Evaluation for the Leaching of Cr from Coal Gangue Using Expansive Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Specimen Preparation
2.2. Experiments Methods
3. Results and Discussions
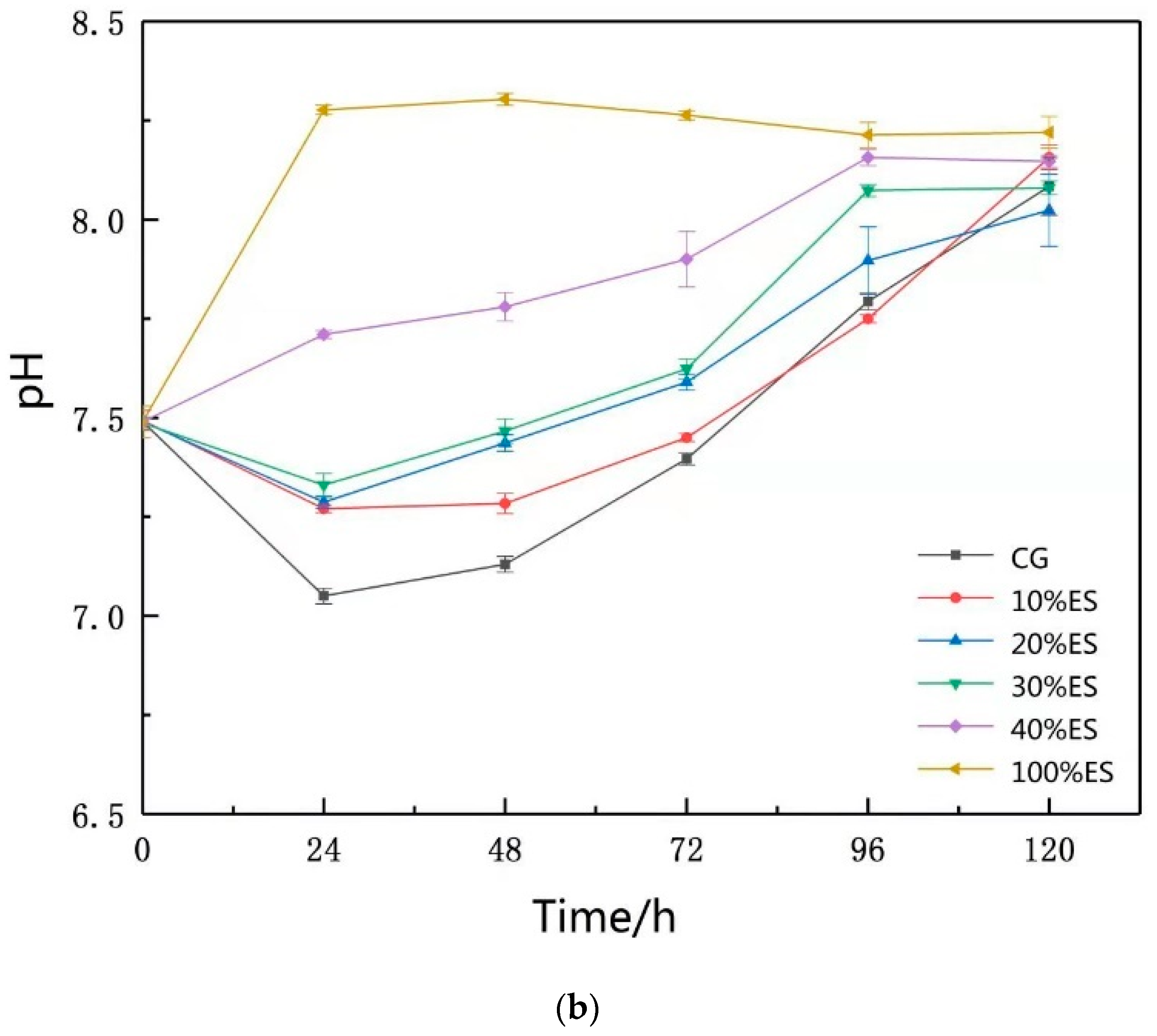
3.1. pH of Leaching Solutions
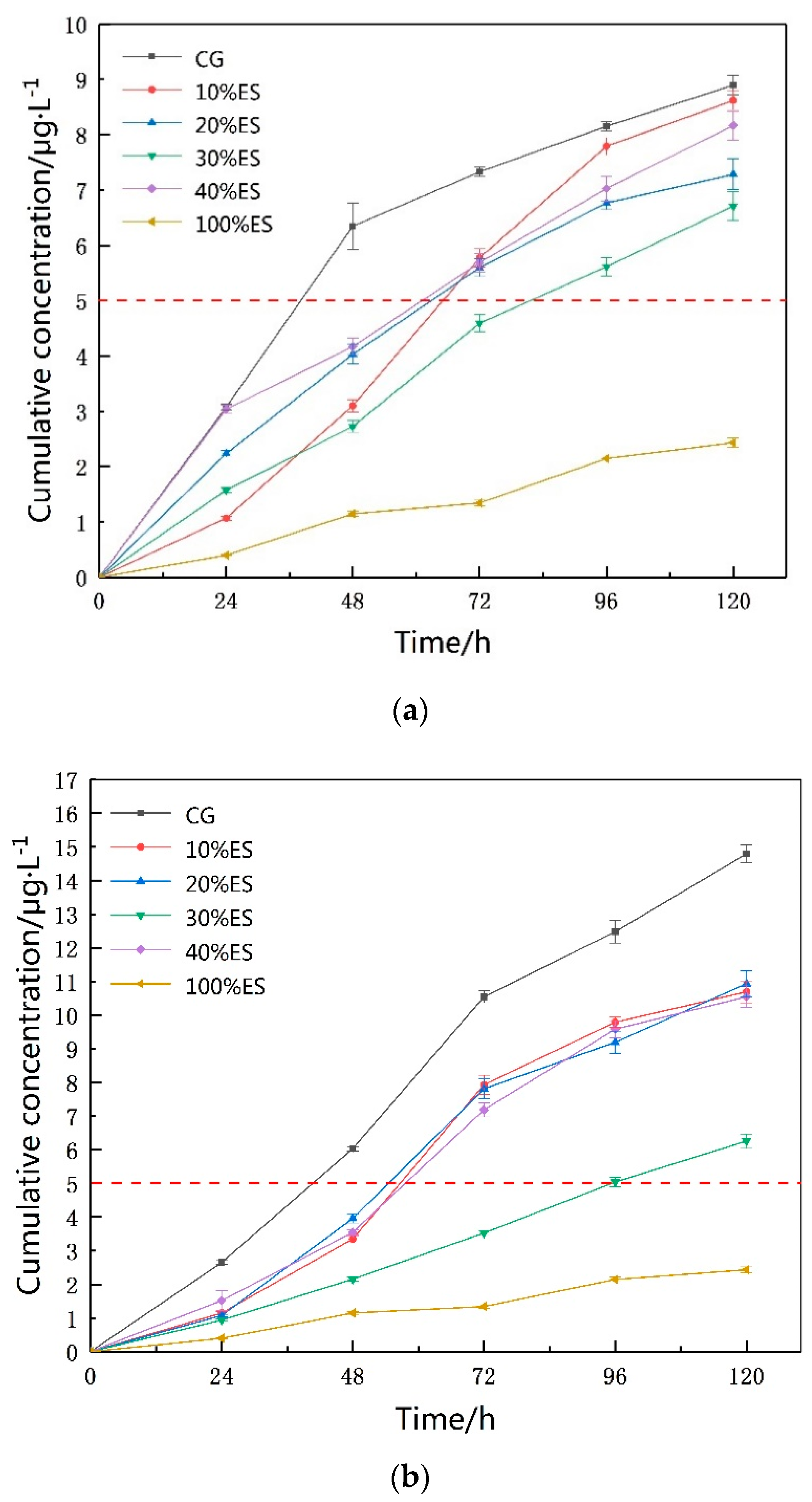
3.2. Concentration of Leaching Solutions
3.3. Leaching Time
3.4. Expansive Soil Amount
4. Evaluation
4.1. Potential Ecological Risk
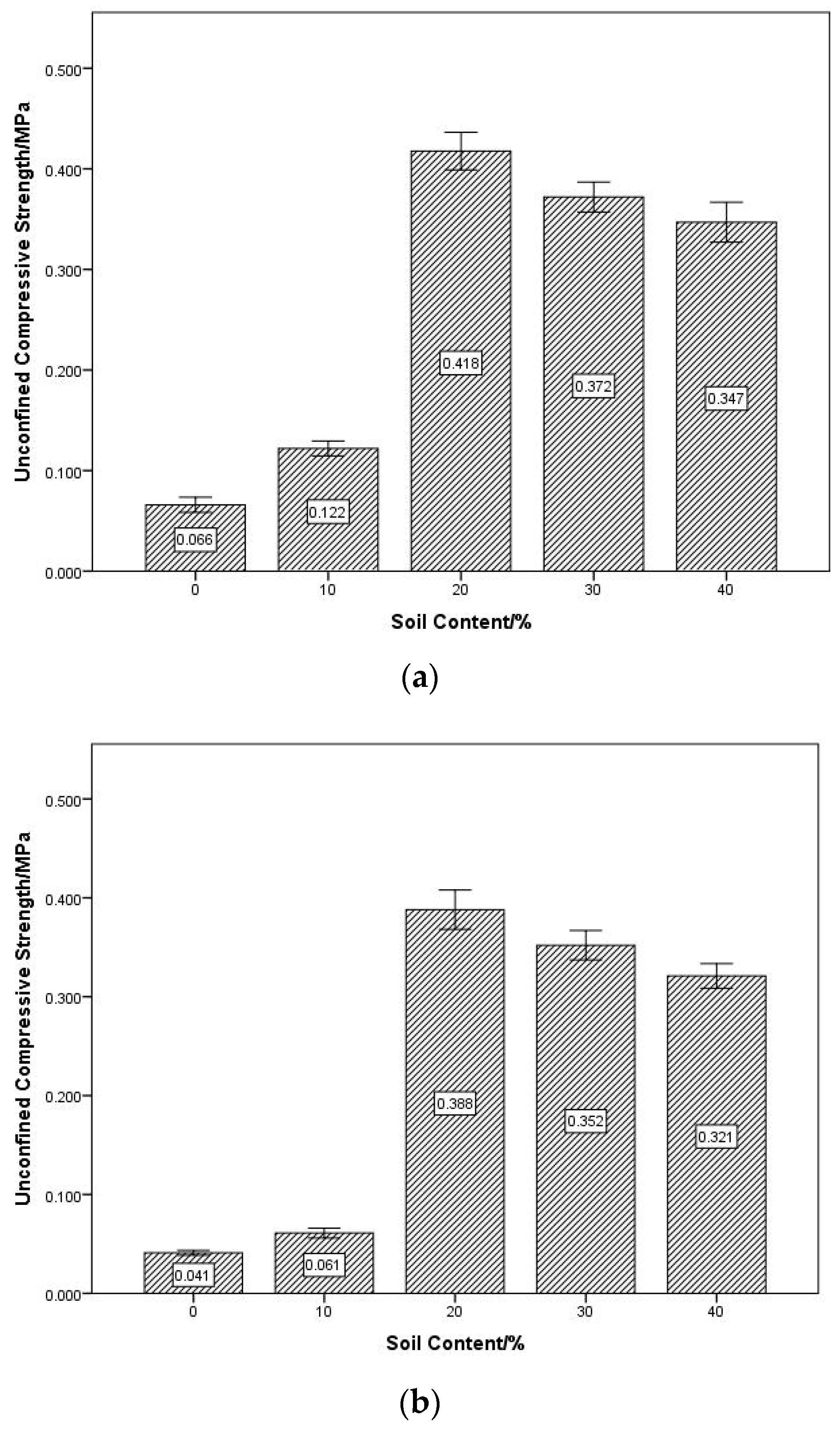
4.2. Unconfined Compressive Strength
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liang, Y.; Liang, H.; Zhu, S. Mercury emission from spontaneously ignited coal gangue hill in Wuda Coalfield, Inner Mongolia, China. Fuel 2016, 182, 525–530. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Zhou, T.; Tang, Q.; Zhang, T. Impact of coal gangue on the level of main trace element in the shallow groundwater of a mine reclamation area. Min. Sci. Technol. China 2011, 21, 715–719. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Wu, D.; Fang, T.; Wang, R.; Fan, X. Mobility behavior and environmental implications of trace elements associated with coal gangue: A case study at the Huainan coalfield in China. Chemosphere 2014, 95, 193–199. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.; Wu, D.; Fang, T.; Wang, R.; Xiang, F. Experimental and modeling study on the volatilization of arsenic during co-combustion of high arsenic lignite blends. Appl. Therm. Eng. 2016, 108, 1336–1343. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Chou, C.; Finkelman, R.; Seredin, V.; Zhou, Y. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Si, H.; Bi, H.; Li, X.; Yang, C. Environmental evaluation for sustainable development of coal mining in Qijiang, Western China. Int. J. Coal Geol. 2010, 81, 163–168. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.; Zhou, Y.; He, C. Spatial distribution and influence analysis of soil heavy metals in a hilly region of Sichuan Basin. Pol. J. Environ. Stud. 2017, 26, 725–732. [Google Scholar] [CrossRef]
- Shi, P.; Schulin, R. Erosion-induced losses of carbon, nitrogen, phosphorus and heavy metals from agricultural soils of contrasting organic matter management. Sci. Total Environ. 2018, 618, 210–218. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Zhou, Y.; Dou, L.; Cai, L.; Mo, L.; You, J. Bioavailability and soil-to-crop transfer of heavy metals in farmland soils: A case study in the Pearl River Delta, South China. Environ. Pollut. 2018, 235, 710–719. [Google Scholar] [CrossRef]
- Qiu, R.; Cheng, F.; Huang, H. Removal of Cd2+ from aqueous solution using hydrothermally modified circulating fluidized bed fly ash resulting from coal gangue power plant. J. Clean. Prod. 2018, 172, 1918–1927. [Google Scholar] [CrossRef]
- Salguero, F.; Grande, J.; Valente, T.; Garrido, R.; Torre, M.; Fortes, J.; Sanchez, A. Recycling of manganese gangue materials from waste-dumps in the Iberian pyrite belt-application as filler for concrete production. Constr. Build. Mater. 2014, 54, 363–368. [Google Scholar] [CrossRef]
- Dong, Z.; Xia, J.; Fan, C.; Cao, J. Activity of calcined coal gangue fine aggregate and its effect on the mechanical behavior of cement mortar. Constr. Build. Mater. 2015, 100, 63–69. [Google Scholar] [CrossRef]
- Wang, C.; Ni, W.; Zhang, S.; Wang, S.; Gai, G.; Wang, W. Preparation and properties of autoclaved aerated concrete using coal gangue and iron ore tailings. Constr. Build. Mater. 2016, 104, 109–115. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Wu, S.; Lam, P. The environmental characteristics of usage of coal gangue in bricking-making: A case study at Huainan, China. Chemosphere 2014, 95, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, B.; Liu, Y. Transportability and pressure drop of fresh cemented coal gangue-fly ash backfill (CGFB) slurry in pipe loop. Powder Technol. 2015, 284, 218–224. [Google Scholar] [CrossRef]
- Wu, D.; Sun, G.; Liu, Y. Modeling the thermo-hydro-chemical behavior of cemented coal gangue-fly ash backfill. Constr. Build. Mater. 2016, 111, 522–528. [Google Scholar]
- Wu, D.; Zhang, Y.; Liu, Y. Mechanical performance and ultrasonic properties of cemented gangue backfill with admixture of fly ash. Ultrasonics 2016, 64, 89–96. [Google Scholar] [CrossRef]
- Yang, L.; Song, J.; Bai, X.; Song, B.; Wang, R.; Zhou, T.; Jia, J.; Pu, H. Leaching behavior and potential environmental effects of trace elements in coal gangue of an open-cast coal mine area, Inner Mongolia, China. Minerals 2016, 6, 50. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, W.; Shen, J.; Zhou, L.; Xia, H.; Shu, W.; Ferris, H.; Fu, S. Nematodes as indicators of soil recovery in tailings of a lead/zinc mine. Soil Biol. Biochem. 2008, 40, 2040–2046. [Google Scholar] [CrossRef]
- Yeganeh, M.; Afyuni, M.; Khoshgoftarmanesh, A.; Khodakarami, L.; Amini, M.; Soffyanian, A.; Schulin, R. Mapping of human health risks arising from soil nickel and mercury contamination. J. Hazard. Mater. 2013, 244, 225–239. [Google Scholar] [CrossRef]
- Wen, X.; Zeng, F.; Zhang, H. Geochemical characteristics of the coal gangue from the Du’erping Coal Mine, Xishan Coalfield, North China. Chin. J. Geochem. 2013, 32, 227–234. [Google Scholar] [CrossRef]
- Shah, B.; Shah, A.; Mistry, C.; Navik, A. Assessment of heavy metals in sediments near Hazira industrial zone at Tapti River Estuary, Surat, India. Environ. Earth Sci. 2013, 69, 2365–2376. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Shi, W.; Li, J. Soil heavy metal contamination and risk assessment around the Fenhe Reservoir, China. Bull. Environ. Contam. Toxicol. 2014, 93, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Amirhossein, P.; Gholamreza, N.; Mojtaba, A.; Mohsen, S.; Akbar, B. A new index for assessing heavy metals contamination in sediments: A case study. Ecol. Indic. 2015, 58, 365–373. [Google Scholar] [CrossRef]
- Ge, H.; Feng, Y.; Li, Y.; Yang, W.; Gong, N. Heavy metal pollution diagnosis and ecological risk assessment of the surrounding soils of coal waste pile at Naluo coal mine, Liupanshui, Guizhou. Int. J. Min. Reclam. Environ. 2016, 30, 312–318. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, S.; Chen, X. Assessment of heavy metal pollution and human health risk in urban soils of a coal mining city in East China. Hum. Ecol. Risk Assess. Int. J. 2016, 22, 1359–1374. [Google Scholar] [CrossRef]
- Wang, W.; Hao, W.; Bian, Z.; Lei, S.; Wang, X.; Sang, S.; Xu, S. Effect of coal mining activities on the environment of Tetraena Mongolica in Wuhai, Inner Mongolia, China—A geochemical perspective. Int. J. Coal Geol. 2014, 132, 94–102. [Google Scholar] [CrossRef]
- Hua, C.; Zhou, G.; Yin, X.; Wang, C.; Chi, B.; Cao, Y.; Wang, Y.; Zheng, Y.; Cheng, Z.; Li, R. Assessment of heavy metal in coal gangue: Distribution, leaching characteristic and potential ecology risk. Environ. Sci. Pollut. Res. 2018, 25, 32321–32331. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, W.; Zhao, H.; Yang, Q.; Yang, Z. Potential ecological risk assessment and prediction of soil heavy-metal pollution around coal gangue damp. Nat. Hazards Earth Syst. Sci. 2014, 14, 1599–1610. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, X.; Wang, X. Adsorption and desorption of Ni2+ on Na-montmorillonite: Effect of pH, ionic strength, fulvic acid, humic acid and addition sequences. Appl. Clay Sci. 2008, 39, 133–141. [Google Scholar] [CrossRef]
- Querol, X.; Zhuang, X.; Font, O.; Izquierdo, M.; Alastuey, A.; Castro, I.; Drooge, B.; Moreno, T.; Grimalt, J.; Betanzos, J.; et al. Influence of soil cover on reducing the environmental impact of spontaneous coal combustion in coal waste gobs: A review and new experimental data. Int. J. Coal Geol. 2011, 85, 2–22. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Liu, X. Hydration mechanism and leaching behavior of bauxite-calcination-method red-coal gangue based cementitious materials. J. Hazard. Mater. 2016, 314, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Quyang, S. Release characteristics of heavy metals from coal gangue under simulation leaching conditions. Energy Explor. Exploit. 2014, 32, 413–422. [Google Scholar] [CrossRef]
- Cao, H.; Li, G.; Zhang, L. The analysis of heavy metal in leaching liquid of coal. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 012213. [Google Scholar] [CrossRef]
- Liu, X.; Luo, T.; Zhu, G.; Chen, Q. Study on the properties of Gaomiaozi Bentonite as the buffer/babkfilling materials for HLW disposal. China Nucl. Sci. Technol. Rep. 2007, 39, 17. [Google Scholar]
- Ministry of Transport of the People’s Republic of China. Test Methods of Soils for Highway Engineering (JTG E40-2007); China Communication Publishing & Media Management Co., Ltd.: Beijing, China, 2007.
- Meng, F.; Zhang, Y.; Zhang, X.; Li, Z. Coal gangue performances of west area in Inner Mongolia. J. Inn. Mong. Agric. Univ. Nat. Sci. Ed. 2012, 33, 174–178. [Google Scholar]
- Meng, F. The Law of Change in Shear Strength and CBR of Coal Gangue Expansive Soil Subgrade. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2012. [Google Scholar]
- Ministry of Transport of the People’s Republic of China. Test Methods of Aggregate for Highway Engineering (JTG E42-2005); China Communication Publishing & Media Management Co., Ltd.: Beijing, China, 2005.
- Guo, W.; Li, D.; Chen, J.; Yang, N. Effect of heat activated and mechanical force activated method on coal gangue cementing performance. J. Build. Mater. 2011, 14, 371–375. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Liu, Z.; Zhou, N. Fractal characteristics of crushed particles of coal gangue under compaction. Powder Technol. 2017, 305, 12–18. [Google Scholar] [CrossRef]
- National Standards Substance Management Committee. People’s Republic of China Standard Substance List; China Quality and Standards Publishing & Media Co. Ltd.: Beijing, China, 2010. [Google Scholar]
- Peng, B.; Wu, D. Leaching characteristics of bromine in coal. J. Fuel Chem. Technol. 2011, 39, 647–651. [Google Scholar] [CrossRef]
- Huggins, F.; Seidu, L.; Shah, N.; Backus, J.; Huffman, G.; Honaker, R. Mobility of elements in long-term leaching tests on Illinois #6 coal rejects. Int. J. Coal Geol. 2011, 94, 326–336. [Google Scholar] [CrossRef]
- Wang, W.; Qin, Y.; Song, D.; Wang, K. Column leaching of coal and its combustion residues, Shizuishan, China. Int. J. Coal Geol. 2008, 75, 81–87. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Li, J.; Sheng, G.; Zhang, Y.; Zheng, X. Enhenced Cr (VI) removal by using the mixture of pillared bentonite and zero-valent iron. Chem. Eng. J. 2012, 185, 243–249. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision. Quality Standard for Ground water (GB/T 14848-1993); General Administration of Quality Supervision: Beijing, China, 1993.
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
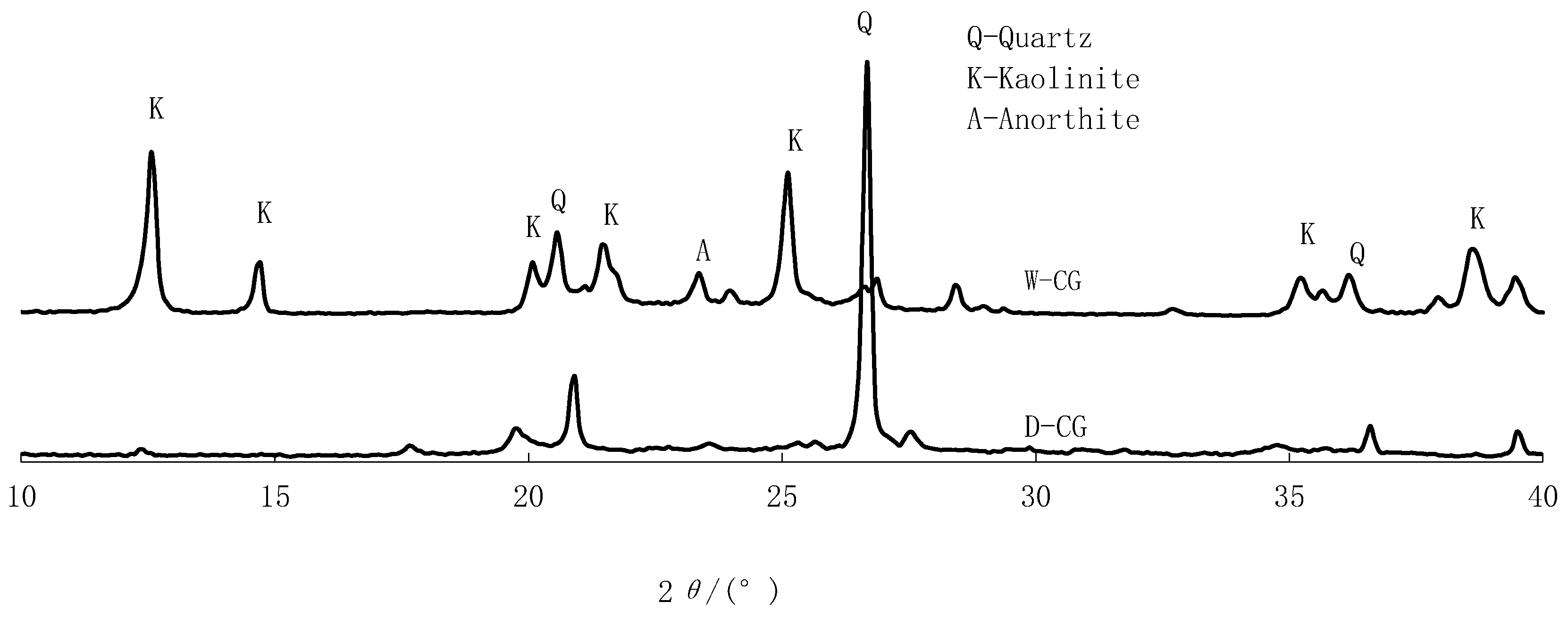
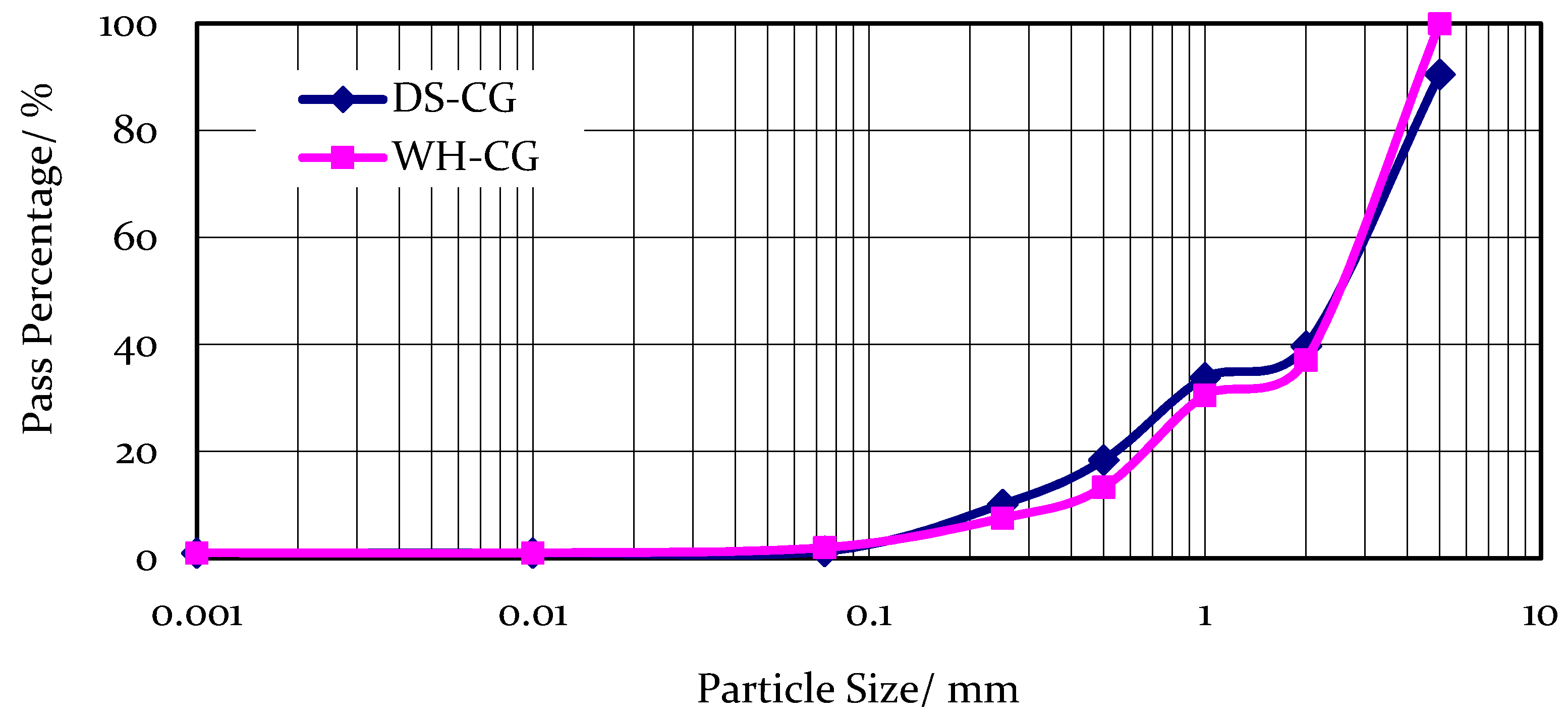
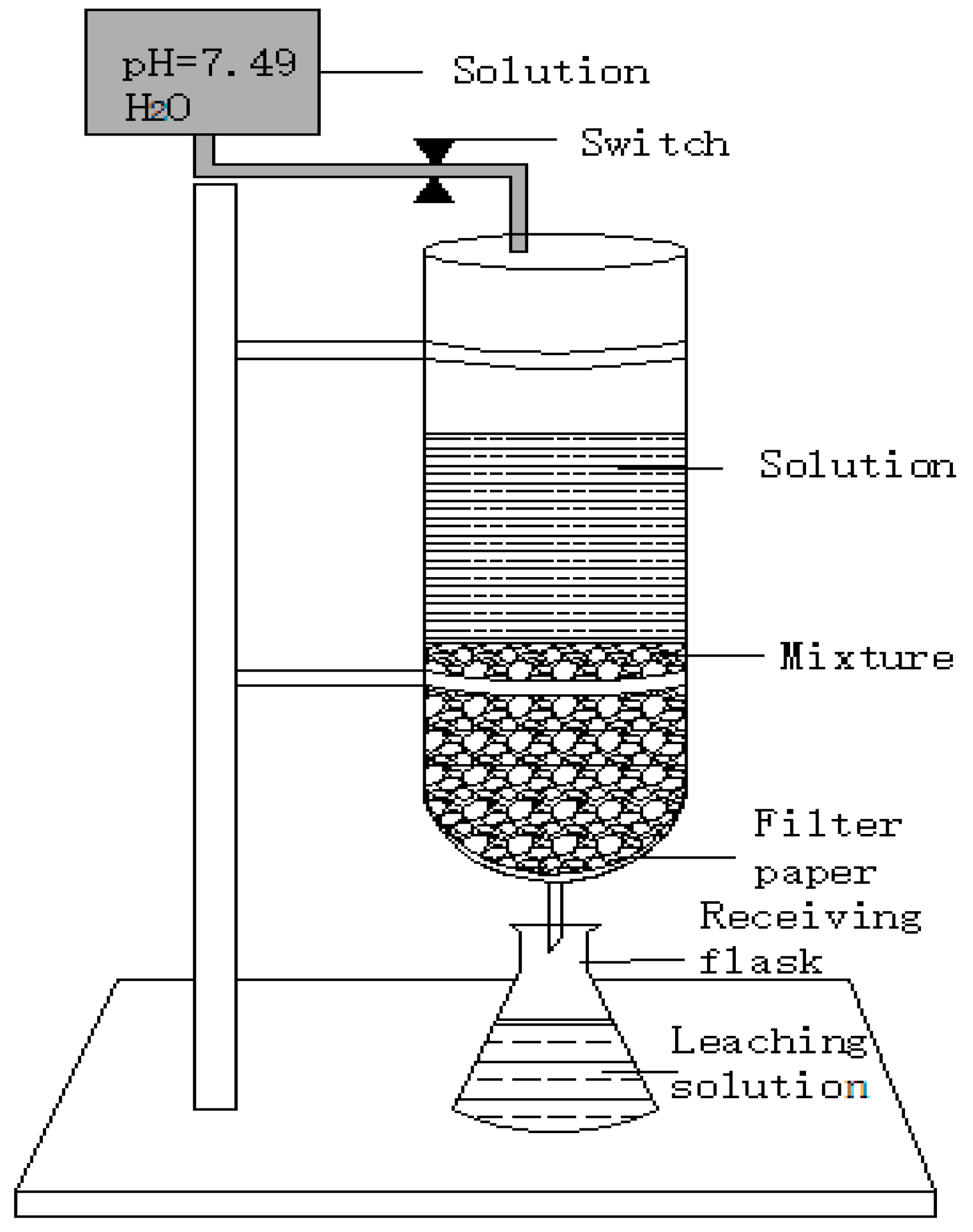
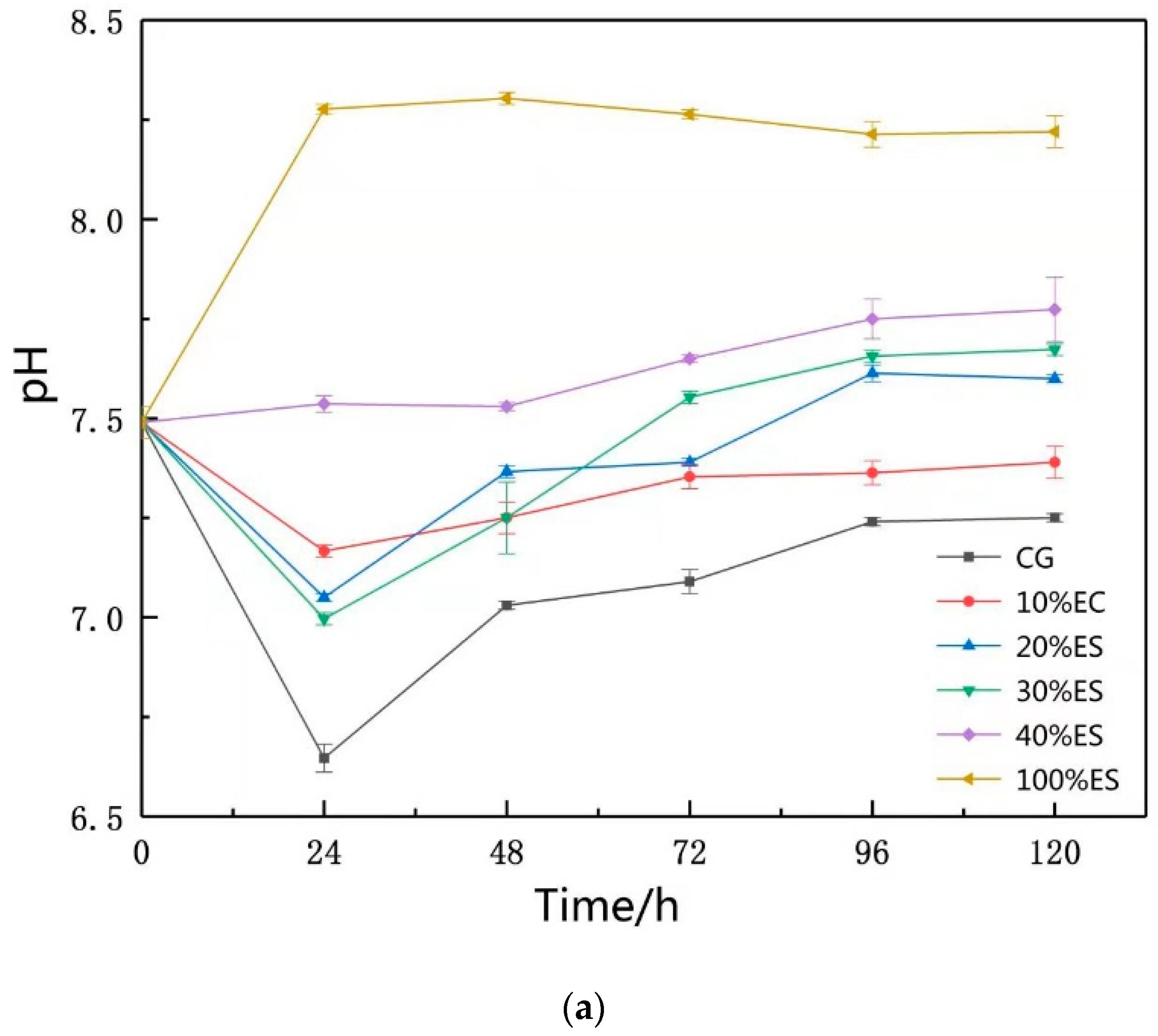
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Cr |
|---|---|---|---|---|---|---|
| ES | 69.17 | 14.43 | 3.12 | 1.29 | 3.31 | 0.0008 |
| Parameter | Liquid Limit/% | Plastic Limit/% | Plasticity Index | Free Expansion Ratio/% |
|---|---|---|---|---|
| Values | 57.2 | 28.7 | 28.5 | 46 |
| Sample | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | C | Cr |
|---|---|---|---|---|---|---|---|
| DS-CG | 58.50 | 25.70 | 6.60 | 1.50 | 1.10 | 3.6 | 0.02 |
| WH-CG | 62.10 | 24.80 | 4.30 | 1.80 | 1.20 | 2.9 | 0.02 |
| Sample | Water Absorption Rate | Burn of Rate | Crushing Value |
|---|---|---|---|
| DS-CG | 3.7 | 13.2 | 24.2 |
| WH-CG | 0.5 | 14.4 | 20.6 |
| Leachate of DS-CG | Leachate of WH-CG | Limitation of the Quality Standard for Groundwater | Limitation of World Health Organization |
|---|---|---|---|
| 8.9 | 14.7 | ≤5 | ≤50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Baaj, H.; Zhao, R. Evaluation for the Leaching of Cr from Coal Gangue Using Expansive Soils. Processes 2019, 7, 478. https://doi.org/10.3390/pr7080478
Zhang Y, Baaj H, Zhao R. Evaluation for the Leaching of Cr from Coal Gangue Using Expansive Soils. Processes. 2019; 7(8):478. https://doi.org/10.3390/pr7080478
Chicago/Turabian StyleZhang, Yan, Hassan Baaj, and Rong Zhao. 2019. "Evaluation for the Leaching of Cr from Coal Gangue Using Expansive Soils" Processes 7, no. 8: 478. https://doi.org/10.3390/pr7080478
APA StyleZhang, Y., Baaj, H., & Zhao, R. (2019). Evaluation for the Leaching of Cr from Coal Gangue Using Expansive Soils. Processes, 7(8), 478. https://doi.org/10.3390/pr7080478




