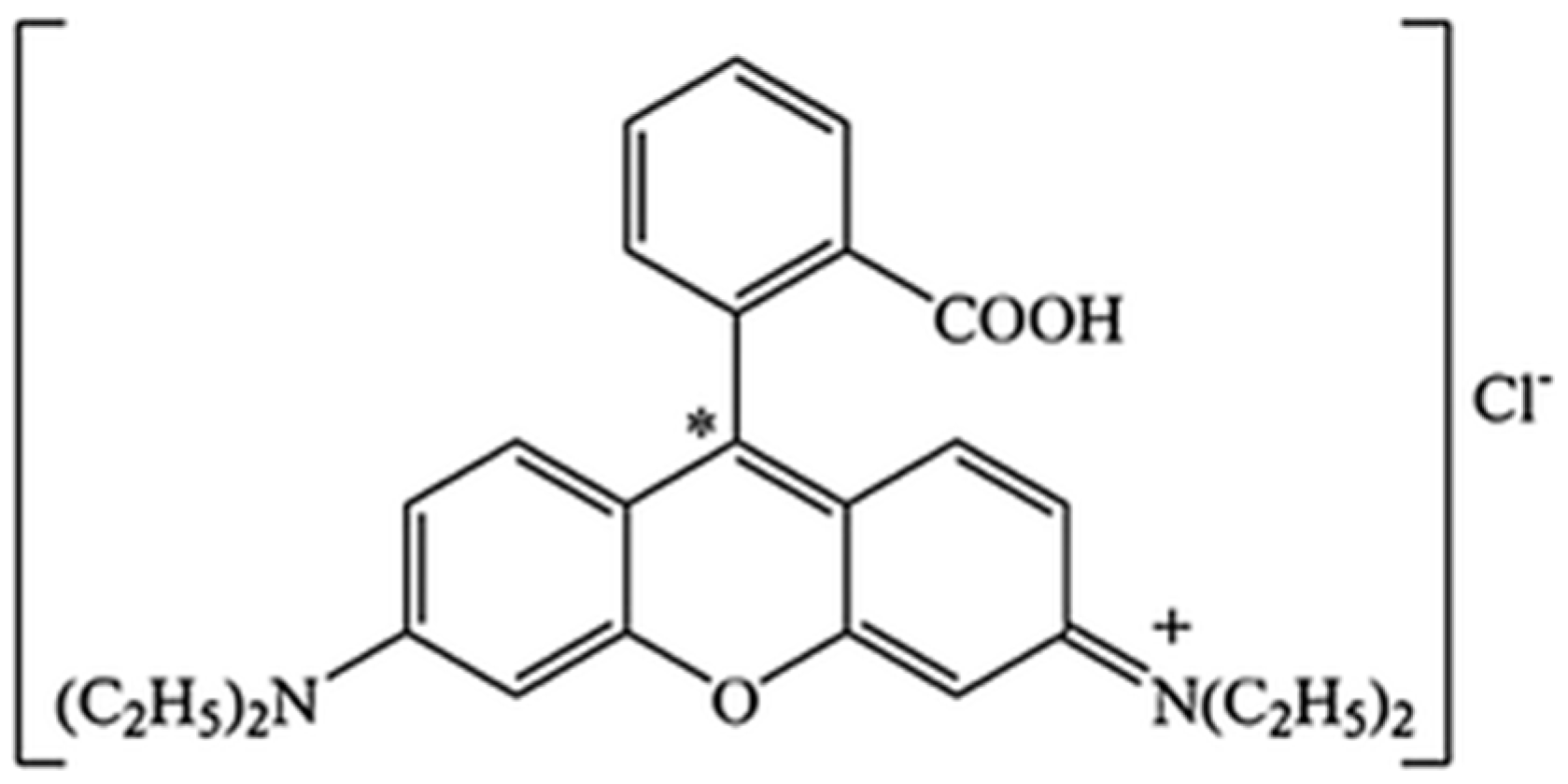
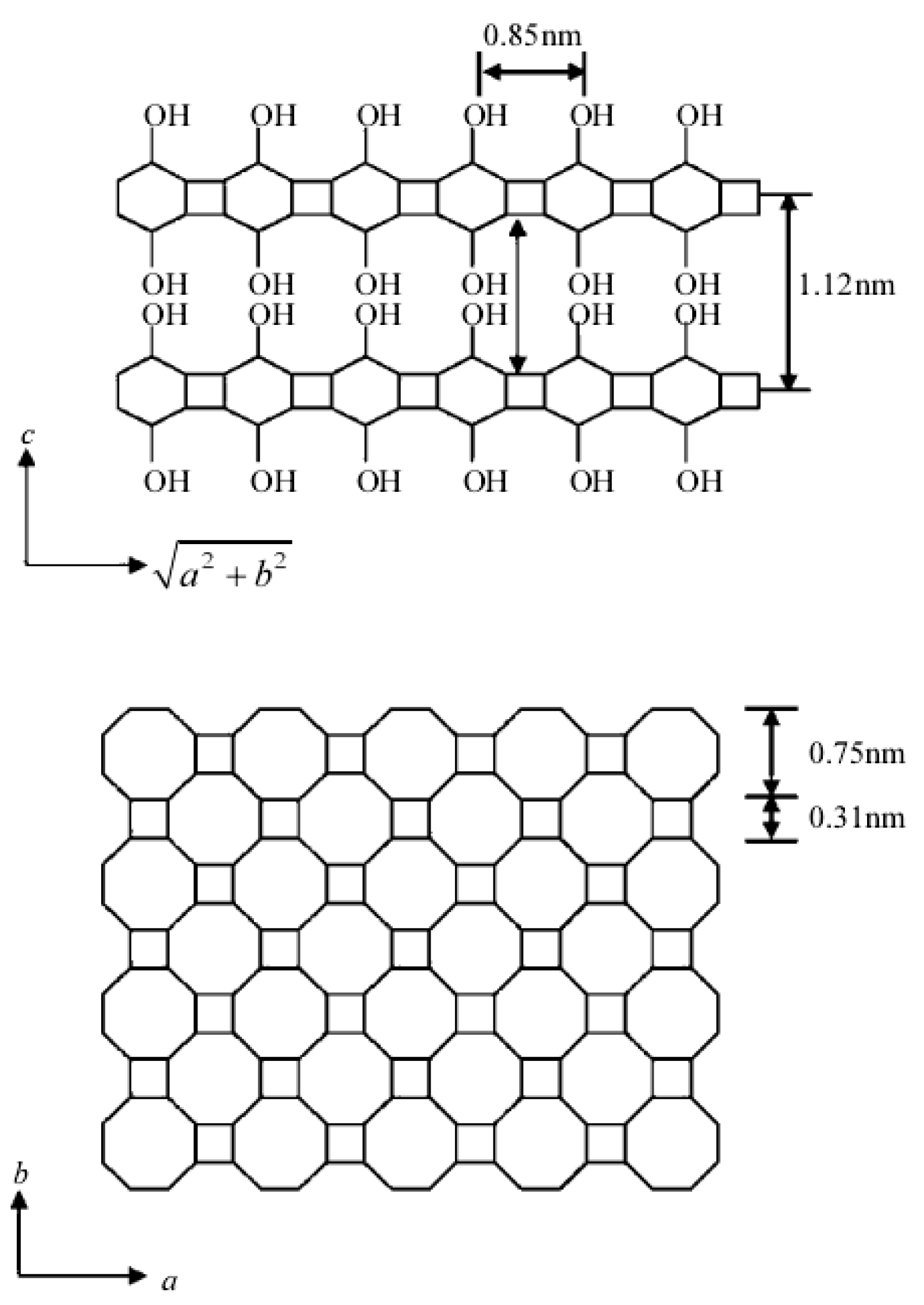
Adsorption Process and Properties Analyses of a Pure Magadiite and a Modified Magadiite on Rhodamine-B from an Aqueous Solution
Abstract
- Intercalated CTAB-MAG was characterized by ion exchange;
- Adsorption kinetics were well fitted in pseudo second order model and adsorption processes were dominated by film diffusion process which belonged to monomolecular layer adsorption;
- Adsorption capacity on Rhodamine-B of CTAB-MAG (67.19 mg/g) was increased by 40% over MAG (48.13 mg/g).
1. Introduction
2. Experimental
2.1. Experimental Reagents
2.2. Measuring Instruments
2.3. Preparation of Sorbents.
2.4. Adsorption Performance Experiment
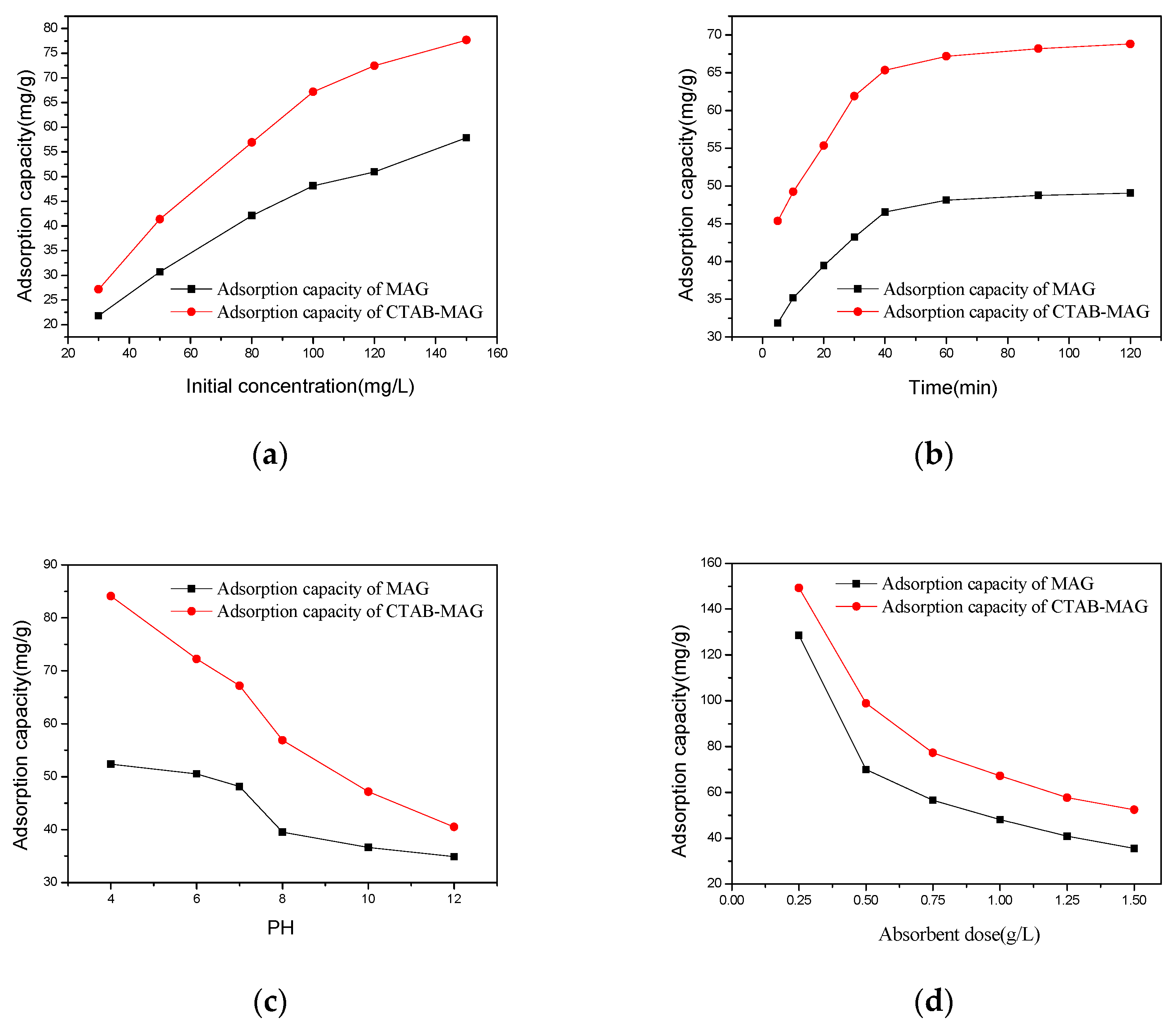
2.4.1. Effect of the Initial Concentration of Rh-B
2.4.2. Effect of Adsorption Time
2.4.3. Effect of pH
2.4.4. Effect of the Absorbent Dosage
3. Results and Discussion
3.1. Characterization of Adsorbents
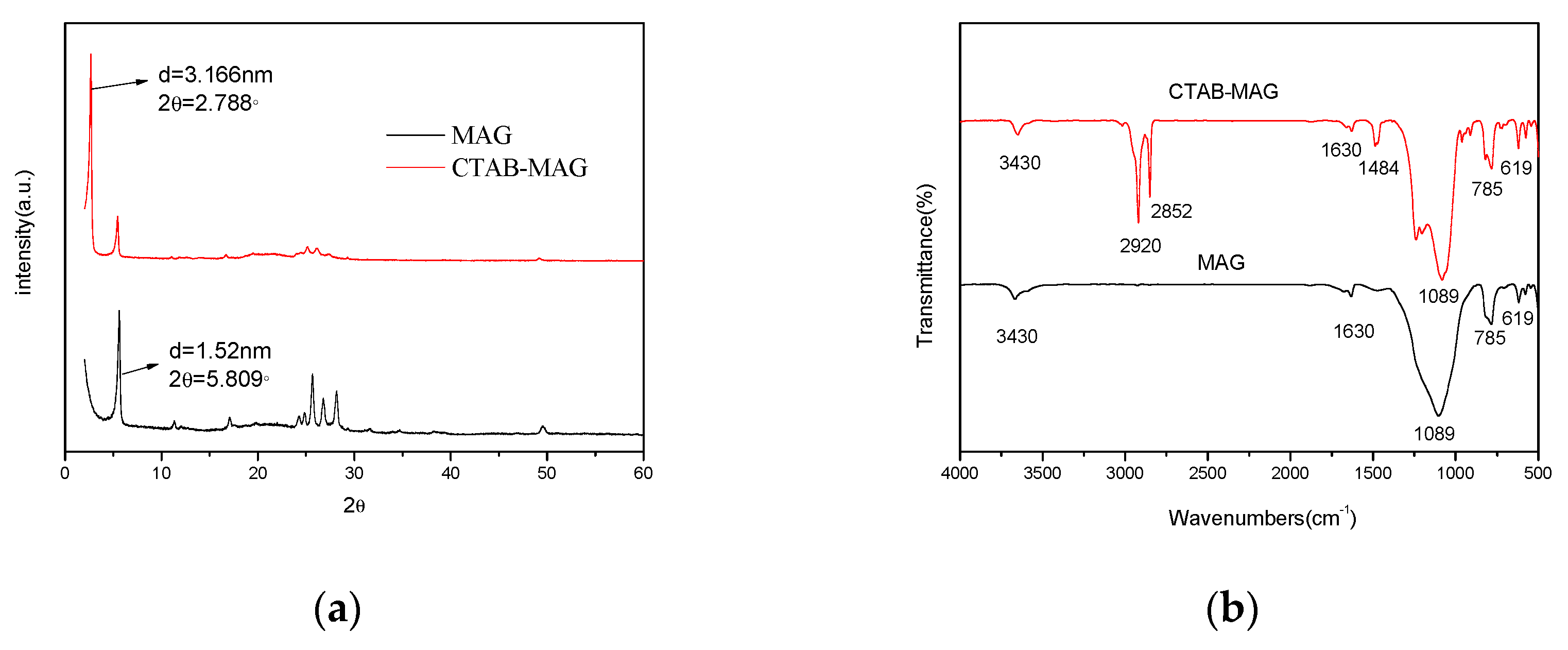
3.1.1. XRD Analyses
3.1.2. FTIR Analyses
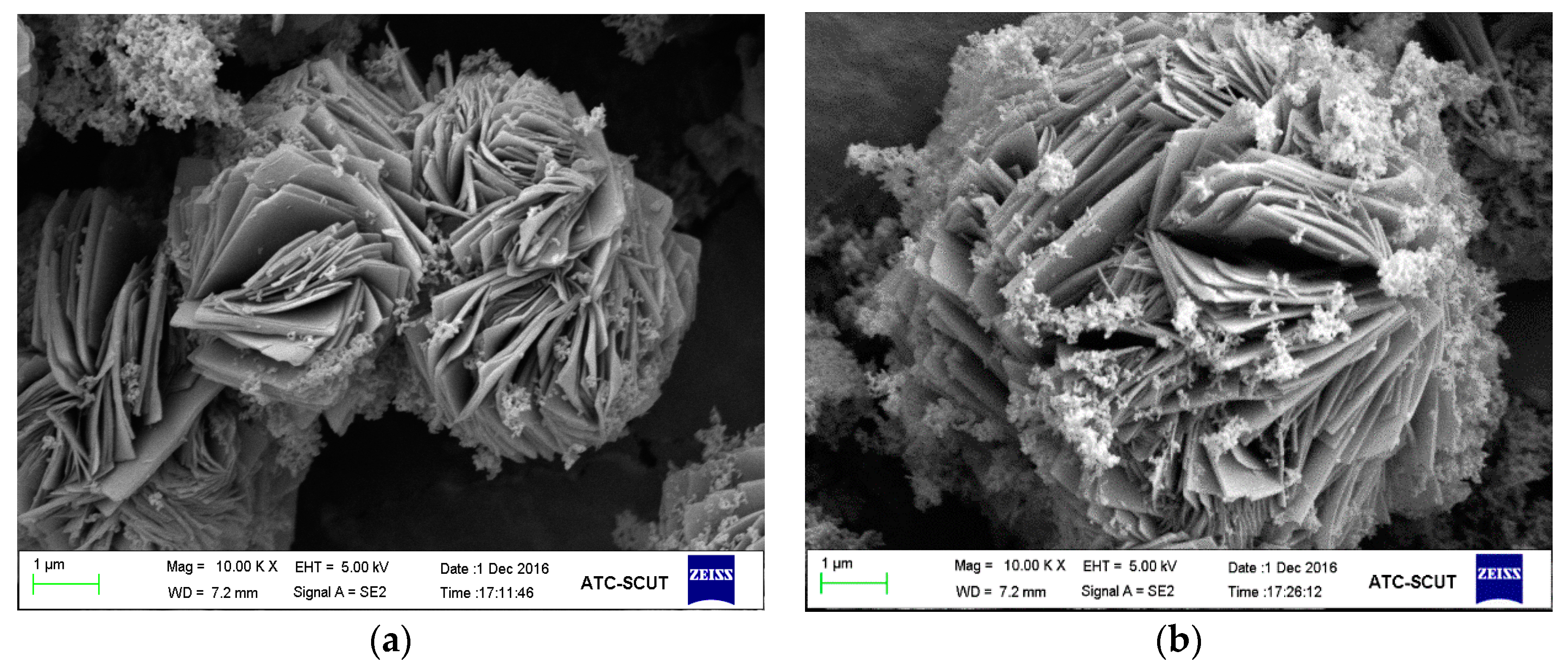
3.1.3. SEM Analysis
3.2. Adsorption Performance
3.2.1. Influencing Factors of the Adsorption Capacity
3.2.2. Isothermal Adsorption Experiment
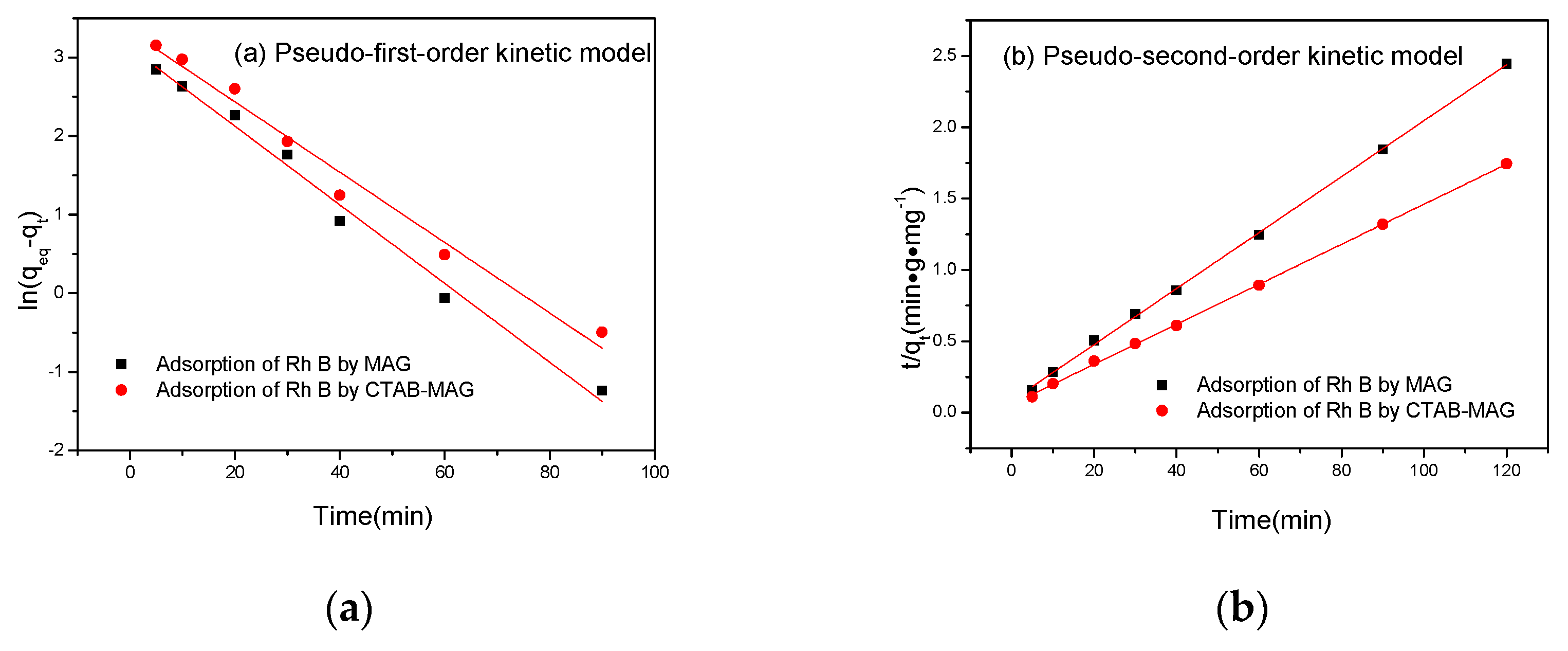
3.2.3. Adsorption Kinetics Model
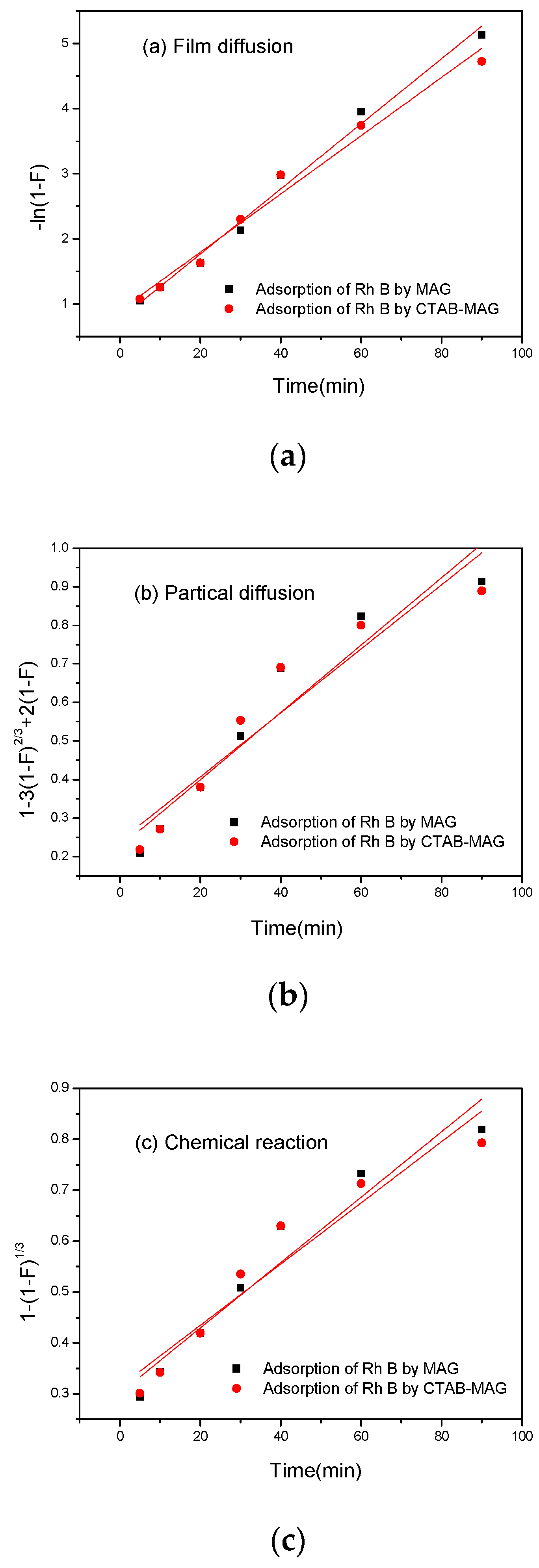
3.2.4. Adsorption Ratio Model
3.2.5. The Greater Adsorption Performance of CTAB-MAG
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baddouh, A.; Garcia Bessegato, G.; Rguiti, M.M.; EI Ibrahimi, B.; Bazzi, L.; Hilali, M.; Boldrin Zanoni, M.V. Electrochemical decolorization of Rhodamine B dye: Influence of anode material, chloride concentration and current density. J. Environ. Chem. Eng. 2018, 6, 2041–2047. [Google Scholar] [CrossRef]
- Zeng, H.X.; Zhang, W.Q.; Deng, L.; Luo, J.M.; Zhou, S.Q.; Liu, X.; Pei, Y.; Shi, Z.; Crittenden, J. Degradation of dyes by peroxymonosulfate activated by ternary CoFeNi-layered double hydroxide: Catalytic performance, mechanism and kinetic modeling. J. Colloid Interface Sci. 2018, 515, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic degradation of rhodamine B and real textile wastewater using Fe-doped TiO2 anchored on reduced graphene oxide (Fe-TiO2/rGO): Characterization and feasibility, mechanism and pathway studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Qiu, L.Y.; Wang, Q.Y.; Liu, Z.Y.; Zhao, Q.Q.; Tian, X.Y.; Li, H.L.; Gao, S.M. Preparation of 3D TiO2 nanotube arrays photoelectrode on Ti mesh for photoelectric conversion and photoelectrocatalytic removal of pollutant. Sep. Purif. Technol. 2018, 207, 206–212. [Google Scholar] [CrossRef]
- Liang, C.; Liu, Y.H.; Li, K.; Wen, J.; Xing, S.T.; Ma, Z.C.; Wu, Y.S. Heterogeneous photo-Fenton degradation of organic pollutants with amorphous Fe-Zn-oxide/hydrochar under visible light irradiation. Sep. Purif. Technol. 2017, 188, 105–111. [Google Scholar] [CrossRef]
- Li, L.; Iqbal, J.; Zhu, Y.; Zhang, P.; Chen, W.C.; Bhatnagar, A.; Du, Y.P. Chitosan/Ag-hydroxyapatite nanocomposite beads as a potential adsorbent for the efficient removal of toxic aquatic pollutants. Int. J. Biol. Macromol. 2018, 120, 1752–1759. [Google Scholar] [CrossRef]
- Eugster, H.P. Hydrous sodium silicates from Lake Magadi, Kenya: Precursors of bedded chert. Science 1967, 157, 1177–1180. [Google Scholar] [CrossRef]
- Homhuan, N.; Bureekaew, S.; Ogawa, M. Efficient Concentration of Indium (III) from Aqueous Solution Using Layered Silicates. Langmuir 2017, 33, 9558–9564. [Google Scholar] [CrossRef]
- Kooli, F.; Liu, Y.; Abboudi, M.; Rakass, S.; Hassani, H.O.; Ibrahim, S.M.; Al-Faze, R. Application of Organo-Magadiites for the Removal of Eosin Dye from Aqueous Solutions: Thermal Treatment Regeneration. Molecules. 2018, 23, 2280. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, Q.S.; Gao, S.N.; Jiang, H.M.; Meng, C.G. Intercalation and in situ formation of coordination compounds with ligand 8-hydroxyquinoline-5-sulfonic acid in the interlayer space of layered silicate magadiite by solid-solid reactions. Microporous Mesoporous Mater. 2018, 266, 14–23. [Google Scholar] [CrossRef]
- Mao, Y.T.; Li, S.G.; Fang, R.L.; Ploehn, H.J. Magadiite/styrene-butadiene rubber composites for tire tread applications: Effects of varying layer spacing and alternate inorganic fillers. J. Appl. Polym. Sci. 2017, 134, 44764. [Google Scholar] [CrossRef]
- Li, S.G.; Mao, Y.T.; Ploehn, H.J. Interlayer functionalization of magadiite with sulfur-containing organosilanes. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 320–330. [Google Scholar] [CrossRef]
- Wang, Q.S.; Zhang, Y.F.; Zheng, J.Q.; Hu, T.; Meng, C.G. Synthesis, structure, optical and magnetic properties of interlamellar decoration of magadiite using vanadium oxide species. Microporous Mesoporous Mater. 2017, 244, 264–277. [Google Scholar] [CrossRef]
- Ge, M.L.; Cao, L.X.; Du, M.Y.; Hu, G.Q.; Jahangir Alam, S.M. Competitive adsorption analyses of a pure magadiite and a new silylated magadiite on methylene blue and phenol from related aqueous solution. Mater. Chem. Phys. 2018, 217, 393–402. [Google Scholar] [CrossRef]
- Lim, W.T.; Jang, J.H.; Park, N.Y.; Paek, S.M.; Kim, W.C.; Park, M. Spontaneous nanoparticle formation coupled with selective adsorption in magadiite. J. Mater. Chem. A 2017, 5, 4144–4149. [Google Scholar] [CrossRef]
- Kosuge, K.; Yamazaki, A.; Tsunashima, A.; Otsuka, R. Hydrothermal synthesis of magaditte and kenyaite. J. Ceram. Soc. Jpn. 1992, 100, 326–331. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.R.; Wang, S.F.; Chang, L.C. Hydrothermal synthesis of magadiite. Appl. Clay Sci. 2006, 33, 73–77. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Park, K.W. Synthesis of layered silicates from sodium silicate solution. Bull. Korean Chem. Soc. 2004, 25, 25–26. [Google Scholar]
- Jeong, S.Y.; Kwon, O.Y.; Suh, J.K.; Jin, H.; Lee, J.M. Preparation of silica-pillared molecular sieves from layered silicates. Stud. Surf. Sci. Catal. 1997, 105, 53–60. [Google Scholar]
- Zhao, J.; Zhang, Y.F.; Zhang, S.Q.; Wang, Q.S.; Chen, M.; Hu, T.; Meng, C.G. Synthesis and characterization of Mn-Silicalite-1 by the hydrothermal conversion of Mn-magadiite under the neutral condition and its catalytic performance on selective oxidation of styrene. Microporous Mesoporous Mater. 2018, 268, 16–24. [Google Scholar] [CrossRef]
- Ge, M.L.; Chen, M. Preparation and Characterization of Magadiite. J. Chin. Ceram. Soc. 2013, 42, 1704–1708. [Google Scholar]
- Ge, M.L.; Wang, Y.W.; Chen, M. Adsorption Characteristics of Zn2+ onto Magadiite. China Environ. Sci. 2015, 35, 2065–2071. [Google Scholar]
- Ge, M.L.; Wang, X.B.; Du, M.Y.; Liang, G.D.; Hu, G.Q.; Jahangir Alam, S.M. Effect on the mechanical properties of nacre-like bio-hybrid membranes with inter-penetrating petal structure based on magadiite. Materials 2019, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.L.; Tang, W.; Du, M.Y.; Liang, G.D.; Hu, G.Q.; Jahangir Alam, S.M. Research on 5-fluorouracil as drug carrier materials with its in vitro release properties on organic modified magadiite. Eur. J. Pharm. Sci. 2019, 130, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Gao, Q.; Wang, Q.G.; Shi, J.L. Layered Structural Heme Protein Magadiite Nanocomposites with High Enzyme-like Peroxidase Activity. Chem. Mater. 2004, 16, 2675–2684. [Google Scholar] [CrossRef]
- Kooli, F.; Yan, L. Thermal Stable Cetyltrimethylammonium-Magadiites: Influence of the Surfactant Solution Type. J. Phys. Chem. C 2009, 113, 1947–1952. [Google Scholar] [CrossRef]
- Kooli, F.; li, M.; Alshahateet, S.F.; Chen, F.X.; Zhu, Y.H. Characterization and thermal stability properties of intercalated Na-magadiite with cetyltrimethylammonium (C16TMA) surfactants. J. Phys. Chem. Solids 2006, 67, 926–931. [Google Scholar] [CrossRef]
- Macedo, T.R.; Airoldi, C. Host lamellar silicic acid magadiite for some heterocyclic amine inclusions and quantitative calorimetric data. Microporous Mesoporous Mater. 2006, 94, 81–88. [Google Scholar] [CrossRef]
- Park, K.W.; Jung, J.H.; Kim, S.K.; Kwon, O.Y. Interlamellar silylation of magadiite by octyl triethoxysilane in the presence of dodecylamine. Appl. Clay Sci. 2009, 46, 251–254. [Google Scholar] [CrossRef]
- Ge, M.L.; Du, M.Y.; Zheng, L.Y.; Wang, B.Y.; Zhou, X.Y.; Jia, Z.X.; Hu, G.Q.; Jahangir Alam, S.M. A maleic anhydride grafted sugarcane bagasse adsorbent and its performance on the removal of methylene blue from related wastewater. Mater. Chem. Phys. 2017, 192, 147–155. [Google Scholar] [CrossRef]
- Ge, M.L.; Cao, L.X.; Du, M.Y.; Hu, G.Q.; Jahangir Alam, S.M. Adsorption characterization of a pure magadiite and an organic modified magadiite on removal of methylene blue from related aqueous solution. Mater. Chem. Phys. 2018, 217, 533–540. [Google Scholar] [CrossRef]
- Mokhtar, M. Application of Synthetic Layered Sodium Silicate Magadiite Nanosheets for Environmental Remediation of Methylene Blue Dye in Water. Materials 2017, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Detrich, A.; Deak, A.; Hild, E.; Kovacs, A.L.; Horvolgyi, Z. Langmuir and Langmuir-Blodgett Films of Bidisperse Silica Nanoparticles. Langmuir 2010, 26, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Factorovich, M.H.; Solveyra, E.G.; Molinero, V. Sorption isotherms of Water in Nanopores: Relationship Between Hydropohobicity, Adsorption Pressure, and Hysteresis. J. Phys. Chem. C 2014, 118, 16290–16300. [Google Scholar] [CrossRef]
- Chen, F.F.; Liu, Q.J.; Xu, Z.M.; Sun, X.W.; Shi, Q.; Zhao, S.Q. Adsorption Kinetics and Thermodynamics of Vanadyl Etioporphyrin on Asphaltene in Pentane. Energy Fuels 2013, 27, 6408–6418. [Google Scholar] [CrossRef]
- Kumar, K.V.; Khaddour, I.A.; Gupta, V.K. A Pseudo Second-Order Kinetic Expression for Dissolution Kinetic Profiles of Solids in Solutions. Ind. Eng. Chem. Res. 2010, 49, 7257–7262. [Google Scholar] [CrossRef]
- Ge, M.L.; Wang, X.B.; Du, M.Y.; Liang, G.D.; Hu, G.Q.; Jahangir Alam, S.M. Adsorption analyses of phenol from aqueous solution using magadiite modified with organo-functional groups: Kinetic and equilibrium studies. Materials 2019, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Guerra, D.J.L.; Mello, I.; Resende, R.; Silva, R. Application as absorbents of natural and functionalized Brazilian bentonite in Pb2+ adsorption: Equilibrium, kinetic, pH, and thermodynamic effects. Water Resour. Ind. 2013, 4, 32–50. [Google Scholar] [CrossRef]
- Ge, M.L.; Xi, Z.Z.; Zhu, C.P.; Liang, G.D.; Hu, G.Q.; Jamal, L.; Jahangir Alam, S.M. Preparation and characterization of magadiite-magnetite nanocomposite with its sorption performance analyses on removal of methylene blue from aqueous solution. Polymers 2019, 11, 607. [Google Scholar] [CrossRef]
- Khan, T.A.; Dahiya, S.; Ali, I. Use of kaolinite as adsorbent: Equilibrium, dynamics and thermodynamic studies on the adsorption of rhodamine B from aqueous solution. Appl. Clay Sci. 2012, 69, 58–66. [Google Scholar] [CrossRef]
- Selvam, P.P.; Preethi, S.; Basakaralingam, P.; Thinakaran, N.; Sivasamy, A.; Sivanesan, S. Removal of rhodamine B from aqueous solution by adsorption onto sodium montmorillonite. J. Hazard. Mater. 2008, 155, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashed, S.M.; Al-Gaid, A.A. Kinetic and thermodynamic studies on the adsorption behavior of rhodamine B dye on duolite C-20 resin. J. Saudi Chem. Soc. 2012, 16, 209–215. [Google Scholar] [CrossRef]
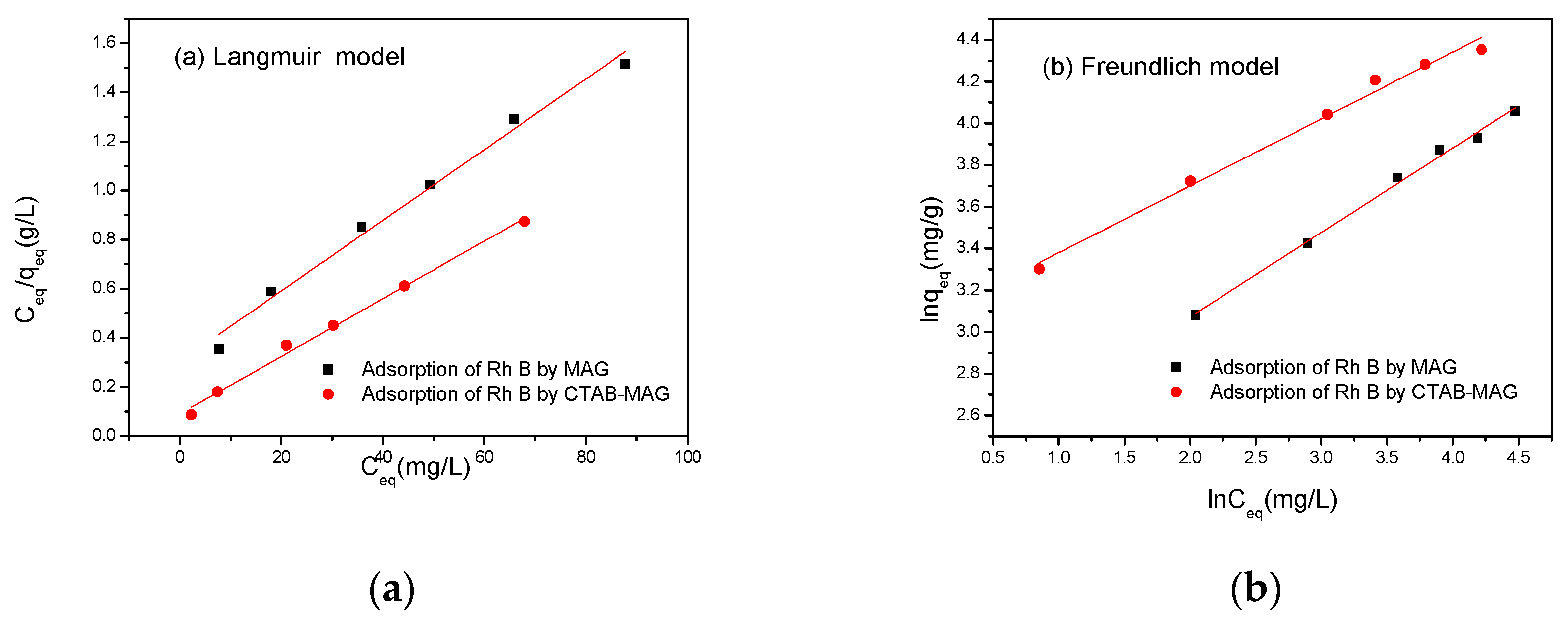
| Langumir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|
| KL | qm | R2 | KF | 1/n | R2 | |
| MAG | 0.0476 | 69.44 | 0.99718 | 9.611 | 0.40486 | 0.98432 |
| CTAB-MAG | 0.1321 | 85.11 | 0.99327 | 21.304 | 0.32086 | 0.98763 |
| Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | Experiment | |||||
|---|---|---|---|---|---|---|---|
| K1 | qeqc | R2 | K2 | qeqc | R2 | qeqe | |
| MAG | 0.0501 | 22.81 | 0.98817 | 0.00449 | 50.994 | 0.99937 | 49.07 |
| CTAB-MAG | 0.0448 | 27.99 | 0.97913 | 0.00336 | 71.327 | 0.99949 | 68.82 |
| Film Diffusion | Particle Diffusion | Chemical Reaction | ||||
|---|---|---|---|---|---|---|
| k | R2 | k | R2 | k | R2 | |
| MAG | 0.051 | 0.988 | 0.009 | 0.909 | 0.006 | 0.934 |
| CTAB-MAG | 0.045 | 0.979 | 0.008 | 0.887 | 0.006 | 0.911 |
| Adsorbents | Conditions | Isotherms | Kinetics | Adsorption Capacity | References |
|---|---|---|---|---|---|
| CTAB-MAG | Ph = 7; dosage 1 g/L; Rh-B concentration 100 mg/L | Langmuir and Freundlich | pseudo-second-order | 67.19 mg/g | This work |
| MAG | Ph = 7; dosage 1 g/L; Rh-B concentration 100 mg/L | Langmuirand Freundlich | pseudo-second-order | 48.13 mg/g | This work |
| Kaolinite | Ph = 7; dosage 3 g/L; Rh-B concentration 90 mg/L | Langmuir | pseudo-second-order | 46.08 mg/g | [40] |
| Sodium montmorillonite | Ph = 7; dosage 0.3 g/L; Rh-B concentration 200 mg/L | Langmuir | pseudo-second-order | 42.19 mg/g | [41] |
| Duolite C-20 resin | Ph = 7; dosage 0.4 g/L; Rh-B concentration 8.129 mg/L | Langmuir and Freundlich | pseudo-first-order | 28.57 mg/g | [42] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, M.; Xi, Z.; Zhu, C.; Liang, G.; Yang, Y.; Hu, G.; Jamal, L.; S.M., J.A. Adsorption Process and Properties Analyses of a Pure Magadiite and a Modified Magadiite on Rhodamine-B from an Aqueous Solution. Processes 2019, 7, 565. https://doi.org/10.3390/pr7090565
Ge M, Xi Z, Zhu C, Liang G, Yang Y, Hu G, Jamal L, S.M. JA. Adsorption Process and Properties Analyses of a Pure Magadiite and a Modified Magadiite on Rhodamine-B from an Aqueous Solution. Processes. 2019; 7(9):565. https://doi.org/10.3390/pr7090565
Chicago/Turabian StyleGe, Mingliang, Zhuangzhuang Xi, Caiping Zhu, Guodong Liang, Yinye Yang, Guoqing Hu, Lafifa Jamal, and Jahangir Alam S.M. 2019. "Adsorption Process and Properties Analyses of a Pure Magadiite and a Modified Magadiite on Rhodamine-B from an Aqueous Solution" Processes 7, no. 9: 565. https://doi.org/10.3390/pr7090565
APA StyleGe, M., Xi, Z., Zhu, C., Liang, G., Yang, Y., Hu, G., Jamal, L., & S.M., J. A. (2019). Adsorption Process and Properties Analyses of a Pure Magadiite and a Modified Magadiite on Rhodamine-B from an Aqueous Solution. Processes, 7(9), 565. https://doi.org/10.3390/pr7090565





