Numerical Investigation of Solid-Fueled Chemical Looping Combustion Process Utilizing Char for Carbon Capture
Abstract
1. Introduction
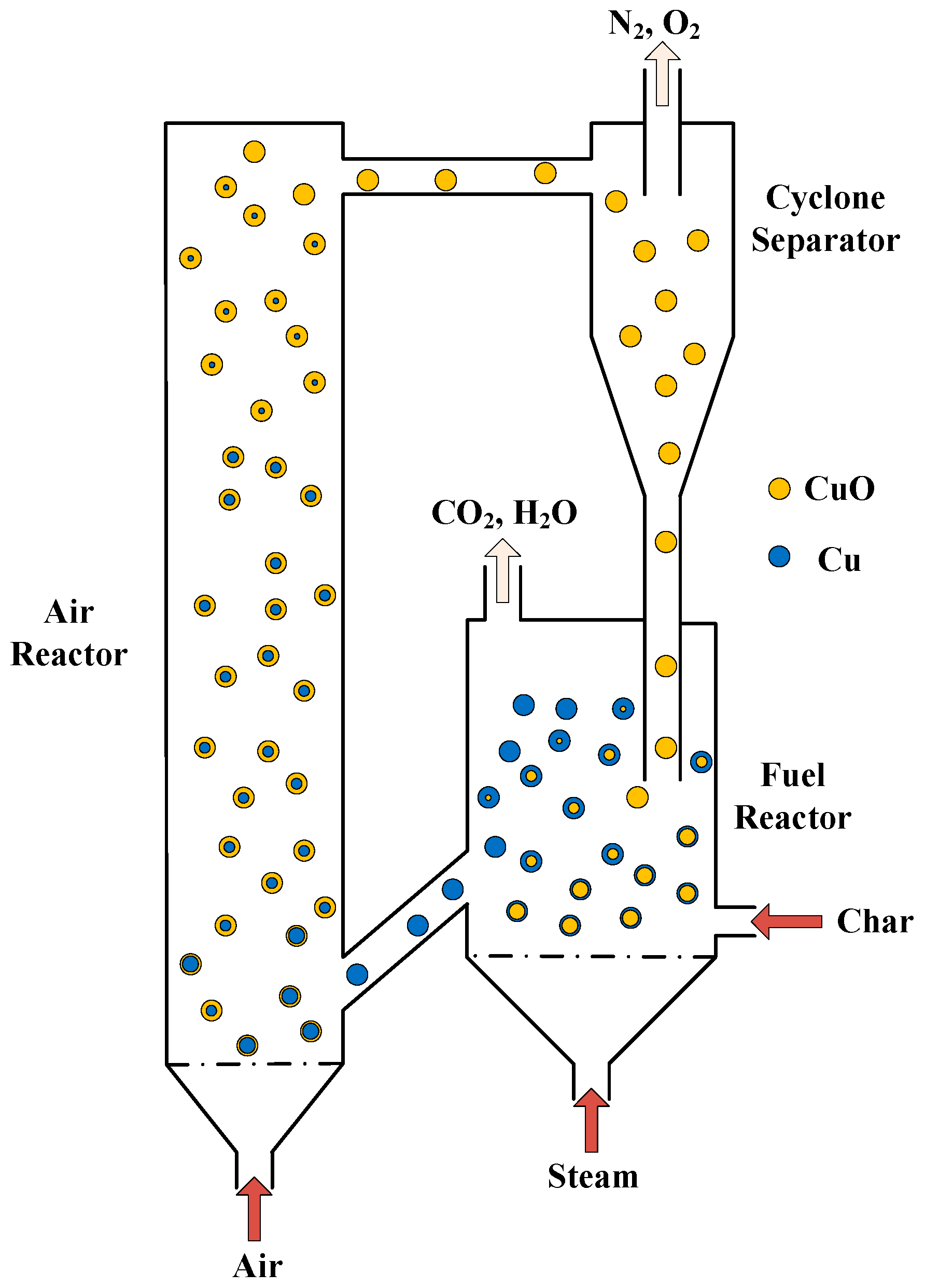
2. Materials and Methods
2.1. Hydrodynamic Models
2.2. Chemical Reaction Models
2.3. Initial Conditions and Boundary Conditions
3. Results and Discussion
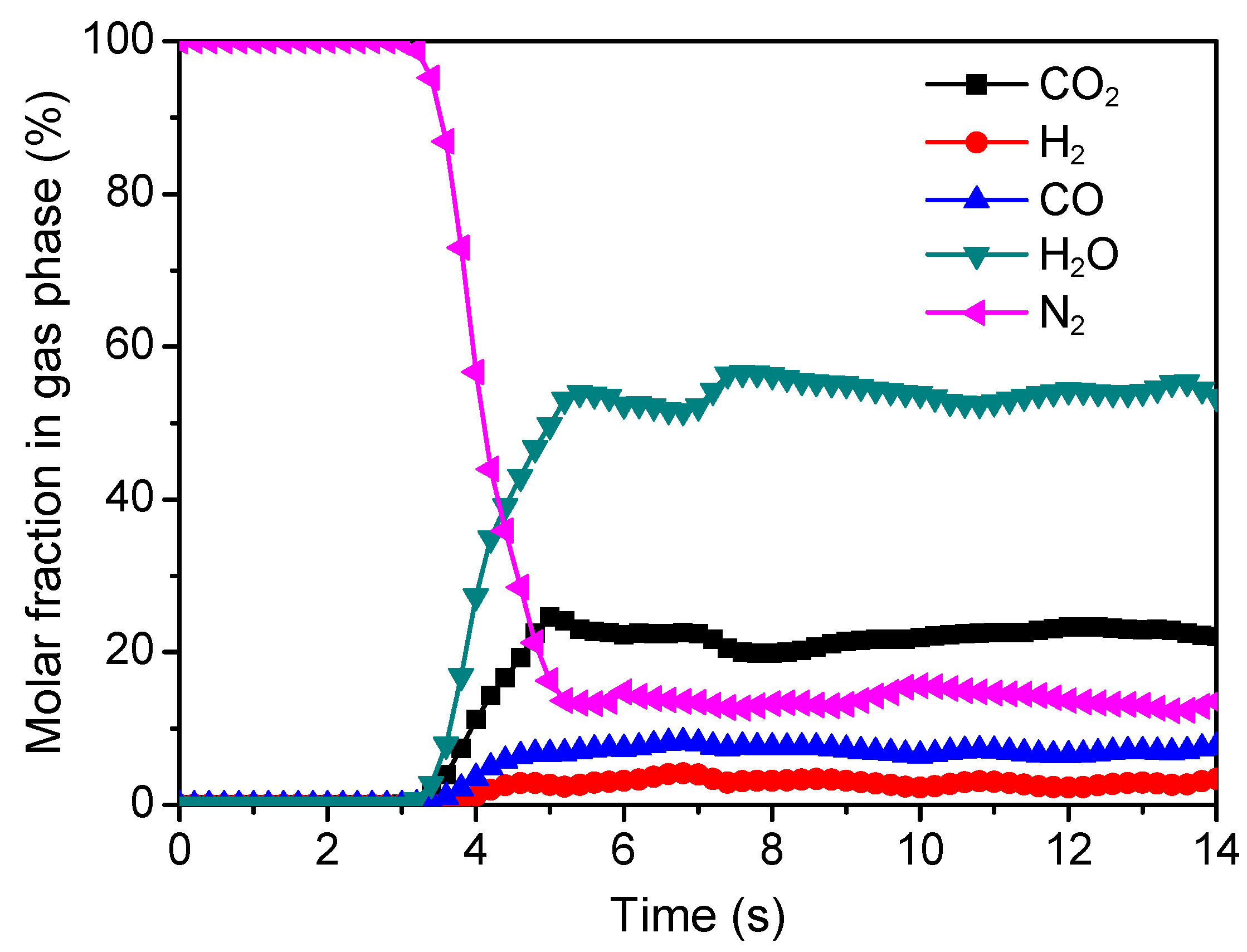
3.1. Flow Patterns and Composition Distributions
3.2. Model Validation and Reaction Performance Evaluation
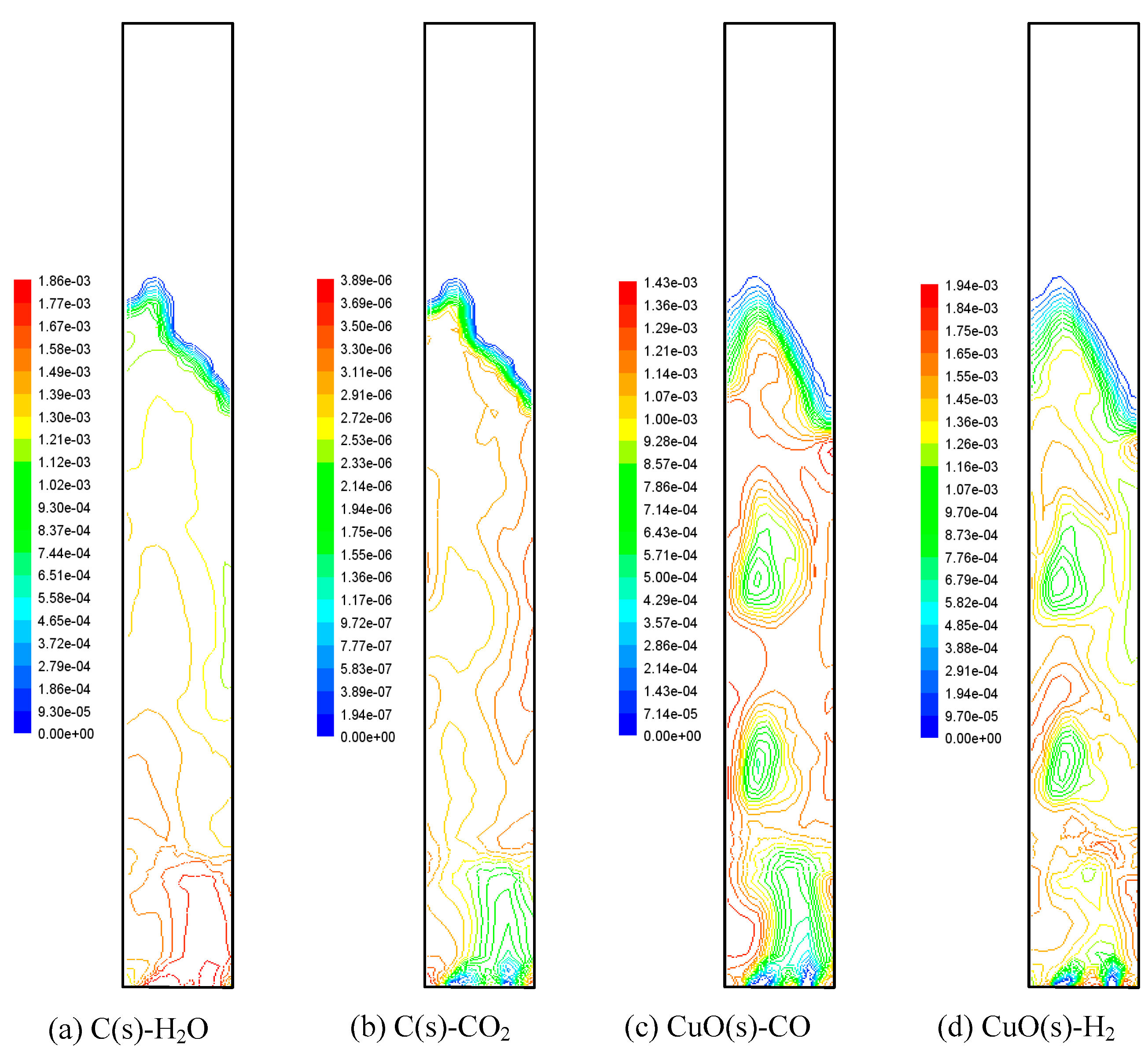
3.3. Patterns of Gas–Solid Reactions
3.3.1. Gasification Reactions of Char
3.3.2. Chemical-Looping Reactions of Oxygen Carrier
3.4. Influence of Solids Inventory
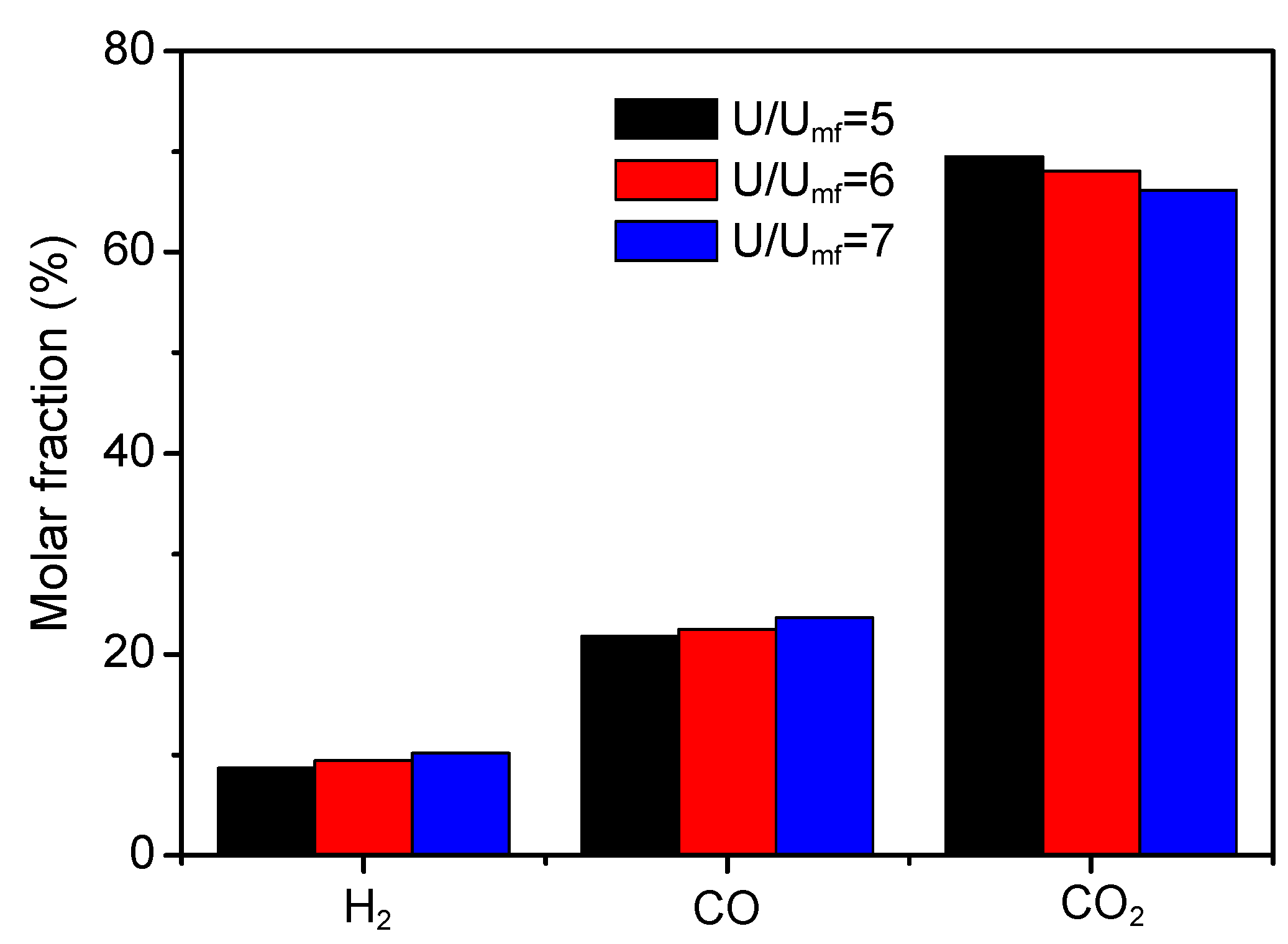
3.5. Influence of Fluidizing Number
4. Conclusions
- (1)
- In the fluidized regime, most of the intermediate products (CO and H2) from gasification are converted to CO2 and H2O by the oxygen carrier. However, the presence of bubble phase shows negative influence on the sufficient contact and reaction between gas–solid reactive components. In the freeboard region, the gas concentrations nearly keep constant because of the lack of solid phase in this region.
- (2)
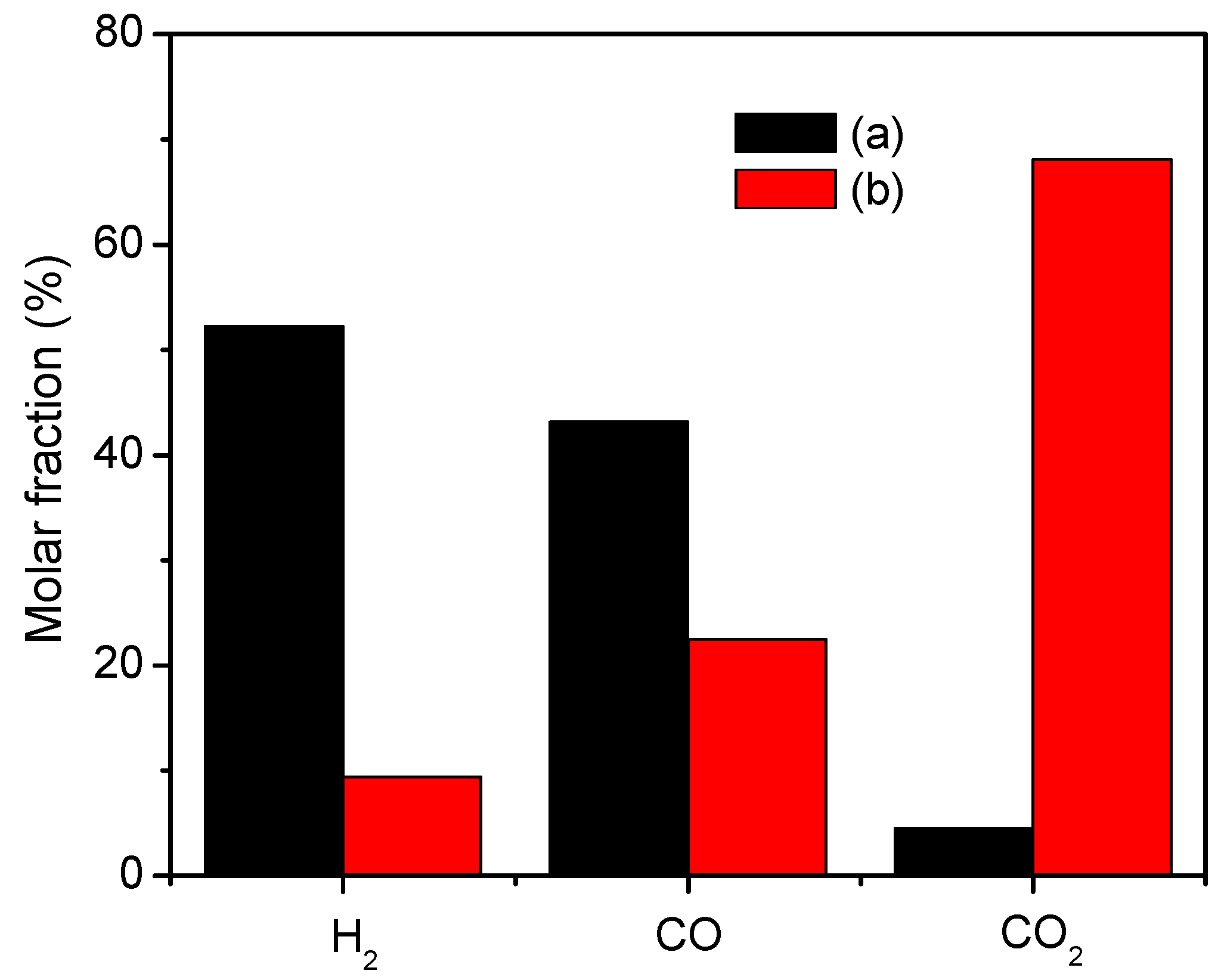
- The N2-excluded CO2 concentration (dry basis) at the outlet for steam-char gasification over sand is 4.5%, which increases to 68.1% for iG-CLC of char. The concentrations of H2 and CO decrease from 52.3% and 43.2% to 9.4% and 22.5%, respectively. This implies that the Cu-based oxygen carrier selected is highly reactive to consume the intermediate gasification products during the iG-CLC process.
- (3)
- The gasification is the rate-determining step of all the reactions. In addition, the performance of the reaction is closely correlated with the flow patterns and the concentration distributions of components.
- (4)
- A higher CO2 concentration at the outlet can be achieved by increasing the solids inventory or decreasing the fluidizing number, which may cause the change of flow patterns such as gas residence time and bubble size. However, the effects of the solids inventory and fluidizing number on the iG-CLC performance are much smaller than those on the gas-fueled CLC because of the restriction from the front-end slow reaction of char gasification.
Author Contributions
Funding
Conflicts of Interest
References
- Abanades, J.C.; Rubin, E.S.; Anthony, E.J. Sorbent cost and performance in CO2 capture systems. Ind. Eng. Chem. Res. 2004, 43, 3462–3466. [Google Scholar] [CrossRef]
- Buhre, B.J.; Elliott, L.K.; Sheng, C.D.; Gupta, R.P.; Wall, T.F. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Lyngfelt, A.; Leckner, B.; Mattisson, T. A fluidized-bed combustion process with inherent CO2 separation; application of chemical-looping combustion. Chem. Eng. Sci. 2001, 56, 3101–3113. [Google Scholar] [CrossRef]
- Li, F.; Fan, L.S. Clean coal conversion processes–progress and challenges. Energy Environ. Sci. 2008, 1, 248–267. [Google Scholar] [CrossRef]
- Toftegaard, M.B.; Brix, J.; Jensen, P.A.; Glarborg, P.; Jensen, A.D. Oxy-fuel combustion of solid fuels. Prog. Energy Combust. 2010, 36, 581–625. [Google Scholar] [CrossRef]
- Fan, L.S.; Zeng, L.; Wang, W.; Luo, S. Chemical looping processes for CO2 capture and carbonaceous fuel conversion–prospect and opportunity. Energy Environ. Sci. 2012, 5, 7254–7280. [Google Scholar] [CrossRef]
- de Diego, L.F.; García-Labiano, F.; Gayán, P.; Celaya, J.; Palacios, J.M.; Adánez, J. Operation of a 10 kWth chemical-looping combustor during 200 h with a CuO–Al2O3 oxygen carrier. Fuel 2007, 86, 1036–1045. [Google Scholar] [CrossRef]
- Forero, C.R.; Gayán, P.; de Diego, L.F.; Abad, A.; García-Labiano, F.; Adánez, J. Syngas combustion in a 500 Wth Chemical-Looping Combustion system using an impregnated Cu-based oxygen carrier. Fuel Process. Technol. 2009, 90, 1471–1479. [Google Scholar] [CrossRef]
- Mattisson, T.; Järdnäs, A.; Lyngfelt, A. Reactivity of some metal oxides supported on alumina with alternating methane and oxygen application for chemical-looping combustion. Energy Fuels 2003, 17, 643–651. [Google Scholar] [CrossRef]
- Jin, H.; Ishida, M. A new type of coal gas fueled chemical-looping combustion. Fuel 2004, 83, 2411–2417. [Google Scholar] [CrossRef]
- Abad, A.; Mattisson, T.; Lyngfelt, A.; Rydén, M. Chemical-looping combustion in a 300W continuously operating reactor system using a manganese-based oxygen carrier. Fuel 2006, 85, 1174–1185. [Google Scholar] [CrossRef]
- Adánez, J.; Dueso, C.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A. Methane combustion in a 500 Wth chemical-looping combustion system using an impregnated Ni-based oxygen carrier. Energy Fuels 2008, 23, 130–142. [Google Scholar] [CrossRef]
- Kolbitsch, P.; Bolhàr-Nordenkampf, J.; Pröll, T.; Hofbauer, H. Operating experience with chemical looping combustion in a 120kW dual circulating fluidized bed (DCFB) unit. Int. J. Greenh. Gas Control 2010, 4, 180–185. [Google Scholar] [CrossRef]
- Hamers, H.P.; Gallucci, F.; Cobden, P.D.; Kimball, E.; van Sint Annaland, M. A novel reactor configuration for packed bed chemical-looping combustion of syngas. Int. J. Greenh. Gas Control 2013, 16, 1–12. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, H.; Tian, X.; Wei, Y.; Zhang, Y.; Zheng, C. Continuous Operation of Interconnected Fluidized Bed Reactor for Chemical Looping Combustion of CH4 Using Hematite as Oxygen Carrier. Energy Fuels 2015, 29, 3257–3267. [Google Scholar] [CrossRef]
- Cao, Y.; Pan, W.P. Investigation of Chemical looping combustion by solid fuels: 1 Process analysis. Energy Fuels 2006, 20, 1836–1844. [Google Scholar] [CrossRef]
- Leion, H.; Mattisson, T.; Lyngfelt, A. Solid fuels in chemical-looping combustion. Int. J. Greenh. Gas Control 2008, 2, 180–193. [Google Scholar] [CrossRef]
- Fan, L.S.; Li, F. Chemical looping technology and its fossil energy conversion applications. Ind. Eng. Chem. Res. 2010, 49. [Google Scholar] [CrossRef]
- Abad, A.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Fuel reactor modelling in chemical-looping combustion of coal: 1. Model formulation. Chem. Eng. Sci. 2013, 87, 277–293. [Google Scholar] [CrossRef]
- Cuadrat, A.; Abad, A.; Gayán, P.; de Diego, L.F.; García-Labiano, F.; Adánez, J. Theoretical approach on the CLC performance with solid fuels: Optimizing the solids inventory. Fuel 2012, 97, 536–551. [Google Scholar] [CrossRef]
- García-Labiano, F.; de Diego, L.F.; Gayán, P.; Abad, A.; Adánez, J. Fuel reactor modelling in chemical-looping combustion of coal: 2-simulation and optimization. Chem. Eng. Sci. 2013, 87, 173–182. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A.; Mendiara, T.; Gayán, P.; de Diego, L.F.; García-Labiano, F. Chemical looping combustion of solid fuels. Prog. Energy Combust. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Xiao, R.; Song, Q.; Song, M.; Lu, Z.; Zhang, S.; Shen, L. Pressurized chemical-looping combustion of coal with an iron ore-based oxygen carrier. Combust. Flame 2010, 157, 1140–1153. [Google Scholar] [CrossRef]
- Berguerand, N.; Lyngfelt, A. Design and operation of a 10 kWth chemical-looping combustor for solid fuels-testing with South African coal. Fuel 2008, 87, 2713–2726. [Google Scholar] [CrossRef]
- Berguerand, N.; Lyngfelt, A. The use of petroleum coke as fuel in a 10 kWth chemical-looping combustor. Int. J. Greenh. Gas Control 2008, 2, 169–179. [Google Scholar] [CrossRef]
- Shen, L.H.; Wu, J.H.; Xiao, J. Experiments on chemical looping combustion of coal with a NiO based oxygen carrier. Combust. Flame 2009, 156, 721–728. [Google Scholar] [CrossRef]
- Bayham, S.; McGiveron, O.; Tong, A.; Chung, E.; Kathe, M.; Wang, D.; Zeng, L.; Fan, L.S. Parametric and dynamic studies of an iron-based 25-kWth coal direct chemical looping unit using sub-bituminous coal. Appl. Energy 2015, 145, 354–363. [Google Scholar] [CrossRef]
- Linderholm, C.; Schmitz, M. Chemical-looping combustion of solid fuels in a 100 kW dual circulating fluidized bed system using iron ore as oxygen carrier. J. Environ. Chem. Eng. 2016, 4, 1029–1039. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, H.; Tian, X.; Wei, Y.; Rajendran, S.; Zhang, Y.; Bhattacharya, S.; Zheng, C. Chemical looping combustion of coal in a 5 kWth interconnected fluidized bed reactor using hematite as oxygen carrier. Appl. Energy 2015, 157, 304–313. [Google Scholar] [CrossRef]
- Pérez-Vega, R.; Abad, A.; García-Labiano, F.; Gayán, P.; de Diego, L.F.; Adánez, J. Coal combustion in a 50 kWth Chemical Looping Combustion unit: Seeking operating conditions to maximize CO2 capture and combustion efficiency. Int. J. Greenh. Gas Control 2016, 50, 80–92. [Google Scholar] [CrossRef]
- Ströhle, J.; Orth, M.; Epple, B. Design and operation of a 1 MWth chemical looping plant. Appl. Energy 2014, 113, 1490–1495. [Google Scholar] [CrossRef]
- Thon, A.; Kramp, M.; Hartge, E.-U.; Heinrich, S.; Werther, J. Operational experience with a system of coupled fluidized beds for chemical looping combustion of solid fuels using ilmenite as oxygen carrier. Appl. Energy 2014, 118, 309–317. [Google Scholar] [CrossRef]
- Xiao, R.; Chen, L.; Saha, C.; Zhang, S.; Bhattacharya, S. Pressurized chemical-looping combustion of coal using an iron ore as oxygen carrier in a pilot-scale unit. Int. J. Greenh. Gas Control 2012, 10, 363–373. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Zhu, X.; Liu, H. Experimental Evaluation of a Novel 20 kWth in Situ Gasification Chemical Looping Combustion Unit with an Iron Ore as the Oxygen Carrier. Ind. Eng. Chem. Res. 2016, 55, 11775–11784. [Google Scholar] [CrossRef]
- Cheng, M.; Sun, H.; Li, Z.; Cai, N. Annular carbon stripper for chemical-looping combustion of coal. Ind. Eng. Chem. Res. 2017, 56, 1580–1593. [Google Scholar] [CrossRef]
- Wang, P.; Means, N.; Howard, B.H.; Shekhawat, D.; Berry, D. The reactivity of CuO oxygen carrier and coal in Chemical-Looping with Oxygen Uncoupled (CLOU) and In-situ Gasification Chemical-Looping Combustion (iG-CLC). Fuel 2018, 217, 642–649. [Google Scholar] [CrossRef]
- Fan, L.S. Chemical Looping Systems for Fossil Energy Conversions; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Adamczyk, W.P.; Bialecki, R.A.; Ditaranto, M.; Gladysz, P.; Haugen, N.E.L.; Katelbach-Wozniak, A.; Klimanek, A.; Sladek, S.; Szlek, A.; Wecel, G. CFD modeling and thermodynamic analysis of a concept of a MILD-OXY combustion large scale pulverized coal boiler. Energy 2017, 140, 1305–1315. [Google Scholar] [CrossRef]
- Liu, B.; Papadikis, K.; Gu, S.; Fidalgo, B.; Longhurst, P.; Li, Z.; Kolios, A. CFD modelling of particle shrinkage in a fluidized bed for biomass fast pyrolysis with quadrature method of moment. Fuel Process. Technol. 2017, 164, 51–68. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, D.; Duan, L.; Ma, J.; Xiong, J.; Chen, X. Three-dimensional CFD simulation of oxy-fuel combustion in a circulating fluidized bed with warm flue gas recycle. Fuel 2018, 216, 596–611. [Google Scholar] [CrossRef]
- Nam, H.; Rodriguez-Alejandro, D.A.; Adhikari, S.; Brodbeck, C.; Taylor, S.; Johnson, J. Experimental investigation of hardwood air gasification in a pilot scale bubbling fluidized bed reactor and CFD simulation of jet/grid and pressure conditions. Energy Convers. Manag. 2018, 168, 599–610. [Google Scholar] [CrossRef]
- Deng, Z.G.; Xiao, R.; Jin, B.S.; Song, Q.L.; Huang, H. Multiphase CFD modeling for a chemical looping combustion process (fuel reactor). Chem. Eng. Technol. 2008, 31, 1754–1766. [Google Scholar] [CrossRef]
- Jung, J.; Gamwo, I. Multiphase CFD-based models for chemical looping combustion process: Fuel reactor modeling. Powder Technol. 2008, 183, 401–409. [Google Scholar] [CrossRef]
- Jin, B.; Xiao, R.; Deng, Z.; Song, Q. Computational fluid dynamics modeling of chemical looping combustion process with calcium sulphate oxygen carrier. Int. J. Chem. React. Eng. 2009, 7. [Google Scholar] [CrossRef]
- Kruggel-Emden, H.; Rickelt, S.; Stepanek, F.; Munjiza, A. Development and testing of an interconnected multiphase CFD-model for chemical looping combustion. Chem. Eng. Sci. 2010, 65, 4732–4745. [Google Scholar] [CrossRef]
- Mahalatkar, K.; Kuhlman, J.; Huckaby, E.D.; O’brien, T. Simulations of a circulating fluidized bed chemical looping combustion system utilizing gaseous fuel. Oil Gas Sci. Technol. 2011, 66, 301–311. [Google Scholar] [CrossRef][Green Version]
- Kruggel-Emden, H.; Stepanek, F.; Munjiza, A. A study on the role of reaction modeling in multi-phase CFD-based simulations of chemical looping combustion. Oil Gas Sci. Technol. 2011, 66, 313–331. [Google Scholar] [CrossRef][Green Version]
- Harichandan, A.B.; Shamim, T. CFD analysis of bubble hydrodynamics in a fuel reactor for a hydrogen-fueled chemical looping combustion system. Energy Convers. Manag. 2014, 86, 1010–1022. [Google Scholar] [CrossRef]
- Wang, S.; Lu, H.; Zhao, F.; Liu, G. CFD studies of dual circulating fluidized bed reactors for chemical looping combustion processes. Chem. Eng. J. 2014, 236, 121–130. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Lu, H.; Liu, G.; Sun, L. Multi-scale simulation of chemical looping combustion in dual circulating fluidized bed. Appl. Energy 2015, 155, 719–727. [Google Scholar] [CrossRef]
- Banerjee, S.; Agarwal, R. Transient reacting flow simulation of spouted fluidized bed for coal-direct chemical looping combustion with different Fe-based oxygen carriers. Appl. Energy 2015, 160, 552–560. [Google Scholar] [CrossRef]
- Zhang, Y.; Chao, Z.; Jakobsen, H.A. Modelling and simulation of chemical looping combustion process in a double loop circulating fluidized bed reactor. Chem. Eng. J. 2017, 320, 271–282. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Agarwal, R. Transient simulations of spouted fluidized bed for coal-direct chemical looping combustion. Energy Fuels 2014, 28, 1548–1560. [Google Scholar] [CrossRef]
- Guan, Y.; Chang, J.; Zhang, K.; Wang, B.; Sun, Q. Three-dimensional CFD simulation of hydrodynamics in an interconnected fluidized bed for chemical looping combustion. Powder Technol. 2014, 268, 316–328. [Google Scholar] [CrossRef]
- Peng, Z.; Doroodchi, E.; Alghamdi, Y.A.; Shah, K.; Luo, C.; Moghtaderi, B. CFD–DEM simulation of solid circulation rate in the cold flow model of chemical looping systems. Chem. Eng. Res. Des. 2015, 95, 262–280. [Google Scholar] [CrossRef]
- Geng, C.; Zhong, W.; Shao, Y.; Chen, D.; Jin, B. Computational study of solid circulation in chemical-looping combustion reactor model. Powder Technol. 2015, 276, 144–155. [Google Scholar] [CrossRef]
- Mahalatkar, K.; Kuhlman, J.; Huckaby, E.D.; O’Brien, T. CFD simulation of a chemical-looping fuel reactor utilizing solid fuel. Chem. Eng. Sci. 2011, 66, 3617–3627. [Google Scholar] [CrossRef]
- Su, M.; Zhao, H.; Ma, J. Computational fluid dynamics simulation for chemical looping combustion of coal in a dual circulation fluidized bed. Energy Convers. Manag. 2015, 105, 1–12. [Google Scholar] [CrossRef]
- Sharma, R.; May, J.; Alobaid, F.; Ohlemueller, P.; Stroehle, J.; Epple, B. Euler-Euler CFD simulation of the fuel reactor of a 1 MWth chemical-looping pilot plant: Influence of the drag models and specularity coefficient. Fuel 2017, 200, 435–446. [Google Scholar] [CrossRef]
- May, J.; Alobaid, F.; Ohlemüller, P.; Stroh, A.; Ströhle, J.; Epple, B. Reactive two–fluid model for chemical–looping combustion–Simulation of fuel and air reactors. Int. J. Greenh. Gas Control 2018, 76, 175–192. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Zhong, W.; Zhang, Y.; Song, M. Three-dimensional simulation of a coal gas fueled chemical looping combustion process. Int. J. Greenh. Gas Control 2011, 5, 1498–1506. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Zhang, Y.; Zhong, W.; Yin, S. Multiphase computational fluid dynamics (CFD) modeling of chemical looping combustion using a CuO/Al2O3 oxygen carrier: effect of operating conditions on coal gas combustion. Energy Fuels 2011, 25, 3815–3824. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Zhang, Y.; Zhang, Y.; Liu, X. Three dimensional modeling of a coal-fired chemical looping combustion process in the circulating fluidized bed fuel reactor. Energy Fuels 2013, 27, 2173–2184. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Liu, H.; Zhang, B.; Zhang, Y. Prediction of In-Situ Gasification Chemical Looping Combustion Effects of Operating Conditions. Catalysts 2018, 8, 526. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Y.; Jin, B.; Zhang, Y. Three-dimensional multiphase full-loop simulation of directional separation of binary particle mixtures in high-flux coal-direct chemical-looping combustion system. Particuology 2019. [Google Scholar] [CrossRef]
- Gordillo, E.D.; Belghit, A. A two phase model of high temperature steam-only gasification of biomass char in bubbling fluidized bed reactors using nuclear heat. Int. J. Hydrogen Energy 2011, 36, 374–381. [Google Scholar] [CrossRef]
- Jin, B.; Wang, X.; Zhong, W.; Tao, H.; Ren, B.; Xiao, R. Modeling on high-flux circulating fluidized bed with Geldart group B particles by kinetic theory of granular flow. Energy Fuels 2010, 24, 3159–3172. [Google Scholar] [CrossRef]
- Benyahia, S.; Syamlal, M.; O’Brien, T.J. Study of the ability of multiphase continuum models to predict core-annulus flow. AIChE J. 2007, 53, 2549–2568. [Google Scholar] [CrossRef]
- Ding, J.; Gidaspow, D. A bubbling fluidization model using kinetic theory of granular flow. AIChE J. 1990, 36, 523–538. [Google Scholar] [CrossRef]
- Gidaspow, D.; Bezburuah, R.; Ding, J. Hydrodynamics of circulating fluidized beds: Kinetic theory approach. In Proceedings of the 7th Engineering Foundation Conference on Fluidization, Brisbane, Australia, 3–8 May 1992. [Google Scholar]
- Umeki, K.; Yamamoto, K.; Namioka, T.; Yoshikawa, K. High temperature steam-only gasification of woody biomass. Appl. Energy 2010, 87, 791–798. [Google Scholar] [CrossRef]
- Abad, A.; Adánez, J.; García-Labiano, F.; de Diego, L.F.; Gayán, P. Modeling of the chemical-looping combustion of methane using a Cu-based oxygen-carrier. Combust. Flame 2010, 157, 602–615. [Google Scholar] [CrossRef]
- Lim, C.N.; Gilbertson, M.A.; Harrison, A.J.L. Bubble distribution and behaviour in bubbling fluidised beds. Chem. Eng. Sci. 2007, 62, 56–69. [Google Scholar] [CrossRef]
- Scott, S.A.; Dennis, J.S.; Hayhurst, A.N.; Brown, T. In situ gasification of a solid fuel and CO2 separation using chemical looping. AIChE J. 2006, 52, 3325–3328. [Google Scholar] [CrossRef]
- Cheng, L.; Basu, P. Effect of pressure on loop seal operation for a pressurized circulating fluidized bed. Powder Technol. 1999, 103, 203–211. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.D. Effects of particle properties on solids recycle in loop-seal of a circulating fluidized bed. Powder Technol. 2002, 124, 76–84. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Liu, X.; Zhang, Y.; Liu, H. Experimental investigation on flow behaviors in a novel in situ gasification chemical looping combustion apparatus. Ind. Eng. Chem. Res. 2013, 52, 14208–14218. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Liu, H.; Wang, W.; Liu, X.; Zhang, Y. Optimization of in situ gasification chemical looping combustion through experimental investigations with a cold experimental system. Ind. Eng. Chem. Res. 2015, 54, 5749–5758. [Google Scholar] [CrossRef]
- Chen, X.Z.; Shi, D.P.; Gao, X.; Luo, Z.H. A fundamental CFD study of the gas–solid flow field in fluidized bed polymerization reactors. Powder Technol. 2011, 205, 276–288. [Google Scholar] [CrossRef]




| No. | Reaction Rate Constant k | Reaction Rate r | Reference |
|---|---|---|---|
| 1 | [71] | ||
| 2 | [71] | ||
| 3 | [66] | ||
| 4 | [7] | ||
| 5 | [7] |
| Description | Unit | Value |
|---|---|---|
| Reactor diameter | m | 0.22 |
| Reactor height | m | 2 |
| Mean particle density | kg/m3 | 1560 |
| Mean particle diameter | mm | 0.5 |
| Initial volume fraction of solid phase | - | 0.55 |
| Maximum packing of solid phase | - | 0.64 |
| Initial bed height | m | 1.1 |
| Char feed rate | kg/h | 1.6 |
| Inlet steam temperature | K | 1073 |
| Fluidizing number | - | 6 |
| Inlet boundary condition | - | velocity-inlet |
| Outlet boundary condition | - | outflow |
| Time step size | s | 10−4 |
| Convergence criteria | - | 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, X.; Zhang, Y.; Zhang, B.; Jin, B. Numerical Investigation of Solid-Fueled Chemical Looping Combustion Process Utilizing Char for Carbon Capture. Processes 2019, 7, 603. https://doi.org/10.3390/pr7090603
Wang X, Liu X, Zhang Y, Zhang B, Jin B. Numerical Investigation of Solid-Fueled Chemical Looping Combustion Process Utilizing Char for Carbon Capture. Processes. 2019; 7(9):603. https://doi.org/10.3390/pr7090603
Chicago/Turabian StyleWang, Xiaojia, Xianli Liu, Yong Zhang, Bo Zhang, and Baosheng Jin. 2019. "Numerical Investigation of Solid-Fueled Chemical Looping Combustion Process Utilizing Char for Carbon Capture" Processes 7, no. 9: 603. https://doi.org/10.3390/pr7090603
APA StyleWang, X., Liu, X., Zhang, Y., Zhang, B., & Jin, B. (2019). Numerical Investigation of Solid-Fueled Chemical Looping Combustion Process Utilizing Char for Carbon Capture. Processes, 7(9), 603. https://doi.org/10.3390/pr7090603





