1. Introduction
The chemical industry builds its value chains on relatively few raw materials. The basic feedstock of the chemical industry is made up of an organic part and an inorganic part. On this basis, German chemical companies, for example, produce around 30,000 different intermediate and end products in numerous chemical plants [
1]. Globally, the chemical industry is a key driver of the demand for crude oil [
2]. Carbon (C) is the basis of the value chain in organic chemistry, and is extracted from natural gas, coal, and, in part, from renewable and thus bio-based raw materials, in addition to crude oil [
1]. Renewable raw materials have a long tradition in the chemical industry, and vegetable oil, animal fat, starch, or sugar still represent the carbon base in special applications. In Germany, renewable raw materials account for about 13% of all carbon-containing raw materials [
3].
A widely used definition of biorefineries was formulated by the International Energy Agency Bioenergy Task 42, namely: “Biorefinery is the sustainable processing of biomass into a spectrum of marketable products (food, feed, materials, and chemicals) and energy (fuels, power, and heat)” [
4]. Therefore, economic and environmental aspects are key to the development of a bio-based chemistry that makes use of biorefineries. In this regard, the development of economically feasible and environmentally benign routes should be prioritized [
5].
The United States Department of Energy identified 5-Hydroxymethyl-Furfural (HMF) and furfural as two of the top ten value-added bio-based chemicals [
6]. HMF and furfural are produced by chemical catalytic processes, while the remaining bio-based platform chemicals are mainly produced by fermentation [
7]. Different production process routes are proposed for these two platform chemicals of the bio-based chemistry, but few are applied on an industrial or near-industrial scale [
8,
9]. Publicly available information about the environmental performance of the chemical production processes is scarce.
In the context of this work, different production process routes for the two chemicals, HMF and furfural, will be analyzed with regard to their structure, the state of the art, and new developments. For two selected process routes, the largest environmental impacts will be analyzed and verified via a detailed process analysis.
The results of the present work will be used to critically examine the development of a new biorefinery in relation to existing processes.
2. Materials and Methods
With the help of a literature review, the current manufacturing processes, the users, and the producers of the bio-based platform chemicals HMF and furfural were identified.
For each molecule, one process was selected for further analysis. Data availability was the basic condition, as it is a necessary foundation for all further analyses. In addition, feasibility on an industrial scale, and, in case of established processes, the market position were included in the selection. The selected production processes of these two processes have subsequently been analyzed in detail. The information on the material and energy flows given in the process description was checked. For this purpose, an input–output table was created for each process step. Based on these data, the resulting environmental impacts were then added for each process step. For the modelling of the environmental impacts, a procedural review was carried out, and information from the relevant databases were included and compared with the process balances at the level of the individual processes. The analysis of the environmental impacts was limited to the energy demand and CO
2 emissions of the respective processes. In Chapter 3.1 an example of the procedure is shown. More details can be found in the
Supplementary Material. Assumptions such as pressure losses, efficiency of pumps and fans, and operating times have been marked. The influence of the estimates on energy consumption and CO
2 emissions was checked by means of sensitivity analyses.
3. Use, Producers, and Process Routes for the Production of 5-Hydroxymethylfurfural and Furfural
3.1. 5-Hydroxmethylfurfural
HMF is an organic compound that forms during the thermal decomposition of carbohydrates. For example, HMF can be detected in many foods that have undergone heat treatment, such as milk, fruit juice, honey, or coffee. When sugar is heated in a frying pan, the compound is noticeable as a caramel-like odor [
10]. HMF is one of the most important platform chemicals in bio-based chemistry [
6].
HMF can be obtained from plant biomass. The synthesis of HMF by the acid-induced elimination of three moles of water from saccharides has been known for many years. The most common catalysts used are mineral acids (such as H
2SO
4, H
3PO
4, and HCl), organic acids (such as oxalic, levulinic, and p-toluene sulfonic acids (PTSAs)), solid acids, transition metal ions, and ion exchange resins [
11]. Sugar cane, sugar beet, sorghum, chicory, Jerusalem artichoke, wheat, corn, and potatoes can be used as raw materials. Some of these have to be enzymatically isomerized into fructose, which can be converted into HMF by water separation [
12].
HMF serves as an intermediate for the production of the biofuel dimethylfuran (DMF), and can also be used for key molecules such as levulinic acid, 2,5-furanedicarboxylic acid (FDA), 2,5-diformylfuran (DFF), dihydroxymethylfuran, and 5-hydroxy-4-keto-2-pentenoic acid [
13]. HMF can serve as a starting material for various innovative materials, especially polymers, with specific properties [
7].
The production of HMF on an industrial scale is a challenge [
10]. Therefore, intensive research is currently underway to develop efficient, cost-effective, and sustainable commercial methods for the production of HMF [
14].
Table 1 shows three process alternatives for the synthesis of HMF from fructose.
In 2018, researchers around Michael-Alexander Kougioumtzis of the National Technical University of Athens developed a process for the production of HMF from a cellulosic biomass. Hydrolysis is carried out in an aqueous medium, while glucose is dehydrated in a dimethyl sulfoxide DMSO/H
2O mixture [
15].
In contrast to other methods of HMF production, the mass and energy balances of this process are readily accessible to the public. For this reason, the process presented in the literature [
15] was subjected to an in-depth process analysis within the scope of this work.
Table 2 shows an example of the procedure for checking the energy and material flows for the hydrolysis preheating step in HMF production, according to the authors of [
15]. In the literature, a power requirement of 3341 kW was stated for this process step. The examination by the authors results in a power requirement of 3397 kW with the given material flow and the process parameters. With the assumed value for energy efficiency being 70% for this process step, this result differs by 2% from the value given in the literature, and was therefore assessed as correct with regard to the verification. With the calculated power value and an assumed operating time of 6500 h per year, the specific power requirement per kilogram of produced HMF was calculated.
3.2. Furfural
Furfural is a natural dehydration product of the C5 sugar xylose, a monosaccharide that often is found in large amounts in the hemicellulose fraction of the lignocellulosic biomass from which it is almost exclusively produced [
9].
Furfural is obtained from the pentosan-containing fraction of lignocellulose. The C5 sugars contained herein (especially xylose) are first separated into monosaccharides by acid hydrolysis. These monosaccharides are then dehydrated and furfural is formed. Both batch and continuous reactors are used industrially [
16].
More than 80 chemicals are directly or indirectly derived from furfural [
17]. It was therefore identified as one of the key chemicals in lignocellulosic biorefineries [
6].
The most important raw materials for furfural production are corncobs, rice husks, flax residues, cottonseed shells, wood, and bagasse obtained from sugar cane [
18]. These plant-based materials with a higher pentosan content allow for a higher yield of furfural. Today, bagasse and corncobs account for more than 98% of all raw materials used in the production of furfural, as they are inexpensive and readily available from sugar cane and corn processing plants, in addition to their high pentosan content [
9].
The current most important use of furfural is as a starting material for the production of furfuryl alcohol and other five-membered oxygen-containing heterocycles, such as furan, methylfuran, furfurylamine, and furoic acid. [
17]. Furfuryl alcohol is a monomer for furan resins, which are mainly used as foundry binding agents. It is produced by the hydrogenation of furfural [
19]. About 60% to 70% of the total furfural production of about 300,000 tons per year (2001) is processed into furfuryl alcohol [
16]. Furfural is also a starting material for tetrahydrofurfuryl alcohol, which is frequently used as a precursor for specialty chemicals. Furfural is also used for the recovery of lubricants from cracked crude oil, pine oils, special adhesives, liquid–liquid extraction, and as a flavoring agent [
18].
The largest producer of furfural is China, with a production capacity of about 220,000 tons per year. Large producers of furfural can also be found in the Dominican Republic, with 32,000 tons per year, and in South Africa, with 20,000 tons per year. These three countries account for about 90% of global furfural production capacity [
18].
In 1921, Quaker Oats implemented the first commercial process for the production of furfural. In the late 1990s, SupraYield, a modification of the Quaker Oats process, was introduced. This process was commercialized in 2009 by the Proserpine Cooperative Sugar Mill in Queensland, Australia [
20]. In Indonesia, a plant with the SupraYield process was planned in 2019 [
21].
Table 3 shows the various furfural production processes currently under development or on an industrial scale that have been identified in this work. The first four processes are already industrially implemented manufacturing processes for furfural. All of the other listed processes are experiments or concepts by companies or scientists.
Currently, continuous processes are used, such as the Huaxia/Westpro processes and the SupraYield process [
22].
The Chinese Huaxia Furfural Technology modified by Westpro is an example of a continuous process technology that can process corncobs, rice husks, and other biogenic raw materials. As this is a proven process in current use, and information on material flows is publicly available, it has been selected for further process analysis in this paper.
The manufacturing process uses fixed-bed reactors and continuous dynamic refining, which enable a furfural yield of between 35% and 50% at low production costs [
18].
4. Results of the Analyses
4.1. Analysis of HMF Production after the Pilot Process of Kougioumtzis
The technological process of HMF production after the pilot process of the authors of [
15] is shown in
Figure 1. The pilot plant processed 1500 kg biomass per hour. The biomass is first crushed to 0.3 to 1 mm and then mixed with diluted sulfuric acid in a ratio of 1:9.
Subsequently, hydrolysis is carried out in a reactor at a temperature of approximately 175 °C. Here, the cellulose part of the biomass is mainly converted into C6-monosaccharides (especially glucose) and other by-products. After drying, the hydrolysate stream undergoes HMF synthesis.
The glucose-rich hydrolysate stream first undergoes an evaporation step to increase the glucose concentration. Then, DMSO as a solvent and a solid catalyst (Sn20/γ-Al
2O
3) are added to allow for the selective synthesis of HMF. DMSO is particularly effective, because it stabilizes the furanose form of fructose and accelerates dewatering. The solvent stream contains only 20% water and 80% DMSO. During synthesis, most of the glucose is dehydrated to HMF, with the simultaneous production of by-products and biotar. After the HMF reactor, the biotar is separated and fed to the incinerator to support the heat demand of the process. The generated HMF stream is first extracted with dichloromethane (DCM) in a liquid–liquid mixture. HMF is mainly transferred into the organic circuit, while the raffinate is generated as waste water (approximately 150 kg/h). The charged organic solvent then flows into a three-stage separation step, in which three expansion tanks operate under vacuum conditions. This is where HMF is separated from the solvent. The latter can be recovered to 98% and is circulated. HMF is recovered as an end product with a purity of over 96.5%. At a yield of approximately 54 kg/h, 3.6% of the biomass starting materials are converted into HMF [
15].
The process balance for the investigated HMF production is very well documented, and the mass and energy flows could be conclusively reconstructed.
Figure 2 shows the input and output flows for the process described above in relation to 1 kg HMF. The catalysts used (H
2SO
4/H
2O and Sn20/γ-Al
2O
3) are not shown in
Figure 2, as they are run in a cycle and have a long service life.
The range of CO
2 emissions is between 326 and 1159 kg of CO
2/kg HMF. The provision and recovery of DCM in particular has an impact here. A 98% recovery of DCM results in a specific CO
2 emission of 208 kg CO
2/kg HMF, and a 90% recovery 1042 kg CO
2/kg HMF (own calculation with values from the literature [
23]). In addition, the variance of the transport distance for the truck (200 to 600 km) and for the water treatment has an impact on CO
2 emissions. The latter, however, are negligible with a 0.01% share of total CO
2 emissions. The DMC supply dominates CO
2 emissions with 64% to 90%, followed by heat supply (7% to 26%). The range of CO
2 emissions is shown in
Table 4.
The process consumes significant amounts of DCM, a CO2 intensive material. Optimization work must therefore primarily focus on DCM consumption, for example, by recycling the DCM as completely as possible in the process.
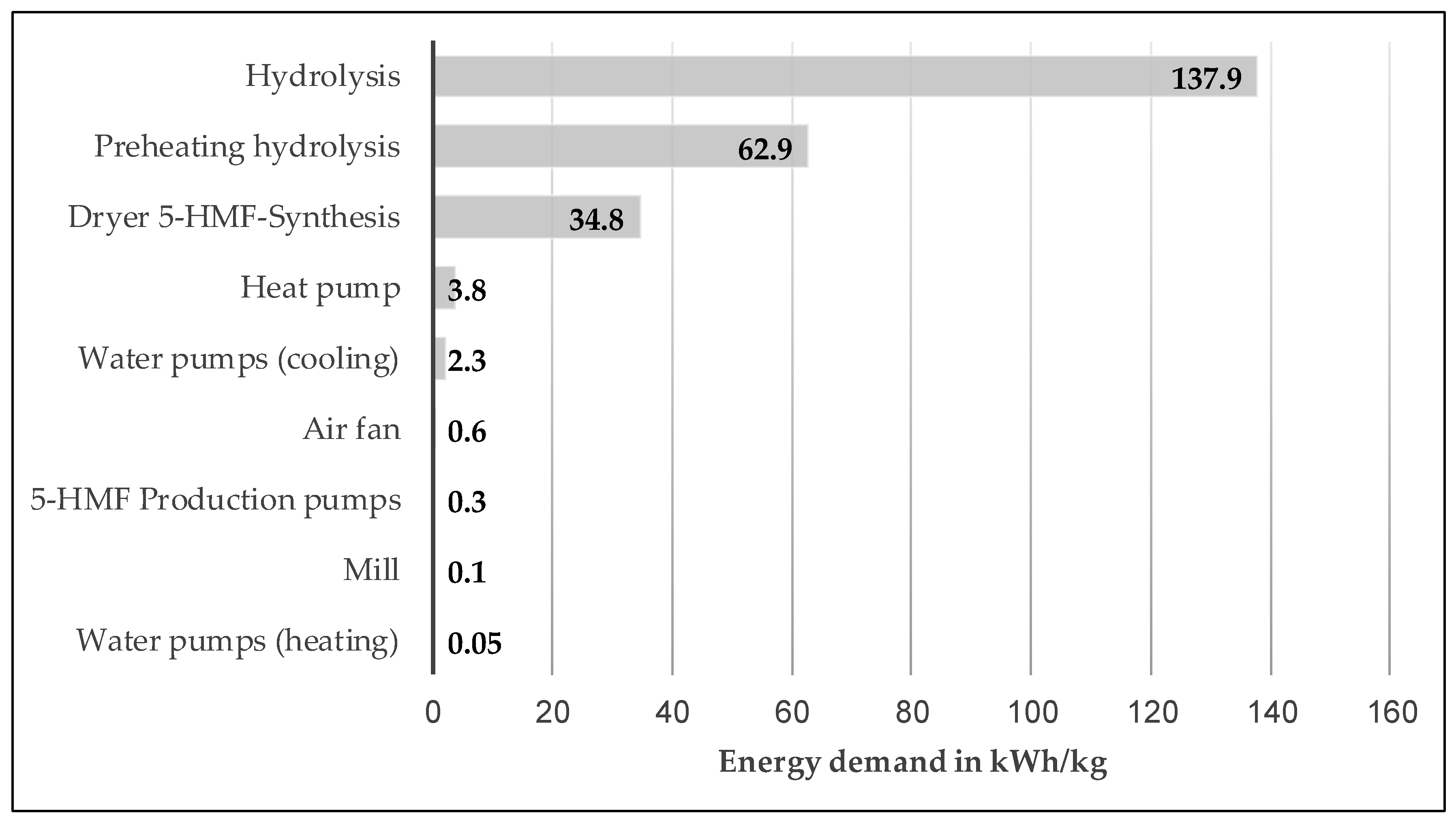
If only the energy demand of the production process without upstream processes is considered (see
Figure 3), it is clear that energy consumption is dominated by steam generation for preheating the hydrolysis with 62.9 kWh/kg HMF and hydrolysis with 137.9 kWh/kg HMF. The drying step prior to HMF synthesis was identified as the third largest energy consumer (34.8 kWh/kg HMF).
The substitution of fossil energy sources during the generation of process heat has already been exhausted by returning the solid fraction to the dual fuel burner. The greatest potential for reducing the primary energy requirements and CO
2 emissions lies in the use of renewable energy or biomass. See
Supplementary Materials for detailed information concerning the process data.
4.2. Analysis of Furfural Production Based on the Huaxia Technology Modified by Westpro
The procedural steps of the Huaxia Furfural Technology modified by Westpro are shown in
Figure 4. They essentially comprise the pretreatment, hydrolysis, refining, and recovery of by-products such as acetic acid and levulinic acid [
18]. In this work, only the processes within the dashed system boundary that are necessary for the production of furfural were investigated. The further processing of residual and by-products, for example levulinic acid, a platform chemical used in textile printing [
24], was not investigated.
The raw materials are pretreated, for example, by grinding corn cobs to a size of 3–10 mm. The pre-treated materials are mixed with sulfuric acid and fed to a steel reactor or fermenter. Furfural is obtained in this reactor at a pressure of 10 bar with hot steam at 180 °C. The furfural-saturated steam ejected from the reactor is filtered to remove solid particles, and is then condensed at 60 °C. The condensate undergoes an azeotropic distillation to achieve a higher furfural concentration. Lime (CaO) is used to remove water. The azeotropic distillation is carried out continuously. The resulting water (H
2O) is used internally, as far as possible. The resulting acid is neutralized with sodium carbonate. By-products from distillation, such as acetone and methanol, are removed. The distillates are collected in storage tanks under a vacuum at different reflux conditions. Furfural has a purity of over 98.5% with a yield of 45%–50% being theoretically possible [
18].
The described production plant produces 1500 tons of furfural per year (t/a) [
18]. This corresponds to about 171 kilograms per hour (kg/h). It was assumed, in the context of the analysis, that the plant is not used at 100% capacity, and is operated at approximately 6500 h per year (i.e., approximately a 74% capacity). With these assumptions, a furfural output of approximately 231 kg/h or 0.06 kg/s will occur.
The furfural production process under investigation was very well documented in the process balance, and could thus be coherently verified.
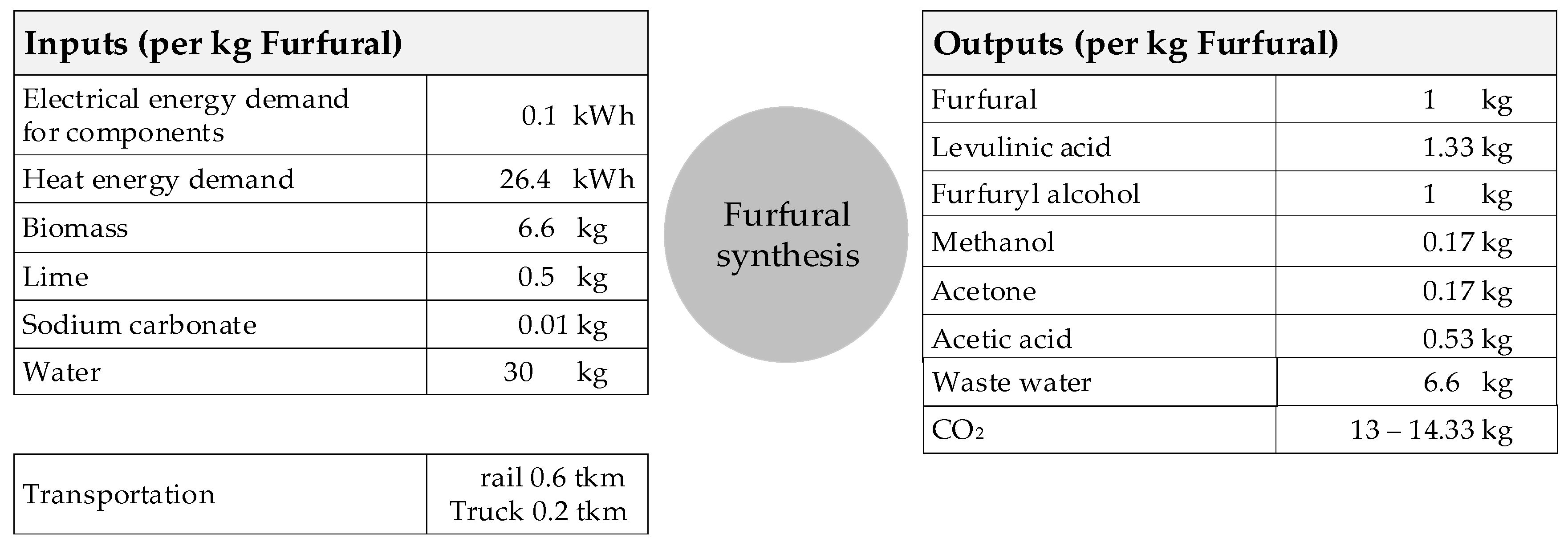
Figure 5 shows the input and output flows for the process described above in relation to 1 kg of furfural.
The range of CO
2 emissions lies between 13.45 and 14.33 kg CO
2/kg furfural, and results from the various fossil fuels used (gas, oil, and coal) for the calcination of limestone. If the truck transport distances are also varied between 200 and 600 km, the distribution of CO
2 emissions shown in
Table 5 occurs. Heat supply dominates the energy demand and thus also CO
2 emissions, followed by the lime production.
If we look exclusively at the energy requirements of the production process, without upstream processes such as lime production (see
Figure 6), it is apparent that both steam generation with 23.2 kWh/kg furfural as well as the operation of the azeotropic distillation plant with 3.3 kWh/kg furfural dominate.
This means that the greatest potential for reducing the primary energy demand and CO
2 emissions lies in the substitution of fossil energy sources, especially in the production of process heat by renewable energy or biomass. To this end, it would make sense to use the solid discharged with the filtering process as an energy source for steam generation, and thus reduce natural gas consumption. This would be possible with the aid of a dual fuel burner, if the calorific value of the solid was sufficient. See
Supplementary Materials for detailed information concerning the process data.
5. Discussion
The process routes for the production of HMF are well known, but there is no established large-scale production process yet. The pilot process of the authors of [
15] for the production of HMF was investigated and verified. The total energy requirement is 181.5 kWh/kg HMF, and between 326 and 1159 kg CO
2/kg HMF is emitted.
Because of the very low yield compared with furfural, the production of HMF is associated with a significantly higher specific energy requirement, and thus also much higher CO2 emissions. In both processes, the heat supply causes the highest energy consumption, and thus the highest CO2 emissions. By recycling the biomass, a considerable part of the energy demand can be covered. Further savings are possible by increasing the efficiency of the aggregates. The general objective should be to develop low-energy processes with higher yields.
In the production of HMF, the consumption of DCM should be minimized in further research work, for example, through improved circulation. The production of DCM is a major CO2 driver in this process. The large-scale and economical production of HMF with a high yield still has to be developed.
For the synthesis of furfural, the Huaxia Furfural Technology modified by Westpro was identified as a large-scale production process route. In addition to the SupraYield process, it is an established process for the production of furfural. The total energy requirement is 26.5 kWh/kg furfural, and between 13.45 and 14.33 kg CO2/kg furfural is emitted.
For the production of furfural, the use of two-phase systems has shown promising results in terms of yields and selectivity. As the recovery of solvents is problematic, the market maturity of these systems is not yet foreseeable [
20]. Current efforts focus on the development of new production technologies to obtain furfural in a cost-effective and environmentally friendly way [
17].
In addition to CO2, other environmental impacts are to be identified through life cycle assessments, for example, caused by biomass provision or by the auxiliary materials used.
6. Conclusions and Outlook
Within the scope of the present study, for two selected processes for the production of HMF and furfural from cellulosic biomass, the main energy consumers and the main sources of CO2 emissions could be clearly identified, despite the limited information available. A robust basis for further investigations, such as the preparation of life cycle assessments and economic considerations, was created. Within the framework of life cycle assessments, further critical environmental burdens and levers for their elimination are to be identified. The operating costs of the investigated processes can be derived directly from the results of the investigations. For the determination of the investment costs, further investigations are planned for the design and dimensioning of the apparatus for both examined processes. The present work and further research should support the development of a novel biorefinery for the production of several platform chemicals in one process. This biorefinery must have both economic and ecological advantages over the competing processes.
Author Contributions
Conceptualization, H.S. and P.K.-M.; methodology, H.S.; validation, H.S., P.K.-M., and R.E.; formal analysis, H.S.; investigation, H.S.; data curation, H.S., P.K.-M., and R.E.; writing (original draft preparation), P.K.-M. and R.E.; writing (review and editing), P.K.-M. and R.E.; visualization, P.K.-M. and R.E.; supervision, P.K.-M.; project administration, P.K.-M.; funding acquisition, P.K.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Bundesministerium für Bildung und Forschung, grant number 031B0664C. The office consortium of the State of Baden-Württemberg in Germany funded 50% of the publication costs in open access. The remaining balance was funded by the research grants of the corresponding author from Reutlingen University.
Acknowledgments
This paper is a result of research work at Reutlingen University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- VCI. VCI-Factbook 05. Die Formel Ressourceneffizienz. 2012. Available online: https://www.vci.de/presse/factbooks/2012-07-25-vci-factbook-05-die-formel-ressourceneffizienz-vci.jsp (accessed on 3 December 2019).
- IEA International Energy Agency. Oil 2018. Analysis and Forecasts to 2023. 2018. Available online: https://www.iea.org/reports/oil-2018 (accessed on 10 January 2020).
- VCI. Einsatz Nachwachsender Rohstoffe in der Chemischen Industrie unter der Anwendung von Massenbilanzansätzen. 2017. Available online: https://www.vci.de/themen/energie-klima-rohstoffe/rohstoffe/einsatz-nachwachsender-rohstoffe-in-der-chemischen-industrie-anwendung-von-massenbilanz-ansaetzen-integration-in-bestehende-produktions-und-lieferketten.jsp (accessed on 3 December 2019).
- Jong, E.D.; Langeveld, H.; van Ree, R. IEA Bioenergy Task 42 Biorefinery. 2009. Available online: https://www.iea-bioenergy.task42-biorefineries.com/en/ieabiorefinery/Publications.htm (accessed on 10 January 2020).
- Kohli, K.; Prajapati, R.; Sharma, B. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals From Biomass. Volume I: Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy: Oak Ridge, TN, USA, 2004.
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- KIT. Chemische Produkte auf Erneuerbarer Grundlage; KIT Entwickelt Neues Verfahren zur Industriellen Herstellung der Plattformchemikalie 5-HMF—Anlage der AVA Biochem Nimmt Produktion auf; Karlsruher Institut für Technologie: Karlsruhe, Germany, 2014. [Google Scholar]
- Salak Asghari, F.; Yoshida, H. Acid-Catalyzed Production of 5-Hydroxymethyl Furfural from d -Fructose in Subcritical Water. Ind. Eng. Chem. Res. 2006, 45, 2163–2173. [Google Scholar] [CrossRef]
- Rapp, K.M.; Daub, J. Herstellung und Derivatisierung von 5-Hydroymethylfurfural. In Nachwachsende Rohstoffe: Perspektiven für die Chemie; Eggersdorfer, M., Warwel, S., Wulff, G., Eds.; VCH: Weinheim, Germany, 1993; pp. 183–196. ISBN 3527290192. [Google Scholar]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Aresta, M.; Di Bitonto, L.; Pastore, C. Organic Carbonates: Efficient Extraction Solvents for the Synthesis of HMF in Aqueous Media with Cerium Phosphates as Catalysts. CHEMSUSCHEM 2016, 9, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Kougioumtzis, M.A.; Marianou, A.; Atsonios, K.; Michailof, C.; Nikolopoulos, N.; Koukouzas, N.; Triantafyllidis, K.; Lappas, A.; Kakaras, E. Production of 5-HMF from Cellulosic Biomass: Experimental Results and Integrated Process Simulation. Waste Biomass Valoriz. 2018, 9, 2433–2445. [Google Scholar] [CrossRef]
- Montané, D.; Salvadó, J.; Torras, C.; Farriol, X. High-temperature dilute-acid hydrolysis of olive stones for furfural production. Biomass Bioenerg. 2002, 22, 295–304. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Win, D.T. Furfural—Gold from Garbage. Assumpt. Univ. J. Technol. 2005, 8, 185–190. [Google Scholar]
- Hayes, D.J.; Fitzpatrick, S.; Hayes, M.H.B.; Ross, J.R.H. The Biofine Process—Production of Levulinic Acid, Furfural, and Formic Acid from Lignocellulosic Feedstocks. In Biorefineries-Industrial Processes and Products; Kamm, B., Gruber, P.R., Kamm, M., Eds.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2005; pp. 139–164. ISBN 9783527619849. [Google Scholar]
- Dashtban, M.; Gilbert, A.; Fatehi, P. Production of Furfural: Overview and Challenges. J. Sci. Technol. For. Prod. Process. 2012, 2, 44–53. [Google Scholar]
- Hidayat, N.; Hidayat, A.N.; Gozan, M. Preliminary design of corncob based furfural plant. In AIP Conference Proceedings 2062, Proceedings of the 10th International Meeting of Advances in Thermofluids (IMAT 2018): Smart City: Advances in Thermofluid Technology in Tropical Urban Development, Bali, Indonesia, 16–17 November 2018; AIP Publishing, Ed.; AIP Publishing: Melville, NY, USA, 2019; pp. 020048-1–020048-9. [Google Scholar]
- Karinen, R.; Vilonen, K.; Niemelä, M. Biorefining: Heterogeneously catalyzed reactions of carbohydrates for the production of furfural and hydroxymethylfurfural. CHEMSUSCHEM 2011, 4, 1002–1016. [Google Scholar] [CrossRef]
- Boustead, I. Ecoprofile of Chloromethanes. 1997. Available online: https://scholar.google.com/scholar?hl=de&as_sdt=0%2C5&q=Ecoprofile+of+Chloromethanes&btnG= (accessed on 3 December 2019).
- Eseyin, A.E.; Steele, P.H. An overview of the applications of furfural and its derivatives. Int. J. Algebra Comput. 2015, 3, 42. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).