standard Testing Methods for Edible Gelatin. Official Procedure of the Gelatin Manufacturers Institute of America, Inc. Available online: http://www.gelatin-gmia.com/images/GMIA_Official_Methods_of_Gelatin_Revised_2013.pdf/ (accessed on 25 September 2020).
- Antony, J. Design of Experiments for Engineers and Scientists, 1st ed.; Butterworth-Heinemann: Oxford, MI, USA, 2003; pp. 54–70. [Google Scholar]
- Widyasari, R.; Rawdkuen, S. Extraction and characterization of gelatin from chicken feet by acid and ultrasound assisted extraction. Food Appl. Biosci. J. 2014, 2, 83–95. [Google Scholar]
- Taufik, M.; Triatmojo, S.; Erwanto, Y.; Santoso, U. Effect of broiler age and extraction temperature on characteristic chicken feet skin gelatin. In Proceedings of the 5th International Seminar on Tropical Animal Production, Yogyakarta, Indonesia, 19–22 October 2010; pp. 649–656. [Google Scholar]
- Almeida, P.F.; Calarge, F.A.; Santana, J.C.C. Production of a product similar to gelatin from chicken feet collagen. Eng. Agrícola 2013, 33, 1289–1300. [Google Scholar] [CrossRef]
- Van Gulick, L.; Saby, C.; Morjani, H.; Beljebbar, A. Age-related changes in molecular organization of type I collagen in tendon as probed by polarized SHG and Raman microspectroscopy. Sci. Rep. 2019, 9, 1–12. Available online: https://www.nature.com/articles/s41598-019-43636-2 (accessed on 2 July 2020). [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Sompie, M.; Triasih, A. 2018: Effect of extraction temperature on characteristics of chicken legskin gelatin. In Proceedings of the IOP Conference Series: Earth and Environmental Science, International Symposium on Food and Agro-Biodiversity (ISFA), Semarang, Indonesia, 26–27 September 2017; p. 12089. [Google Scholar] [CrossRef]
- Gelatine Manufacturers of Europe. Available online: https://www.gelatine.org/ (accessed on 1 April 2020).
- Gelatin Manufacturers Institute of America. Available online: http://www.gelatin-gmia.com/ (accessed on 1 April 2020).
- European Pharmacopoeia 9.0. European Directorate for the Quality of Medicines & Health Care. 2017. Available online: https://www.edqm.eu/en/news/shutdown-european-pharmacopoeia-9th-edition (accessed on 15 December 2019).
- Food Chemical Codex 12. Available online: https://www.foodchemicalscodex.org/ (accessed on 10 April 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).