Rapid Quality Control of Woodchip Parameters Using a Hand-Held Near Infrared Spectrophotometer
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation of Woodchip Samples
2.2. Laboratory Analysis
2.3. Near-Infrared Spectral Acquisition
2.4. Data Processing and Partial Least Square Regression
3. Results
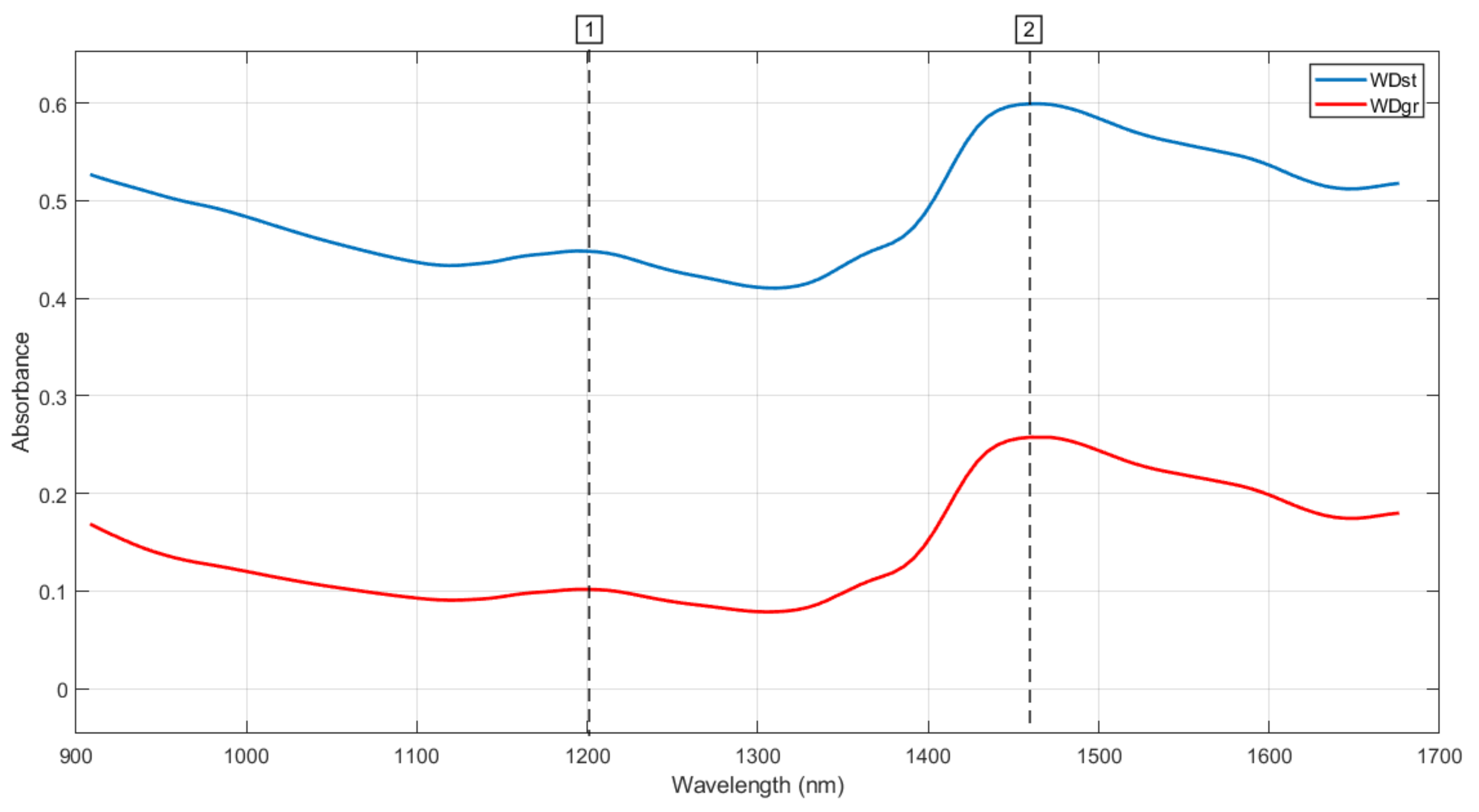
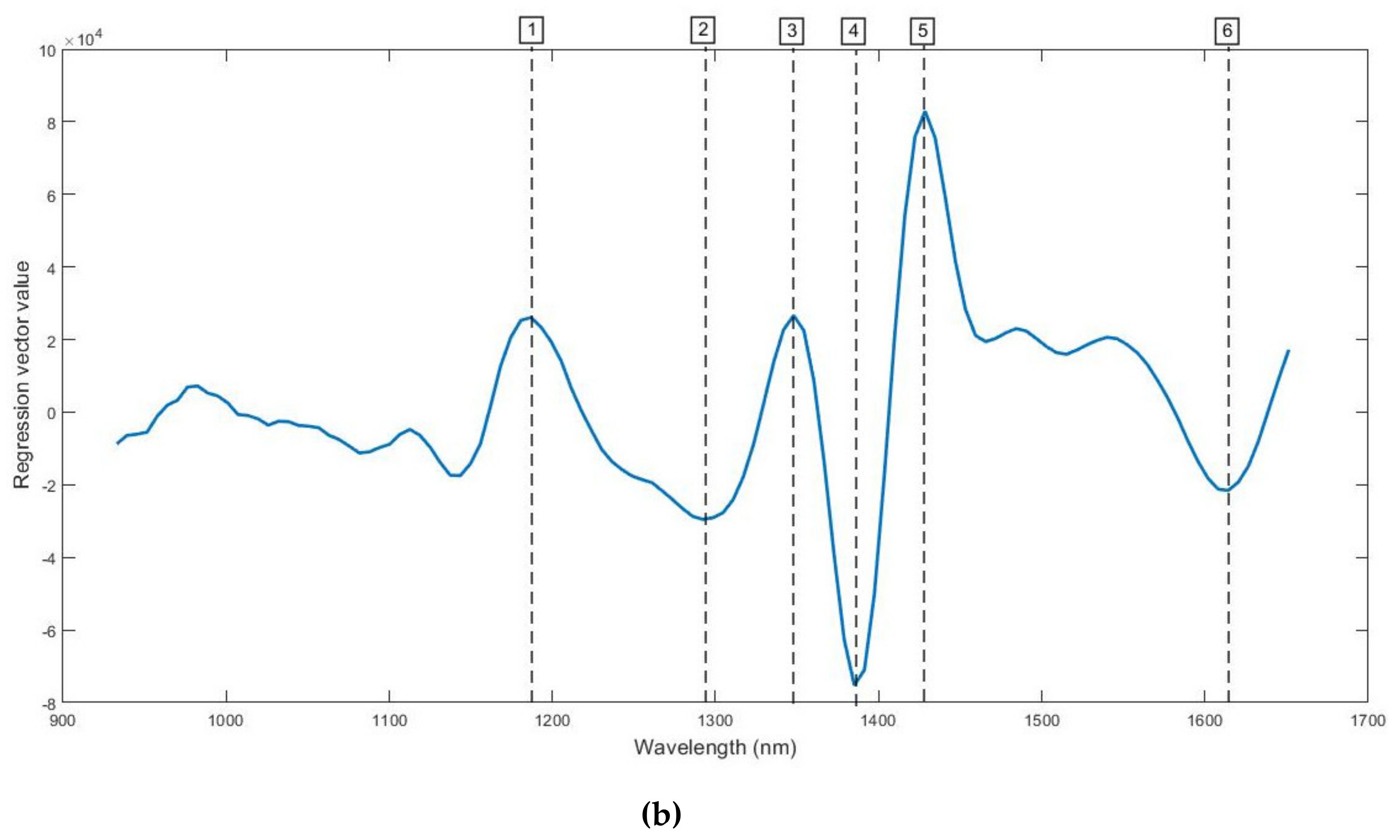
3.1. Spectra
3.2. General Statistical Analysis
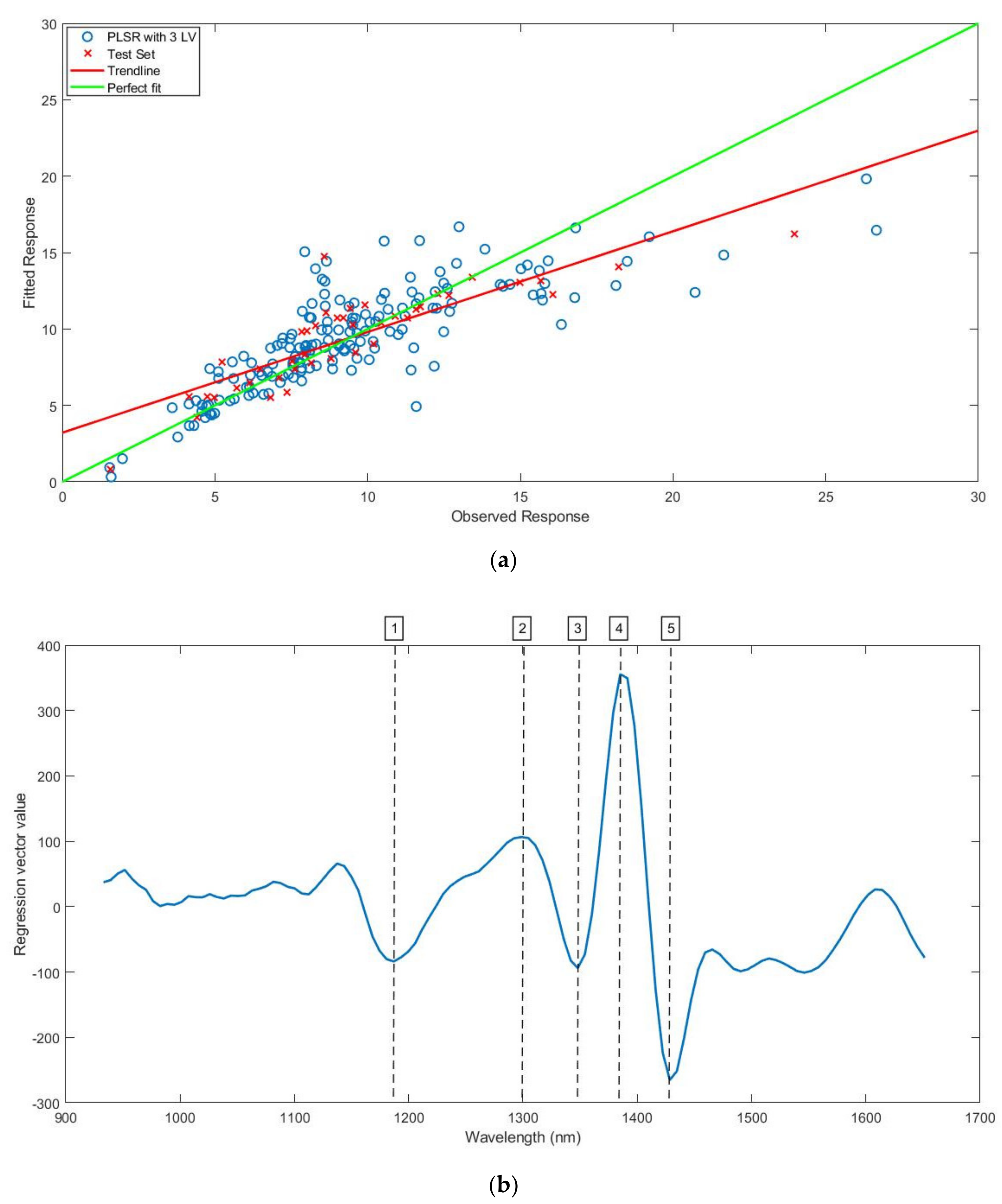
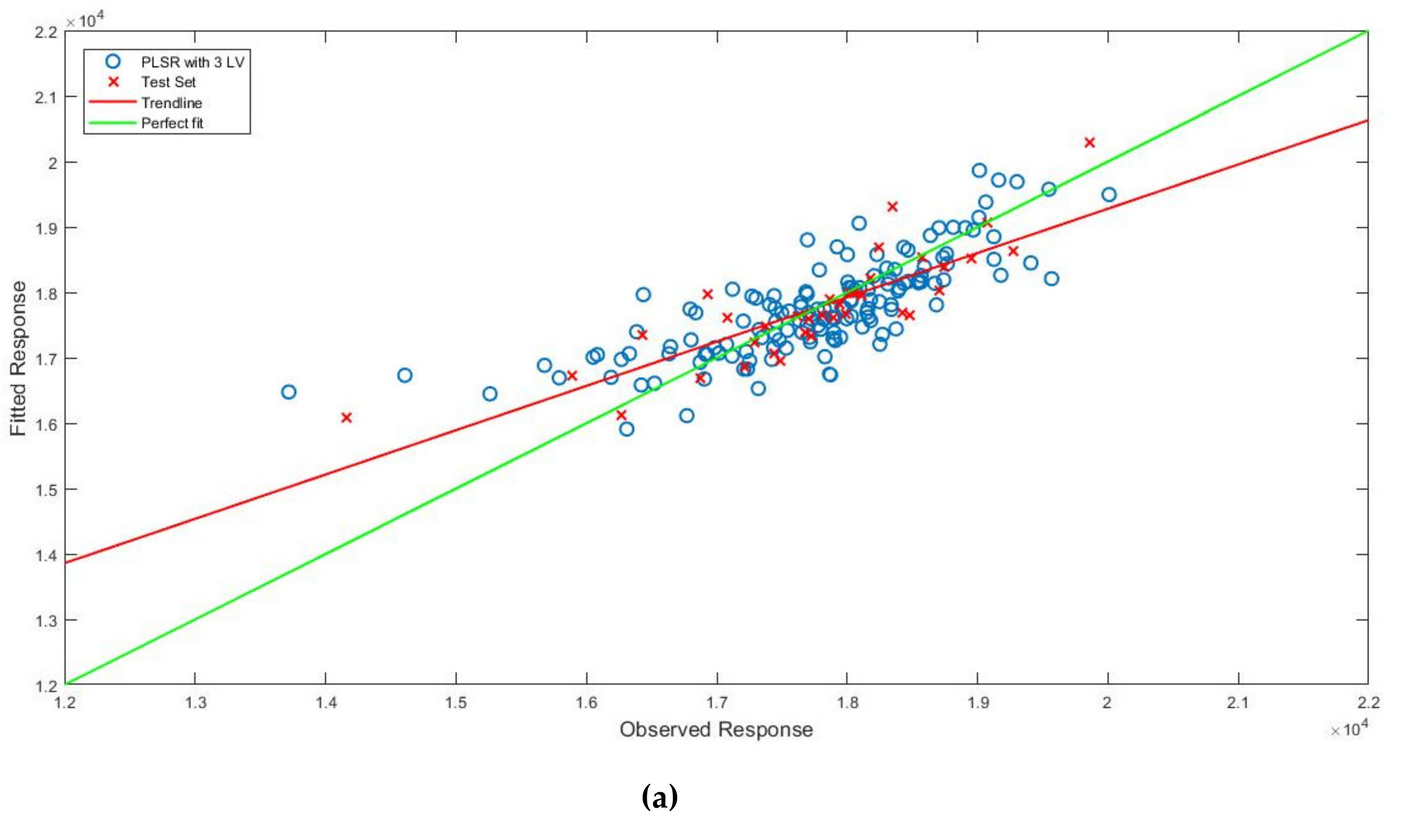
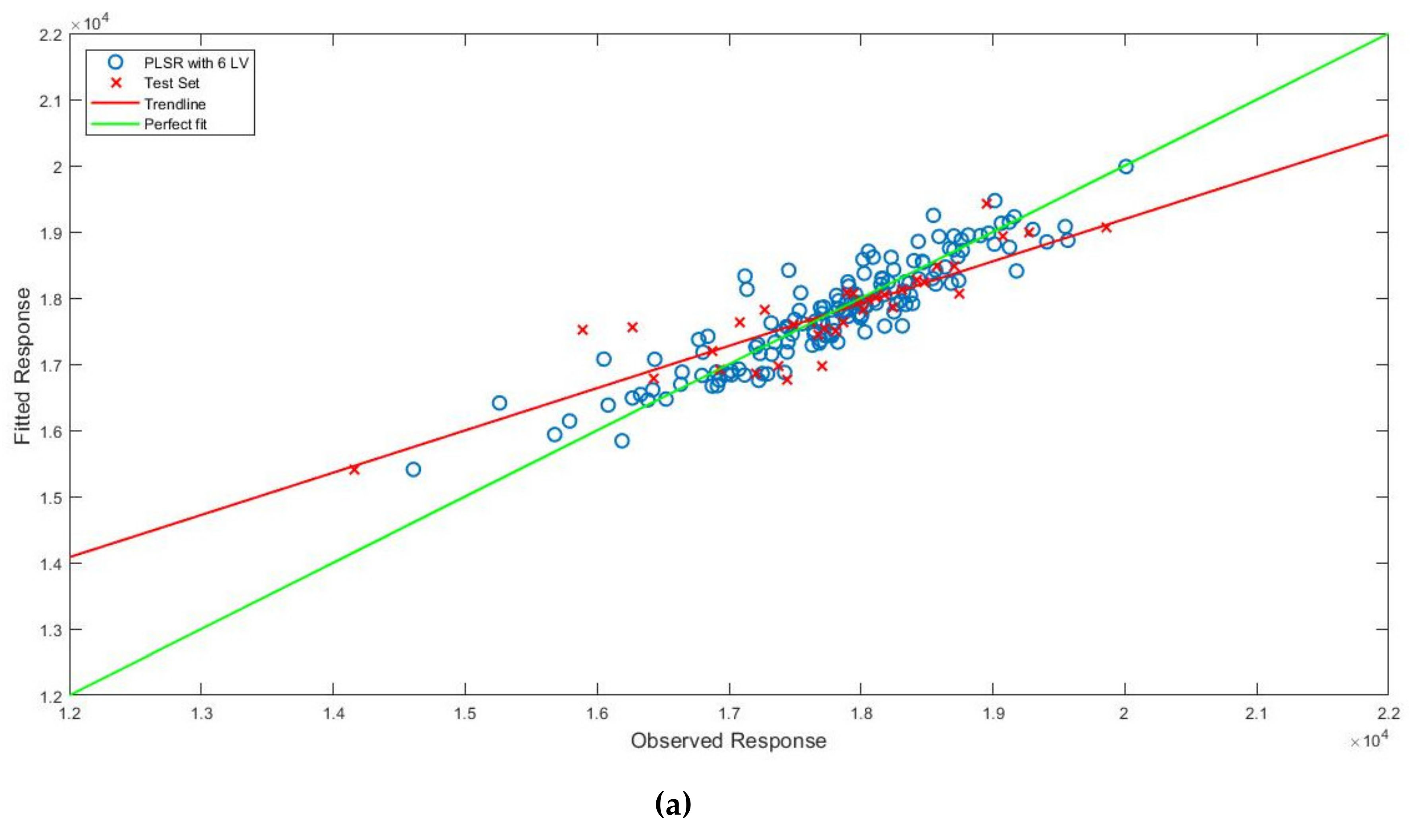
3.3. Prediction of Bound Water Content
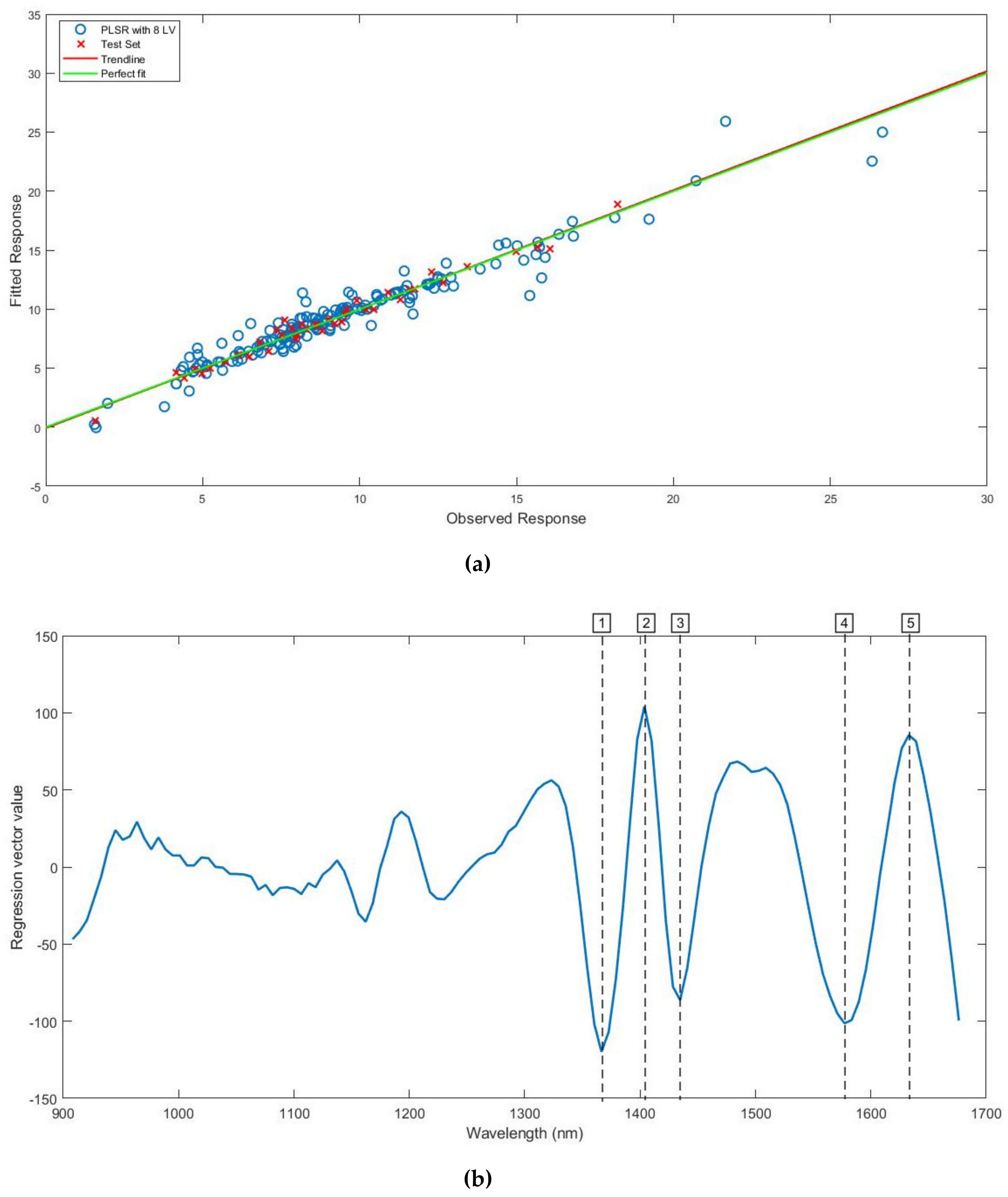
3.4. Prediction of Ash Content
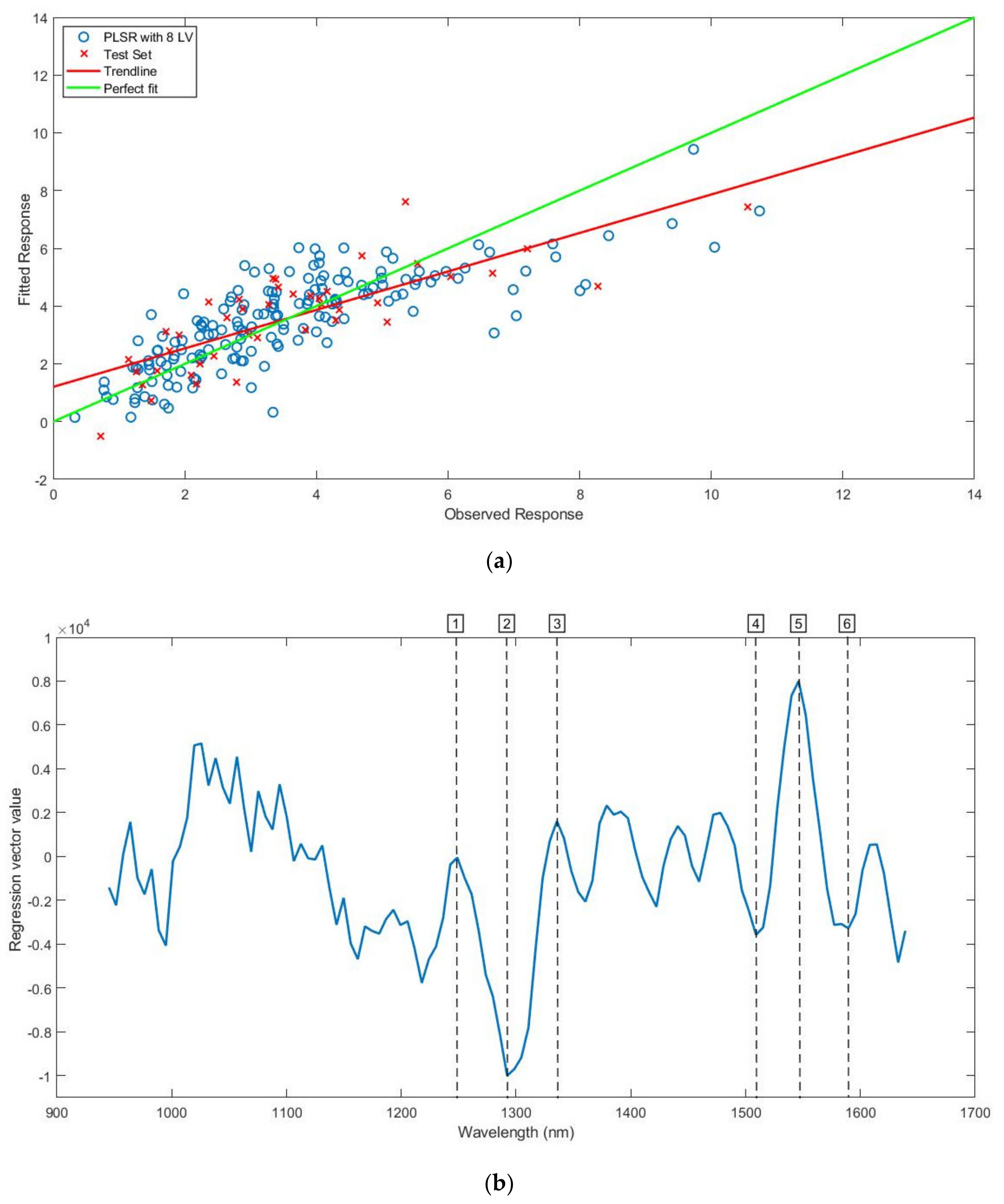
3.5. Prediction of Gross Calorific Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- PricewaterhouseCoopers. Sustainable and Optimal Use of Biomass for Energy in the EU beyond 2020; VITO: Mol, Belgium; Utrecht University: Utrecht, The Netherlands; TU Vienna: Vienna, Austria; INFRO: Celle, Germany; Rütter Soceco & PwC: Rüschlikon, Switzerland, 2017. [Google Scholar]
- Mancini, M.; Duca, D.; Toscano, G. Laboratory customized online measurements for the prediction of the key-parameters of biomass quality control. J. Near Infrared Spectrosc. 2019, 27, 15–25. [Google Scholar] [CrossRef]
- Manzone, M.; Calvo, A. Woodchip transportation: Climatic and congestion influence on productivity, energy and CO2 emission of agricultural and industrial convoys. Renew. Energy 2017, 108, 250–259. [Google Scholar] [CrossRef]
- Fagan, C.C.; Everard, C.D.; McDonnell, K. Prediction of moisture, calorific value, ash and carbon content of two dedicated bioenergy crops using near-infrared spectroscopy. Bioresour. Technol. 2011, 102, 5200–5206. [Google Scholar] [CrossRef]
- Toscano, G.; Leoni, E.; Feliciangeli, G.; Duca, D.; Mancini, M. Application of ISO standards on sampling and effects on the quality assessment of solid biofuel employed in a real power plant. Fuel 2020, 278, 118142. [Google Scholar] [CrossRef]
- Mancini, M.; Rinnan, Å.; Pizzi, A.; Toscano, G. Prediction of gross calorific value and ash content of woodchip samples by means of FT-NIR spectroscopy. Fuel Process. Technol. 2018, 169, 77–83. [Google Scholar] [CrossRef]
- Toscano, G.; Duca, D.; Pedretti, E.F.; Pizzi, A.; Rossini, G.; Mengarelli, C.; Mancini, M. Investigation of woodchip quality: Relationship between the most important chemical and physical parameters. Energy 2016, 106, 38–44. [Google Scholar] [CrossRef]
- Demirbas, A. Relationships between Heating Value and Lignin, Moisture, Ash and Extractive Contents of Biomass Fuels. Energy Explor. Exploit. 2002, 20, 105–111. [Google Scholar] [CrossRef]
- Mancini, M.; Toscano, G.; Rinnan, Å. Study of the scattering effects on NIR data for the prediction of ash content using EMSC correction factors. J. Chemom. 2019, 33, e3111. [Google Scholar] [CrossRef]
- Kelley, S.S.; Rials, T.G.; Snell, R.; Groom, L.H.; Sluiter, A. Use of near infrared spectroscopy to measure the chemical and mechanical properties of solid wood. Wood Sci. Technol. 2004, 38, 257–276. [Google Scholar] [CrossRef]
- Georgieva, M.; Nebojan, I.; Mihalev, K.; Yoncheva, N. Application of NIR spectroscopy and chemometrics in quality control of wild berry fruit extracts during storage. Croat. J. Food Technol. Biotechnol. Nutr. 2013, 8, 67–73. [Google Scholar]
- Mancini, M.; Taavitsainen, V.-M.; Toscano, G. Comparison of three different classification methods performance for the determination of biofuel quality by means of NIR spectroscopy. J. Chemom. 2019, 33, e3145. [Google Scholar] [CrossRef]
- Blanco, M.; Villarroya, I. NIR spectroscopy: A rapid-response analytical tool. TrAC Trends Anal. Chem. 2002, 21, 240–250. [Google Scholar] [CrossRef]
- UNI. Biocombustibili Solidi—Linee Guida per la Determinazione della Qualità Mediante Spettrometria nel Vicino Infrarosso; UNI/TS 11765:2019; Ente Nazionale Italiano di Unificazione: Milano, Italy, 2019. [Google Scholar]
- Posom, J.; Sirisomboon, P. Evaluation of the moisture content of Jatropha curcas kernels and the heating value of the oil-extracted residue using near-infrared spectroscopy. Biosyst. Eng. 2015, 130, 52–59. [Google Scholar] [CrossRef]
- Everard, C.D.; McDonnell, K.P.; Fagan, C.C. Prediction of biomass gross calorific values using visible and near infrared spectroscopy. Biomass Bioenergy 2012, 45, 203–211. [Google Scholar] [CrossRef]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Correia, R.M.; Domingos, E.; Cáo, V.M.; Araujo, B.R.; Sena, S.; Pinheiro, L.U.; Fontes, A.M.; Aquino, L.F.M.; Ferreira, E.C.; Filgueiras, P.R.; et al. Portable near infrared spectroscopy applied to fuel quality control. Talanta 2018, 176, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Basri, K.N.; Hussain, M.N.; Bakar, J.; Sharif, Z.; Khir, M.F.A.; Zoolfakar, A.S.; Basri, K.N. Classification and quantification of palm oil adulteration via portable NIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 335–342. [Google Scholar] [CrossRef]
- Yan, H.; Lu, D.-L.; Chen, B.; Hansen, W.G. Development of a Hand-Held near Infrared System Based on an Android OS and MicroNIR, and its Application in Measuring Soluble Solids Content in Fuji Apples. NIR News 2014, 25, 16–19. [Google Scholar] [CrossRef]
- Mancini, M.; Mazzoni, L.; Gagliardi, F.; Balducci, F.; Duca, D.; Toscano, G.; Mezzetti, B.; Capocasa, F. Application of the Non-Destructive NIR Technique Quality Parameters. Foods 2020, 9, 441. [Google Scholar] [CrossRef]
- Sun, Z.; Li, C.; Li, L.; Nie, L.; Dong, Q.; Li, D.; Gao, L.; Zang, H. Study on feasibility of determination of glucosamine content of fermentation process using a micro NIR spectrometer. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 153–160. [Google Scholar] [CrossRef]
- Alcalà, M.; Blanco, M.; Moyano, D.; Broad, N.W.; O’Brien, N.; Friedrich, D.; Pfeifer, F.; Siesler, H.W. Qualitative and Quantitative Pharmaceutical Analysis with a Novel Hand-Held Miniature near Infrared Spectrometer. J. Near Infrared Spectrosc. 2013, 21, 445–457. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, R.; Fu, F.; Fei, B.; Jiang, Z. Prediction of mechanical properties of Chinese fir wood by near infrared spectroscopy. Front. For. China 2009, 4, 368–373. [Google Scholar] [CrossRef]
- Pasquini, C. Near Infrared Spectroscopy: Fundamentals, practical aspects and analytical applications. J. Braz. Chem. Soc. 2003, 14, 198–219. [Google Scholar] [CrossRef]
- Tsuchikawa, S.; Kobori, H. A review of recent application of near infrared spectroscopy to wood science and technology. J. Wood Sci. 2015, 61, 213–220. [Google Scholar] [CrossRef]
- Feng, X.; Jianming, Y.; Tesfaye, T.; Floyd, D.; Donghai, W. Qualitative and quantitative analysis of lignocellulosic biomass using infrared spectroscopy. Appl. Energy 2013, 40, 1–10. [Google Scholar] [CrossRef]
- Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Studies on bound water of cellulose by differential scanning calorimetry. Text. Res. J. 1981, 51. [Google Scholar] [CrossRef]
- Park, S.; Venditti, R.A.; Jameel, H.; Pawlak, J.J. Hard to remove water in cellulose fibers characterized by high resolution thermogravimetric analysis—Methods development. Cellulose 2006, 13, 23–30. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Xu, J.; Xu, N.; He, Y. Quantitative visualization of lignocellulose components in transverse sections of moso bamboo based on FTIR macro- and micro-spectroscopy coupled with chemometrics. Biotechnol. Biofuels 2018, 11, 263. [Google Scholar] [CrossRef]
- Diniz, C.P.; Grattapaglia, D.; Mansfield, S.D.; Figueiredo, L.F.D.A. Near-infrared-based models for lignin syringyl/guaiacyl ratio of Eucalyptus benthamii and E. pellita using a streamlined thioacidolysis procedure as the reference method. Wood Sci. Technol. 2019, 53, 521–533. [Google Scholar] [CrossRef]
- Sandak, J.; Sandak, A.; Zitek, A.; Hintestoisser, B.; Picchi, G. Development of Low-Cost Portable Spectrometers for Detection of Wood Defects. Sensors 2020, 20, 545. [Google Scholar] [CrossRef]
- Huang, C.; Han, L.; Yang, Z.; Liu, X. Ultimate analysis and heating value prediction of straw by near infrared spectroscopy. Waste Manag. 2009, 29, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Posom, J.; Saechua, W. Prediction of elemental components of ground bamboo using micro-NIR spectrometer. IOP Conf. Series Earth Environ. Sci. 2019, 301, 012063. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K. Near-Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 1987; ISBN 091325049X. [Google Scholar]
- Fagan, C.; Everard, C.; O’Donnell, C.P.; Downey, G.; Sheehan, E.; Delahunty, C.; O’Callaghan, D.J. Evaluating Mid-infrared Spectroscopy as a New Technique for Predicting Sensory Texture Attributes of Processed Cheese. J. Dairy Sci. 2007, 90, 1122–1132. [Google Scholar] [CrossRef]
- Sørensen, L.K. Application of reflectance near infrared spectroscopy for bread analyses. Food Chem. 2009, 113, 1318–1322. [Google Scholar] [CrossRef]
- Davrieux, F.; Baillères, H.; Ham-Pichavant, F. Near infrared analysis as a tool for rapid screening of some major wood characteristics in a eucalyptus breeding program. Ann. For. Sci. 2002, 59, 479–490. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Fackler, K. A Review of Band Assignments in near Infrared Spectra of Wood and Wood Components. J. Near Infrared Spectrosc. 2011, 19, 287–308. [Google Scholar] [CrossRef]
- Alves, A.; Santos, A.J.; Rozenberg, P.; Pâques, L.E.; Charpentier, J.-P.; Schwanninger, M.; Rodrigues, J. A common near infrared—Based partial least squares regression model for the prediction of wood density of Pinus pinaster and Larix × eurolepis. Wood Sci. Technol. 2010, 46, 157–175. [Google Scholar] [CrossRef]
- Sandak, A.; Różańska, A.; Sandak, J.; Riggio, M. Near infrared spectroscopic studies on coatings of 19th century wooden parquets from manor houses in South-Eastern Poland. J. Cult. Heritage 2015, 16, 508–517. [Google Scholar] [CrossRef]
- Sandak, J.; Sandak, A.; Pauliny, D.; Krasnoshlyk, V.; Hagman, O. Near Infrared Spectroscopy as a Tool for Estimation of Mechanical Stresses in Wood. Adv. Mater. Res. 2013, 778, 448–453. [Google Scholar] [CrossRef]
- Fujimoto, T.; Kurata, Y.; Matsumoto, K.; Tsuchikawa, S. Feasibility of near-infrared spectroscopy for online multiple trait assessment of sawn lumber. J. Wood Sci. 2010, 56, 452–459. [Google Scholar] [CrossRef]
- Glass, S.V.; Zelinka, S.L. Physical Properties and Moisture Relations of Wood. Wood Handbook: Wood as an engeneering material. In General Technical Report FPL; US Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2010. [Google Scholar]
- Andrés, J.M.; Bona, M. Analysis of coal by diffuse reflectance near-infrared spectroscopy. Anal. Chim. Acta 2005, 535, 123–132. [Google Scholar] [CrossRef]
- Stuart, B.H. Inorganic molecules. In Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; pp. 95–111. ISBN 9780470011140. [Google Scholar]
- Gillespie, G.D.; Everard, C.D.; McDonnell, K.P. Prediction of biomass pellet quality indices using near infrared spectroscopy. Energy 2015, 80, 582–588. [Google Scholar] [CrossRef]

| GCVad (J/g) | GCVdb (J/g) | ACad (%) | ACdb (%) | BWC (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Data | Train | Test | Data | Train | Test | Data | Train | Test | Data | Train | Test | Data | Train | Test | |
| MEAN | 17766 | 17770 | 17754 | 19706 | 19707 | 19701 | 3.65 | 3.66 | 3.61 | 4.05 | 4.06 | 4.02 | 9.5 | 9.5 | 9.4 |
| STD1 | 954 | 937 | 1030 | 634 | 634 | 645 | 2.15 | 2.18 | 2.03 | 2.45 | 2.48 | 2.34 | 4.1 | 4.1 | 4.1 |
| MIN | 13717 | 13717 | 14163 | 17742 | 17742 | 18044 | 0.33 | 0.33 | 0.72 | 0.36 | 0.36 | 0.80 | 1.6 | 1.6 | 1.6 |
| MAX | 20008 | 20008 | 19856 | 22138 | 22138 | 21165 | 16.51 | 16.51 | 10.56 | 18.00 | 18.00 | 12.80 | 26.7 | 26.7 | 24.0 |
| RANGE | 6291 | 6291 | 5693 | 4396 | 4396 | 3121 | 16.18 | 16.18 | 9.84 | 17.64 | 17.64 | 12.00 | 25.1 | 25.1 | 22.4 |
| N | LVs5 | R2cv | RMSECV | N | LVs | R2val | RMSEP | Bias | Slope | RER | RPD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (A) | 166 | 5 | 0.62 | 2.5 | 42 | 5 | 0.73 | 2.2 | 2.99 × 10−14 | 0.642 | 10.40 | 1.91 |
| SNV1 | 167 | 3 | 0.50 | 2.9 | 42 | 3 | 0.72 | 2.2 | −1.61 × 10−15 | 0.629 | 10.12 | 1.86 |
| MSC2 | 167 | 3 | 0.43 | 3.1 | 42 | 3 | 0.77 | 2.2 | 7.32 × 10−15 | 0.581 | 10.43 | 1.92 |
| 9der13 | 166 | 3 | 0.65 | 2.4 | 42 | 3 | 0.74 | 2.1 | 1.69 × 10−16 | 0.659 | 10.55 | 1.94 |
| 13der1 | 166 | 3 | 0.54 | 2.8 | 42 | 3 | 0.73 | 2.2 | −5.92 × 10−16 | 0.583 | 10.06 | 1.85 |
| 21der1 | 166 | 5 | 0.62 | 2.5 | 42 | 5 | 0.75 | 2.1 | −4.23 × 10−16 | 0.655 | 10.77 | 1.98 |
| 9der24 | 166 | 3 | 0.65 | 2.4 | 42 | 3 | 0.72 | 2.2 | −4.23 × 10−17 | 0.633 | 10.14 | 1.87 |
| 13der2 | 165 | 2 | 0.66 | 2.4 | 42 | 2 | 0.70 | 2.3 | −6.34 × 10−16 | 0.640 | 9.93 | 1.83 |
| 21der2 | 165 | 2 | 0.63 | 2.5 | 42 | 2 | 0.69 | 2.3 | 8.88 × 10−16 | 0.619 | 9.73 | 1.79 |
| Mean (B) | 166 | 8 | 0.95 | 0.9 | 42 | 8 | 0.96 | 1.0 | 1.27 × 10−15 | 0.837 | 23.17 | 4.26 |
| SNV1 | 166 | 7 | 0.91 | 1.2 | 42 | 7 | 0.97 | 0.7 | −4.63 × 10−15 | 1.016 | 32.64 | 3.55 |
| MSC2 | 165 | 8 | 0.94 | 1.0 | 42 | 8 | 0.94 | 1.4 | −1.07 × 10−14 | 1.183 | 16.07 | 2.96 |
| 9der13 | 166 | 6 | 0.95 | 0.9 | 42 | 6 | 0.96 | 1.0 | 9.31 × 10−16 | 0.824 | 22.33 | 4.11 |
| 13der1 | 166 | 5 | 0.94 | 1.0 | 42 | 5 | 0.90 | 1.4 | −1.90 × 10−15 | 0.799 | 16.59 | 3.05 |
| 21der1 | 166 | 5 | 0.91 | 1.2 | 42 | 5 | 0.87 | 1.5 | 7.61 × 10−16 | 0.770 | 14.58 | 2.68 |
| 9der24 | 166 | 6 | 0.95 | 0.9 | 42 | 6 | 0.92 | 1.3 | −5.29 × 10−15 | 0.780 | 17.46 | 3.21 |
| 13der2 | 166 | 6 | 0.95 | 0.9 | 42 | 6 | 0.93 | 1.2 | −2.96 × 10−15 | 0.803 | 18.55 | 3.41 |
| 21der2 | 166 | 6 | 0.95 | 0.9 | 42 | 6 | 0.95 | 1.1 | −8.46 × 10−16 | 0.813 | 20.36 | 3.75 |
| N | LVs5 | R2cv | RMSECV | N | LVs | R2val | RMSEP | Bias | Slope | RER | RPD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 166 | 6 | 0.57 | 1.28 | 42 | 6 | 0.52 | 1.39 | −3.40 × 10−15 | 0.526 | 7.08 | 1.46 |
| SNV1 | 166 | 8 | 0.62 | 1.20 | 42 | 8 | 0.54 | 1.37 | −3.64 × 10−15 | 0.499 | 7.20 | 1.48 |
| MSC2 | 166 | 5 | 0.59 | 1.24 | 42 | 5 | 0.51 | 1.41 | −2.24 × 10−15 | 0.461 | 6.98 | 1.44 |
| 9der13 | 166 | 9 | 0.61 | 1.21 | 42 | 9 | 0.53 | 1.38 | 2.45 × 10−15 | 0.546 | 7.15 | 1.47 |
| 13der1 | 166 | 9 | 0.61 | 1.22 | 42 | 9 | 0.55 | 1.35 | −1.48 × 10−15 | 0.546 | 7.31 | 1.51 |
| 21der1 | 166 | 7 | 0.60 | 1.23 | 42 | 7 | 0.54 | 1.35 | −1.71 × 10−15 | 0.530 | 7.27 | 1.50 |
| 9der24 | 166 | 9 | 0.65 | 1.14 | 42 | 9 | 0.55 | 1.36 | −3.72 × 10−15 | 0.655 | 7.22 | 1.49 |
| 13der2 | 166 | 8 | 0.62 | 1.19 | 42 | 8 | 0.62 | 1.23 | −1.12 × 10−15 | 0.666 | 7.98 | 1.64 |
| 21der2 | 166 | 8 | 0.61 | 1.22 | 42 | 8 | 0.57 | 1.31 | −5.29 × 10−15 | 0.596 | 7.49 | 1.54 |
| N | LVs5 | R2cv | RMSECV | N | LVs | R2val | RMSEP | Bias | Slope | RER | RPD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (A) | 149 | 5 | 0.58 | 604 | 36 | 5 | 0.70 | 561 | −1.21 × 1012 | 0.661 | 10.14 | 1.85 |
| SNV1 | 150 | 3 | 0.50 | 662 | 38 | 3 | 0.51 | 713 | −1.77 × 10−12 | 0.542 | 7.57 | 1.44 |
| MSC2 | 149 | 4 | 0.54 | 634 | 37 | 4 | 0.50 | 732 | 4.57 × 10−12 | 0.513 | 7.78 | 1.43 |
| 9der13 | 149 | 3 | 0.59 | 596 | 36 | 3 | 0.70 | 563 | −3.54 × 10−13 | 0.678 | 10.12 | 1.84 |
| 13der1 | 149 | 3 | 0.58 | 605 | 37 | 3 | 0.62 | 622 | 2.26 × 10−12 | 0.650 | 9.15 | 1.65 |
| 21der1 | 149 | 3 | 0.55 | 628 | 37 | 3 | 0.62 | 624 | 1.52 × 10−12 | 0.629 | 9.13 | 1.65 |
| 9der24 | 149 | 3 | 0.60 | 589 | 36 | 3 | 0.68 | 579 | −2.02 × 10−13 | 0.687 | 9.84 | 1.79 |
| 13der2 | 149 | 3 | 0.59 | 596 | 36 | 3 | 0.69 | 570 | −1.01 × 10−13 | 0.668 | 9.99 | 1.82 |
| 21der2 | 149 | 3 | 0.58 | 604 | 37 | 3 | 0.61 | 631 | 1.72 × 10−12 | 0.638 | 9.03 | 1.63 |
| Mean (B) | 149 | 6 | 0.78 | 434 | 37 | 6 | 0.64 | 624 | −9.34 × 10−13 | 0.744 | 9.12 | 1.65 |
| SNV1 | 150 | 5 | 0.83 | 388 | 37 | 5 | 0.71 | 550 | −3.44 × 10−13 | 0.723 | 10.35 | 1.87 |
| MSC2 | 150 | 6 | 0.84 | 374 | 37 | 6 | 0.71 | 544 | 9.83 × 10−14 | 0.708 | 10.47 | 1.89 |
| 9der13 | 149 | 7 | 0.84 | 378 | 37 | 7 | 0.69 | 571 | −3.44 × 10−13 | 0.785 | 9.96 | 1.80 |
| 13der1 | 149 | 7 | 0.82 | 395 | 37 | 7 | 0.67 | 603 | −1.97 × 10−13 | 0.787 | 9.44 | 1.70 |
| 21der1 | 149 | 8 | 0.82 | 400 | 37 | 8 | 0.64 | 629 | 8.36 × 10−13 | 0.768 | 9.06 | 1.63 |
| 9der24 | 148 | 6 | 0.83 | 358 | 36 | 6 | 0.74 | 533 | 1.97 × 10−12 | 0.641 | 10.68 | 1.95 |
| 13der2 | 148 | 6 | 0.83 | 360 | 36 | 6 | 0.75 | 528 | 1.92 × 10−12 | 0.639 | 10.78 | 1.97 |
| 21der2 | 149 | 7 | 0.83 | 384 | 37 | 7 | 0.71 | 545 | 5.90 × 10−13 | 0.757 | 10.45 | 1.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leoni, E.; Mancini, M.; Duca, D.; Toscano, G. Rapid Quality Control of Woodchip Parameters Using a Hand-Held Near Infrared Spectrophotometer. Processes 2020, 8, 1413. https://doi.org/10.3390/pr8111413
Leoni E, Mancini M, Duca D, Toscano G. Rapid Quality Control of Woodchip Parameters Using a Hand-Held Near Infrared Spectrophotometer. Processes. 2020; 8(11):1413. https://doi.org/10.3390/pr8111413
Chicago/Turabian StyleLeoni, Elena, Manuela Mancini, Daniele Duca, and Giuseppe Toscano. 2020. "Rapid Quality Control of Woodchip Parameters Using a Hand-Held Near Infrared Spectrophotometer" Processes 8, no. 11: 1413. https://doi.org/10.3390/pr8111413
APA StyleLeoni, E., Mancini, M., Duca, D., & Toscano, G. (2020). Rapid Quality Control of Woodchip Parameters Using a Hand-Held Near Infrared Spectrophotometer. Processes, 8(11), 1413. https://doi.org/10.3390/pr8111413







