Biotechnological Processing of Laying Hen Paw Collagen into Gelatins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Analytical Methods and Appliances
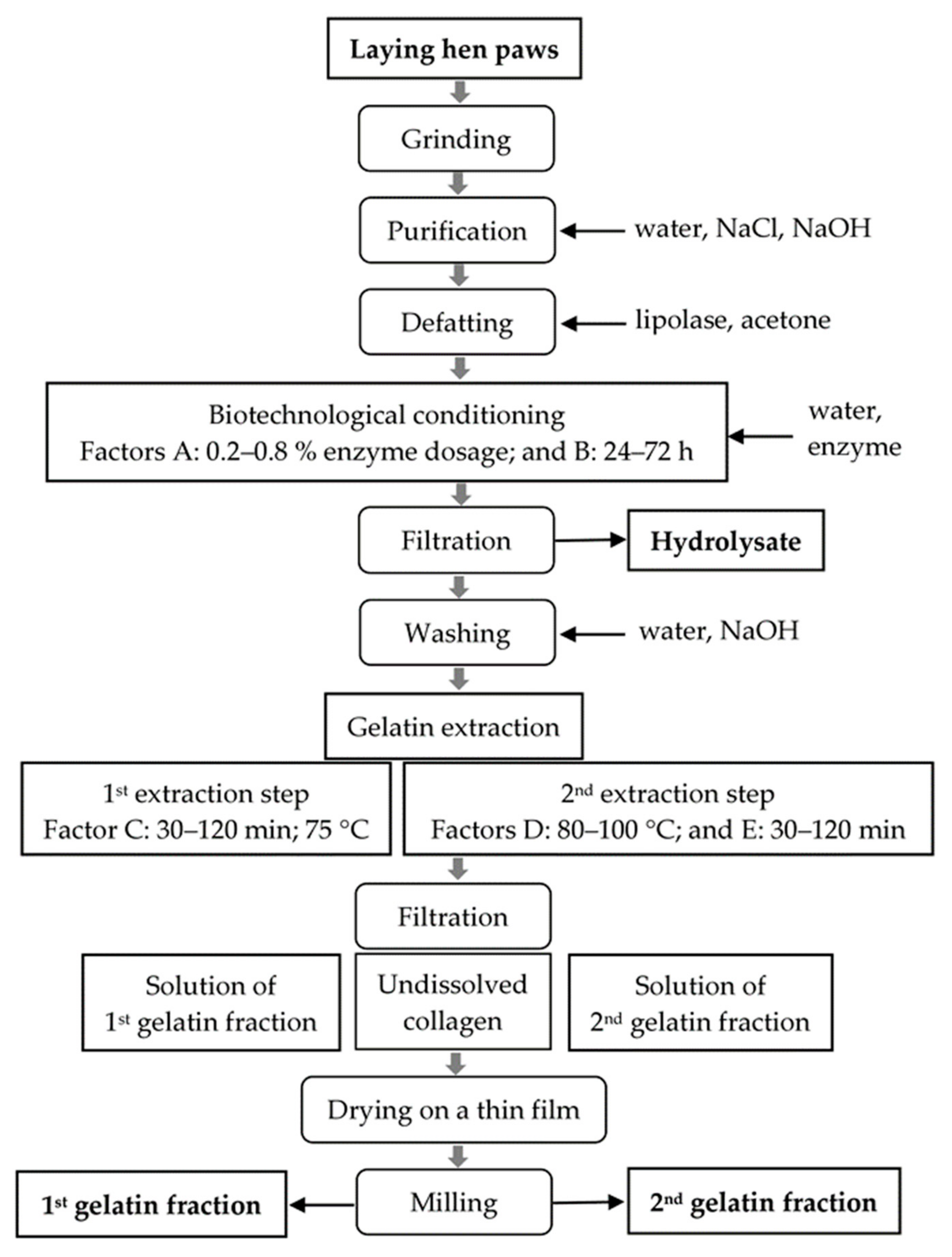
2.2. General Process Layout
2.3. Workflow of Processing of Laying Hen Paw Collagen into Gelatins
2.4. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Gelatins of the 1st Fraction
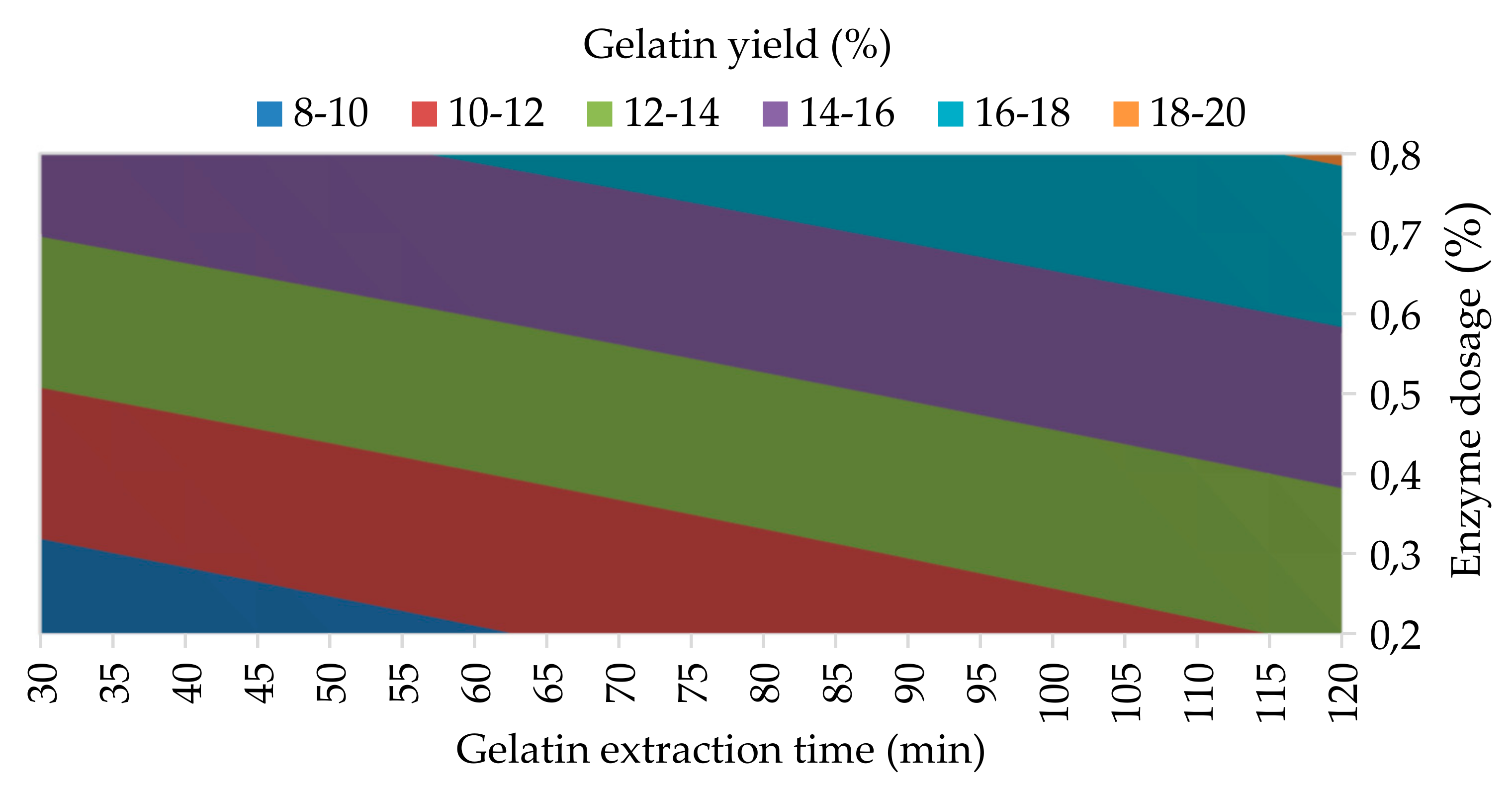
3.1.1. Gelatin Yield
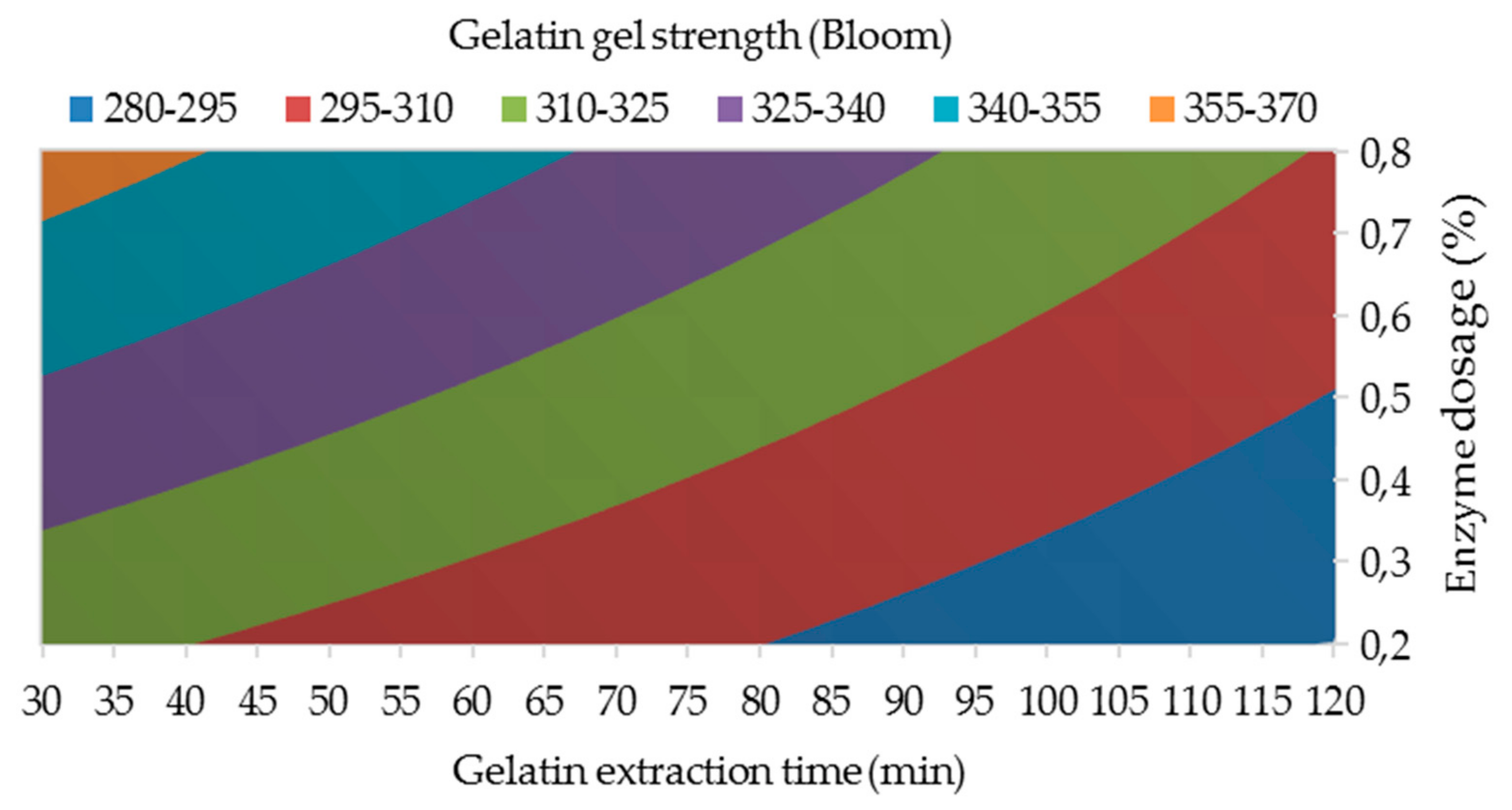
3.1.2. Gelatin Gel Strength
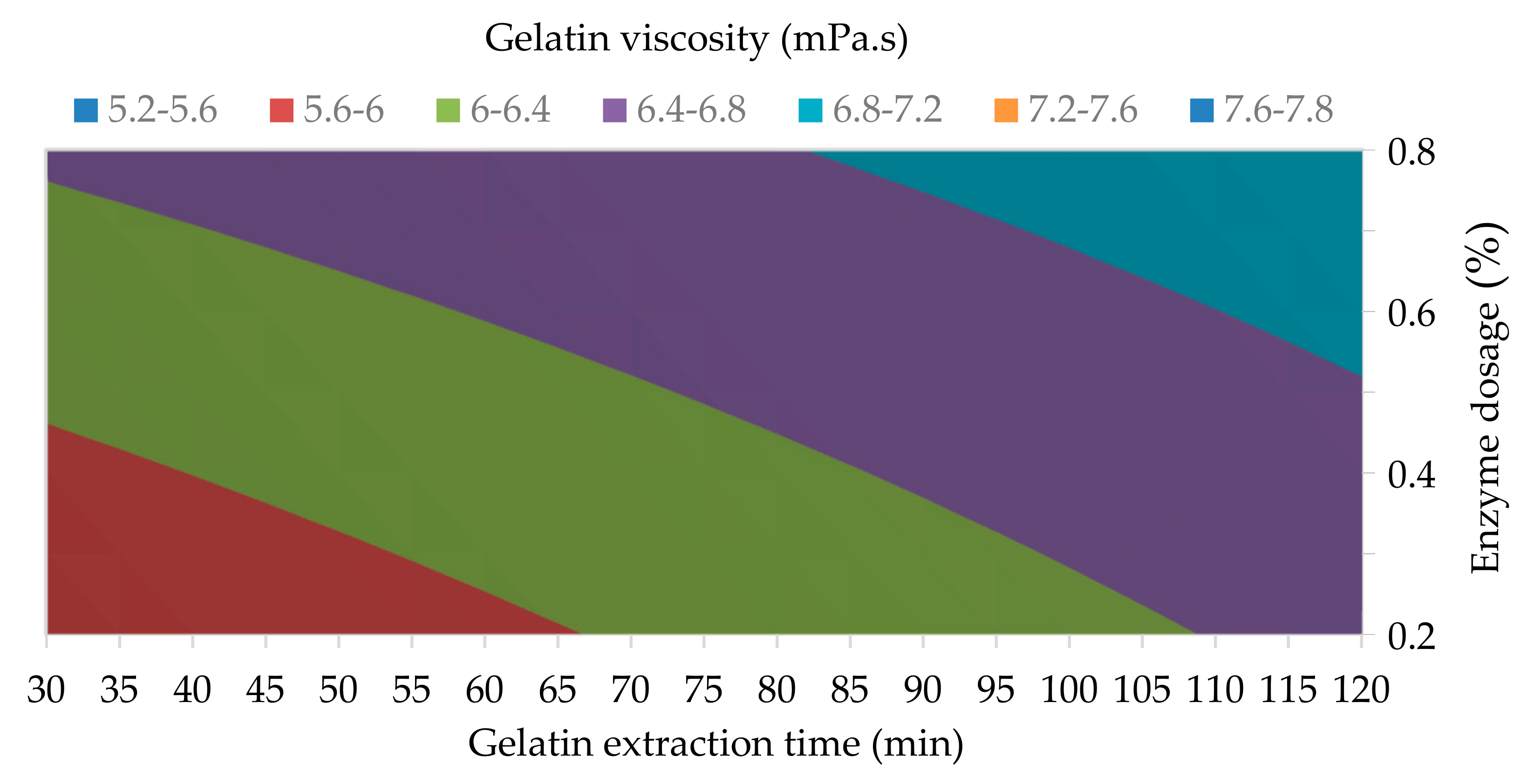
3.1.3. Gelatin Viscosity and Gelatin Ash Content
3.2. Preparation of Gelatins of the 2nd Fraction
3.3. Proposition of Optimal Process Conditions
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poultry Trends: The Statistical Reference for Poultry Executives. Available online: http://www.poultrytrends.com/201911/ (accessed on 15 March 2020).
- OECD-FAO Agricultural Outlook 2019–2028. Available online: http://www.fao.org/3/ca4076en/ca4076en.pdf (accessed on 16 March 2020).
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Bawa, A.S. Utilization of by-products and waste materials from meat, poultry and fish processing industries: A review. J. Food Sci. Technol. 2012, 49, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, A.; Abu Bakar, F.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef]
- Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 Laying Down Health Rules as Regards Animal By-Products and Derived Products Not Intended for Human Consumption and Repealing Regulation (EC) No 1774/2002 (Animal By-Products Regulation). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R1069 (accessed on 3 March 2020).
- Toldra, F.; Mora, L.; Reig, M. New insights into meat by-product utilization. Meat Sci. 2016, 120, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Seidavi, A.; Zaker-Esteghamati, H.; Scanes, C.G. Poultry byproducts. In Byproducts from Agriculture and Fisheries, 1st ed.; Simpson, B.K., Aryee, A.N., Toldrá, F., Eds.; John Wiley & Sons: Chichester, UK, 2020; pp. 123–146. [Google Scholar]
- Hsieh, Y.-H.P.; Ofori, J.A. Blood-derived products for human consumption. Revel. Sci. 2011, 1, 14–21. [Google Scholar]
- Ofori, J.A.; Hsieh, Y.H. Issues related to the use of blood in food and animal feed. Crit. Rev. Food Sci. Nutr. 2014, 54, 687–697. [Google Scholar] [CrossRef]
- McGovern, V. Recycling poultry feathers: More bang for the cluck. Environ. Health Perspect. 2000, 108, A336–A339. [Google Scholar] [CrossRef][Green Version]
- Bertsch, A.; Cello, N. A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Bioresour. Technol. 2005, 96, 1703–1708. [Google Scholar] [CrossRef]
- Grazziotin, A.; Pimentel, F.A.; de Jong, E.V.; Brandelli, A. Nutritional improvement of feather protein by treatment with microbial keratinase. Anim. Feed Sci. Technol. 2006, 126, 135–144. [Google Scholar] [CrossRef]
- Ścibisz, M.; Arct, J.; Pytkowska, K. Hydrolysed proteins in cosmetic production, part II. SOFW J. Polish Ed. 2008, 1, 12–20. [Google Scholar]
- Teglia, A.; Secchi, G. Proteins in cosmetics. In Principles of Polymer Science and Technology in Cosmetics and Personal Care, 1st ed.; Goddard, E.D., Gruber, J.V., Eds.; Marcel Dekker: New York, NY, USA, 1999; Chapter 9; pp. 404–477. [Google Scholar]
- Mokrejš, P.; Huťťa, M.; Pavlačková, J.; Egner, P.; Beníček, L. The cosmetic and dermatological potential of keratin hydrolysate. J. Cosmet. Dermatol. 2017, 16, e21–e27. [Google Scholar] [CrossRef]
- Ockerman, H.W.; Hansen, C.I. Animal By-Product Processing and Utilization, 1st ed.; CRC Press: London, UK, 2000; pp. 23–83. [Google Scholar]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Characterization of antioxidant and angiotensin-converting enzyme inhibitory peptides derived from cauliflower by-products by multidimensional liquid chromatography and bioinformatics. J. Funct. Foods 2018, 44, 40–47. [Google Scholar] [CrossRef]
- Caliceti, C.; Capriotti, A.L.; Calabria, D.; Bonvicini, F.; Zenezini Chiozzi, R.; Montone, C.M.; Piovesana, S.; Zangheri, M.; Mirasoli, M.; Simoni, P.; et al. Peptides from cauliflower by-products, obtained by an efficient, ecosustainable, and semi-industrial method, exert protective effects on endothelial function. Oxid. Med. Cell. Longev. 2019, 2019, 13. Available online: https://www.hindawi.com/journals/omcl/2019/1046504/ (accessed on 2 July 2020). [CrossRef]
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2016, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Norziah, M.H.; Al-Hassan, A.; Khairulnizam, A.B.; Mordi, M.N.; Norita, M. Characterization of fish gelatin from surimi processing wastes: Thermal analysis and effect of transglutaminase on gel properties. Food Hydrocoll. 2009, 23, 1610–1616. [Google Scholar] [CrossRef]
- Kasankala, L.M.; Xue, Y.; Weilong, Y.; Hong, S.D.; He, Q. Optimization of gelatine extraction from grass carp (Catenopharyngodon idella) fish skin by response surface methodology. Bioresour. Technol. 2007, 98, 3338–3343. [Google Scholar] [CrossRef]
- Mariod, A.A.; Adam, H.F. Review: Gelatin, source, extraction and industrial applications. Acta Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Peterková, P.; Lapčík, L., Jr. Kolagen—Vlastnosti, modifikace a aplikace. Chem. Listy 2000, 94, 371–379. [Google Scholar]
- Schrieber, R.; Gareis, H. Gelatine Handbook—Theory and Industrial Practice, 1st ed.; Wiley-VCH: Weinheim, Germany, 2007; pp. 45–117. [Google Scholar]
- Mokrejš, P.; Gál, R.; Janáčová, D.; Plšková, M.; Brychtová, M. Chicken paws by-products as an alternative source of proteins. Orient. J. Chem. 2017, 33, 2209–2216. [Google Scholar] [CrossRef]
- Mokrejš, P.; Mrázek, P.; Gál, R.; Pavlačková, J. Biotechnological preparation of gelatines from chicken paws. Polymers 2019, 11, 1060. [Google Scholar] [CrossRef]
- Mokrejš, P.; Gál, R.; Pavlačková, J.; Janáčová, D.; Mrázek, P.; Orsavová, J. Utilisation of chicken slaughterhouse collagen by-products for preparation of gelatines and hydrolysates. Chem. Listy 2019, 113, 121–125. [Google Scholar]
- Gál, R.; Mokrejš, P.; Mrázek, P.; Pavlačková, J.; Janáčová, D.; Orsavová, J. Chicken heads as a promising by-product for preparation of food gelatins. Molecules 2020, 25, 494. [Google Scholar] [CrossRef]
- Mrázek, P.; Mokrejš, P.; Gál, R.; Orsavová, J. Chicken skin gelatine as an alternative to pork and beef gelatins. Potravinarstvo Slovak J. Food Sci. 2019, 13, 224–233. [Google Scholar] [CrossRef]
- Nollet, L.M.L.; Toldrá, F. Handbook of Food Analysis, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 357–754. [Google Scholar]
- ISO 937:1978. Meat and Meat Products—Determination of Nitrogen Content. Available online: https://law.resource.org/pub/in/bis/S06/is.5960.1.1996.pdf (accessed on 15 February 2020).
- ISO 3496:1994. Meat and Meat Products—Determination of Hydroxyproline Content. Available online: https://cdn.standards.iteh.ai/samples/8848/908d030b1d6a4807bc2ac15fee8d51f9/SIST-ISO-3496-1995.pdf (accessed on 15 February 2020).
- Standard Testing Methods for Edible Gelatin. Official Procedure of the Gelatin Manufacturers Institute of America, Inc. Available online: http://www.gelatin-gmia.com/images/GMIA_Official_Methods_of_Gelatin_Revised_2013.pdf/ (accessed on 25 September 2020).
- Antony, J. Design of Experiments for Engineers and Scientists, 1st ed.; Butterworth-Heinemann: Oxford, MI, USA, 2003; pp. 54–70. [Google Scholar]
- Widyasari, R.; Rawdkuen, S. Extraction and characterization of gelatin from chicken feet by acid and ultrasound assisted extraction. Food Appl. Biosci. J. 2014, 2, 83–95. [Google Scholar]
- Taufik, M.; Triatmojo, S.; Erwanto, Y.; Santoso, U. Effect of broiler age and extraction temperature on characteristic chicken feet skin gelatin. In Proceedings of the 5th International Seminar on Tropical Animal Production, Yogyakarta, Indonesia, 19–22 October 2010; pp. 649–656. [Google Scholar]
- Almeida, P.F.; Calarge, F.A.; Santana, J.C.C. Production of a product similar to gelatin from chicken feet collagen. Eng. Agrícola 2013, 33, 1289–1300. [Google Scholar] [CrossRef]
- Van Gulick, L.; Saby, C.; Morjani, H.; Beljebbar, A. Age-related changes in molecular organization of type I collagen in tendon as probed by polarized SHG and Raman microspectroscopy. Sci. Rep. 2019, 9, 1–12. Available online: https://www.nature.com/articles/s41598-019-43636-2 (accessed on 2 July 2020). [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Sompie, M.; Triasih, A. 2018: Effect of extraction temperature on characteristics of chicken legskin gelatin. In Proceedings of the IOP Conference Series: Earth and Environmental Science, International Symposium on Food and Agro-Biodiversity (ISFA), Semarang, Indonesia, 26–27 September 2017; p. 12089. [Google Scholar] [CrossRef]
- Gelatine Manufacturers of Europe. Available online: https://www.gelatine.org/ (accessed on 1 April 2020).
- Gelatin Manufacturers Institute of America. Available online: http://www.gelatin-gmia.com/ (accessed on 1 April 2020).
- European Pharmacopoeia 9.0. European Directorate for the Quality of Medicines & Health Care. 2017. Available online: https://www.edqm.eu/en/news/shutdown-european-pharmacopoeia-9th-edition (accessed on 15 December 2019).
- Food Chemical Codex 12. Available online: https://www.foodchemicalscodex.org/ (accessed on 10 April 2020).

| Processing Parameters | |||||
|---|---|---|---|---|---|
| Exp. No. | Factor A (%) | Factor B (h) | Factor C (min) | Factor D (°C) | Factor E (min) |
| 1 | 0.2 | 24 | 30 | 80 | 30 |
| 2 | 0.2 | 24 | 120 | 80 | 120 |
| 3 | 0.2 | 72 | 30 | 80 | 30 |
| 4 | 0.2 | 72 | 120 | 80 | 120 |
| 5 | 0.8 | 24 | 30 | 100 | 30 |
| 6 | 0.8 | 24 | 120 | 100 | 120 |
| 7 | 0.8 | 72 | 30 | 100 | 30 |
| 8 | 0.8 | 72 | 120 | 100 | 120 |
| 9 | 0.5 | 48 | 75 | 90 | 75 |
| 10 | 0.5 | 48 | 75 | 90 | 75 |
| 11 | 0 | 48 | 75 | 90 | 75 |
| Mass Balance | 1st Gelatin Fractions | 2nd Gelatin Fractions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. No. | YH (%) | YG1 (%) | YG2 (%) | ∑Y (%) | BE (%) | F1 (Bloom) ± SD | ν1 (mPa.s) ± SD | Ash1 (%) ± SD | F2 (Bloom) ± SD | ν2 (mPa.s) ± SD | Ash2 (%) ± SD |
| 1 | 3.3 | 8.2 | 3.3 | 11.5 | 2.2 | 302 ± 4 | 5.3 ± 0.07 | 2.0 ± 0.01 | 190 ± 3 | 3.4 ± 0.04 | 2.6 ± 0.02 |
| 2 | 3.5 | 11.5 | 5.5 | 17.0 | 5.4 | 275 ± 3 | 6.3 ± 0.08 | 2.5 ± 0.03 | 185 ± 3 | 3.5 ± 0.04 | 2.5 ± 0.01 |
| 3 | 4.5 | 9.3 | 4.9 | 14.2 | 4.4 | 326 ± 4 | 5.7 ± 0.08 | 2.0 ± 0.02 | 187 ± 4 | 3.6 ± 0.03 | 2.6 ± 0.02 |
| 4 | 4.4 | 12.9 | 6.7 | 19.6 | 4.7 | 285 ± 2 | 5.5 ± 0.06 | 2.1 ± 0.01 | 191 ± 2 | 3.4 ± 0.04 | 2.6 ± 0.02 |
| 5 | 6.1 | 14.3 | 5.4 | 19.7 | 5.5 | 344 ± 3 | 6.8 ± 0.04 | 2.3 ± 0.02 | 214 ± 3 | 4.4 ± 0.04 | 2.7 ± 0.03 |
| 6 | 6.5 | 14.9 | 7.1 | 22.0 | 3.9 | 305 ± 1 | 7.3 ± 0.04 | 1.4 ± 0.01 | 205 ± 3 | 4.6 ± 0.03 | 2.6 ± 0.01 |
| 7 | 7.2 | 15.9 | 5.5 | 21.4 | 5.9 | 380 ± 4 | 5.8 ± 0.05 | 1.6 ± 0.01 | 215 ± 4 | 4.6 ± 0.05 | 2.7 ± 0.02 |
| 8 | 6.9 | 21.4 | 7.7 | 29.1 | 4.4 | 313 ± 3 | 5.6 ± 0.07 | 1.7 ± 0.02 | 208 ± 3 | 4.5 ± 0.05 | 2.8 ± 0.03 |
| 9 | 5.5 | 13.3 | 6.2 | 19.5 | 6.0 | 341 ± 4 | 6.5 ± 0.06 | 1.7 ± 0.01 | 261 ± 2 | 5.6 ± 0.04 | 2.5 ± 0.01 |
| 10 | 5.4 | 13.1 | 6.1 | 19.2 | 5.5 | 339 ± 4 | 6.3 ± 0.06 | 1.8 ± 0.02 | 263 ± 1 | 5.5 ± 0.04 | 2.5 ± 0.01 |
| 11 | 1.7 | 9.9 | 4.7 | 14.6 | 1.7 | 362 ± 3 | 7.8 ± 0.08 | 1.9 ± 0.02 | 273 ± 3 | 6.0 ± 0.05 | 2.5 ± 0.02 |
| Degree of Freedom | Sum of Squares | Mean Squares | F-Value | P-Value | |
|---|---|---|---|---|---|
| Gelatin yield | |||||
| Model | 8 | 121.036 | 15.1295 | 756.47 | 0.028 |
| Factor A (Enzyme dosage) | 1 | 75.645 | 75.6450 | 3782.25 | 0.010 |
| Factor B (Enzyme processing time) | 1 | 14.045 | 14.0450 | 702.25 | 0.024 |
| Factor C (Gelatin extraction time) | 1 | 21.125 | 21.1250 | 1056.25 | 0.020 |
| 2-way interaction AB | 1 | 3.920 | 3.9200 | 196.00 | 0.045 |
| 2-way interaction AC | 1 | 0.080 | 0.0800 | 4.00 | 0.295 |
| 2-way interaction BC | 1 | 3.380 | 3.3800 | 169.00 | 0.049 |
| 3-way interaction ABC | 1 | 2.645 | 2.6450 | 132.25 | 0.056 |
| Curvature | 1 | 0.196 | 0.1960 | 9.80 | 0.197 |
| Error | 1 | 0.020 | 0.0200 | ||
| Total | 9 | 121.056 | |||
| Gelatin gel strength | |||||
| Model | 8 | 8850.00 | 1106.25 | 553.13 | 0.033 |
| Factor A (Enzyme dosage) | 1 | 2964.50 | 2964.50 | 1482.25 | 0.017 |
| Factor B (Enzyme processing time) | 1 | 760.50 | 760.50 | 380.25 | 0.033 |
| Factor C (Gelatin extraction time) | 1 | 3784.50 | 3784.50 | 1892.25 | 0.015 |
| 2-way interaction AB | 1 | 12.50 | 12.50 | 6.25 | 0.242 |
| 2-way interaction AC | 1 | 180.50 | 180.50 | 90.25 | 0.067 |
| 2-way interaction BC | 1 | 220.50 | 220.50 | 110.25 | 0.060 |
| 3-way interaction ABC | 1 | 24.50 | 24.50 | 12.25 | 0.177 |
| Curvature | 1 | 902.50 | 902.50 | 451.25 | 0.030 |
| Error | 1 | 2.00 | 2.00 | ||
| Total | 9 | 8852.00 | |||
| Gelatin viscosity | |||||
| Model | 8 | 3.64900 | 0.45612 | 22.81 | 0.161 |
| Factor A (Enzyme dosage) | 1 | 0.91125 | 0.91125 | 45.56 | 0.094 |
| Factor B (Enzyme processing time) | 1 | 1.20125 | 1.20125 | 60.06 | 0.082 |
| Factor C (Gelatin extraction time) | 1 | 0.15125 | 0.15125 | 7.56 | 0.222 |
| 2-way interaction AB | 1 | 0.66125 | 0.66125 | 33.06 | 0.110 |
| 2-way interaction AC | 1 | 0.03125 | 0.03125 | 1.56 | 0.430 |
| 2-way interaction BC | 1 | 0.45125 | 0.45125 | 22.56 | 0.132 |
| 3-way interaction ABC | 1 | 0.03125 | 0.03125 | 1.56 | 0.430 |
| Curvature | 1 | 0.21025 | 0.21025 | 10.51 | 0.190 |
| Error | 1 | 0.02000 | 0.02000 | ||
| Total | 9 | 3.66900 | |||
| Gelatin ash content | |||||
| Model | 8 | 1.00400 | 0.125500 | 25.10 | 0.153 |
| Factor A (Enzyme dosage) | 1 | 0.32000 | 0.320000 | 64.00 | 0.079 |
| Factor B (Enzyme processing time) | 1 | 0.08000 | 0.080000 | 16.00 | 0.156 |
| Factor C (Gelatin extraction time) | 1 | 0.00500 | 0.005000 | 1.00 | 0.500 |
| 2-way interaction AB | 1 | 0.00000 | 0.000000 | 0.00 | 1.000 |
| 2-way interaction AC | 1 | 0.24500 | 0.245000 | 49.00 | 0.090 |
| 2-way interaction BC | 1 | 0.04500 | 0.045000 | 9.00 | 0.205 |
| 3-way interaction ABC | 1 | 0.24500 | 0.245000 | 19.00 | 0.090 |
| Curvature | 1 | 0.06400 | 0.064000 | 12.80 | 0.174 |
| Error | 1 | 0.00500 | 0.005000 | ||
| Total | 9 | 1.00900 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gál, R.; Mokrejš, P.; Pavlačková, J.; Linh, N.T.H.; Mlček, J. Biotechnological Processing of Laying Hen Paw Collagen into Gelatins. Processes 2020, 8, 1415. https://doi.org/10.3390/pr8111415
Gál R, Mokrejš P, Pavlačková J, Linh NTH, Mlček J. Biotechnological Processing of Laying Hen Paw Collagen into Gelatins. Processes. 2020; 8(11):1415. https://doi.org/10.3390/pr8111415
Chicago/Turabian StyleGál, Robert, Pavel Mokrejš, Jana Pavlačková, Ngo Thi Hong Linh, and Jiří Mlček. 2020. "Biotechnological Processing of Laying Hen Paw Collagen into Gelatins" Processes 8, no. 11: 1415. https://doi.org/10.3390/pr8111415
APA StyleGál, R., Mokrejš, P., Pavlačková, J., Linh, N. T. H., & Mlček, J. (2020). Biotechnological Processing of Laying Hen Paw Collagen into Gelatins. Processes, 8(11), 1415. https://doi.org/10.3390/pr8111415







