Influence of Particle Charge and Size Distribution on Triboelectric Separation—New Evidence Revealed by In Situ Particle Size Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
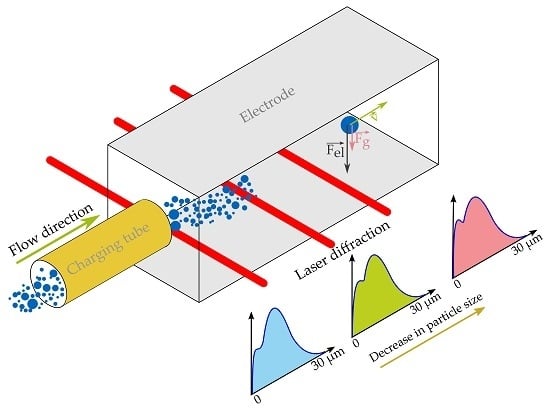
2.2.1. Separation Setup
2.2.2. In Situ Particle Size Analysis
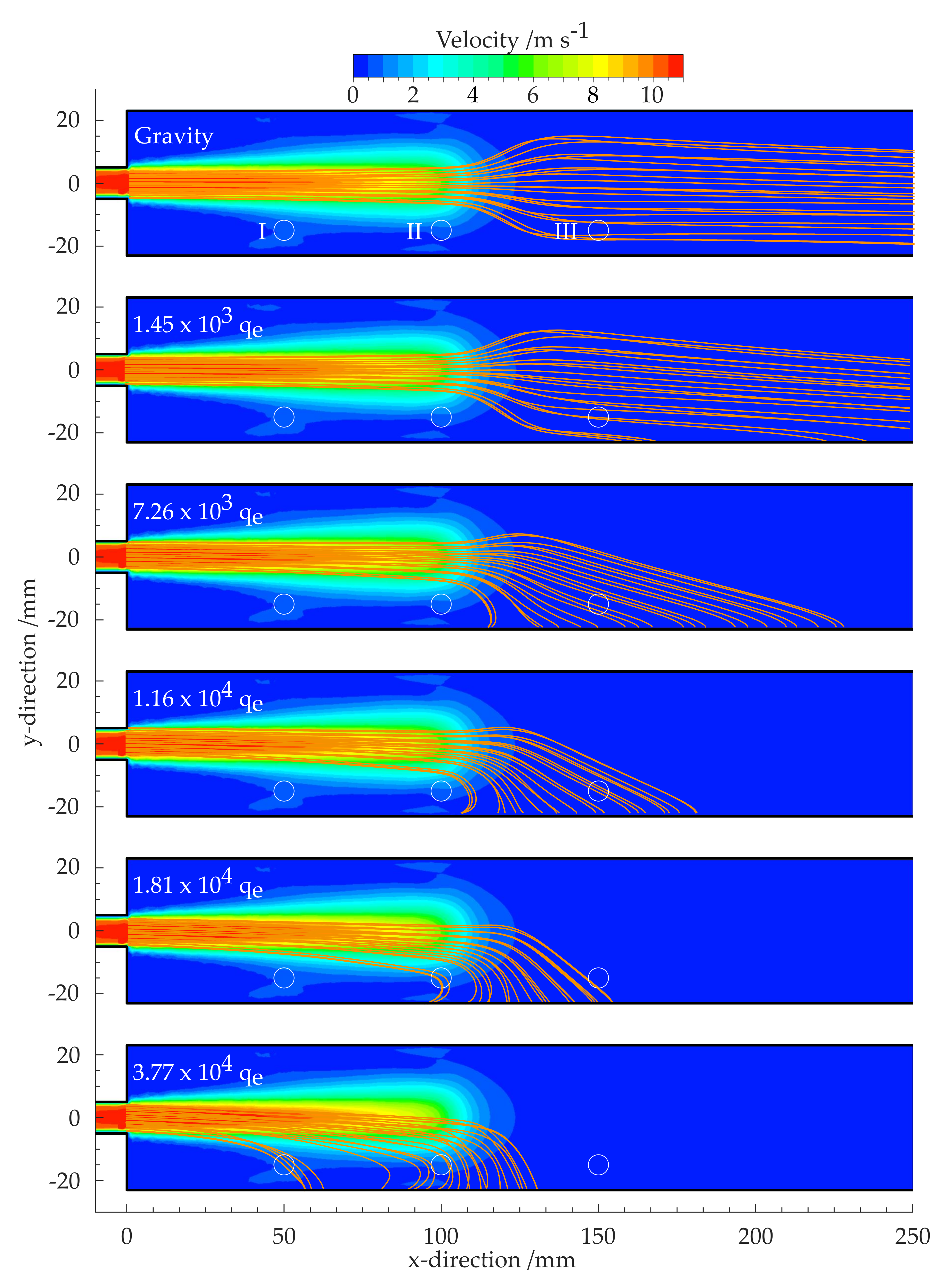
2.2.3. Flow Simulation and Estimation of the Particle Charge
3. Results
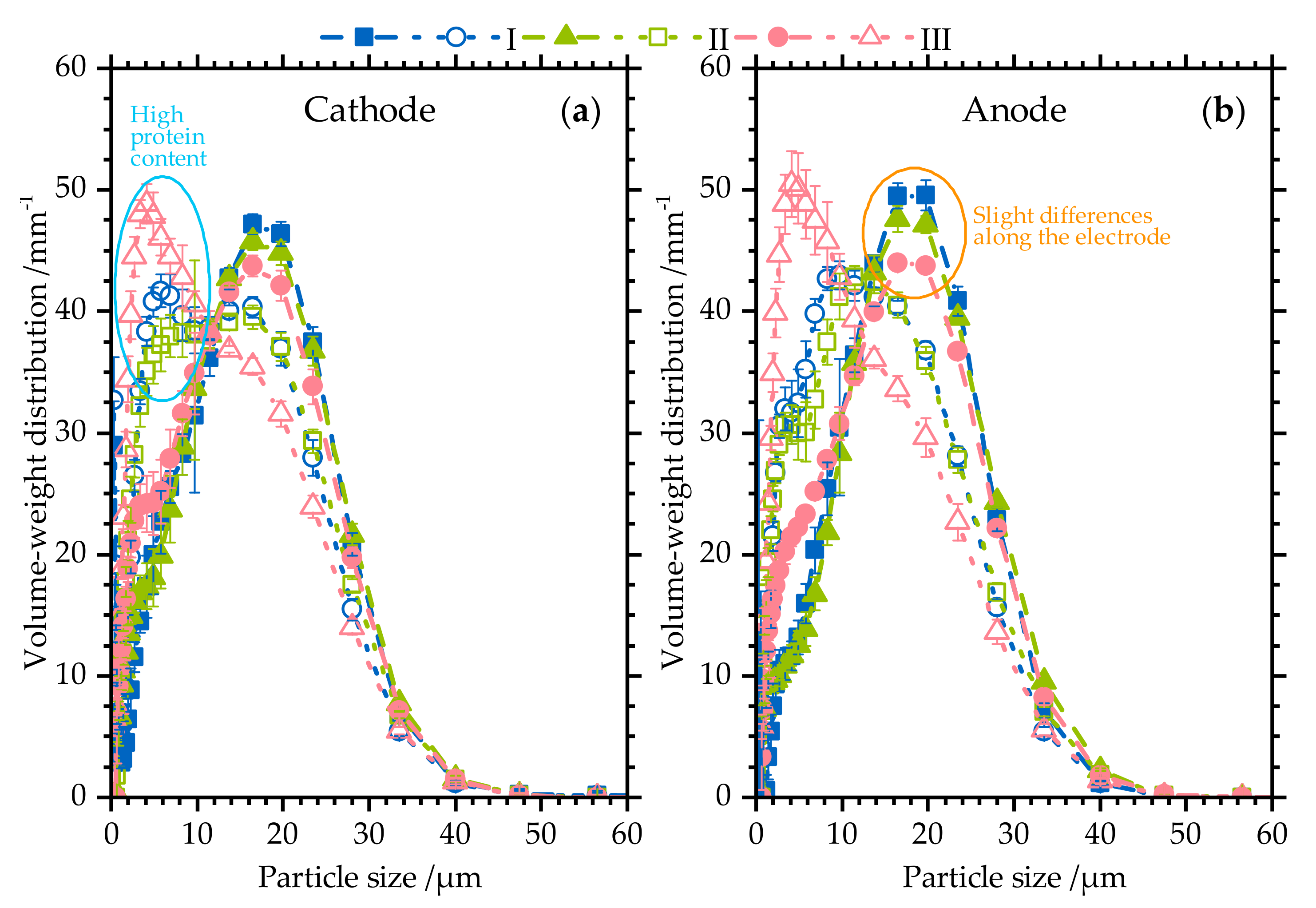
3.1. Particle Size Distribution
3.1.1. Agglomeration within the Charging Tube
3.1.2. Particle Size Distribution along the Electrodes
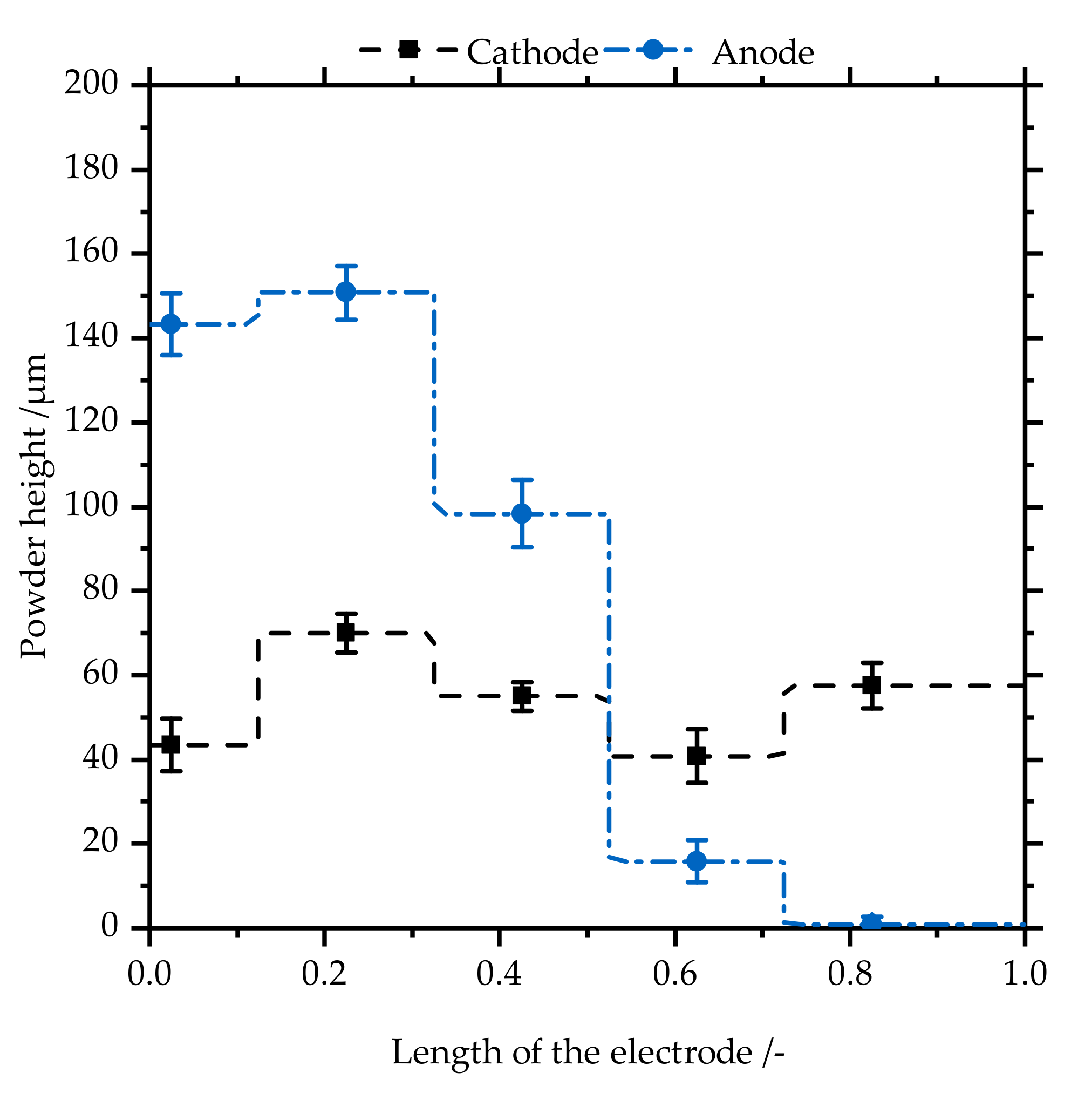
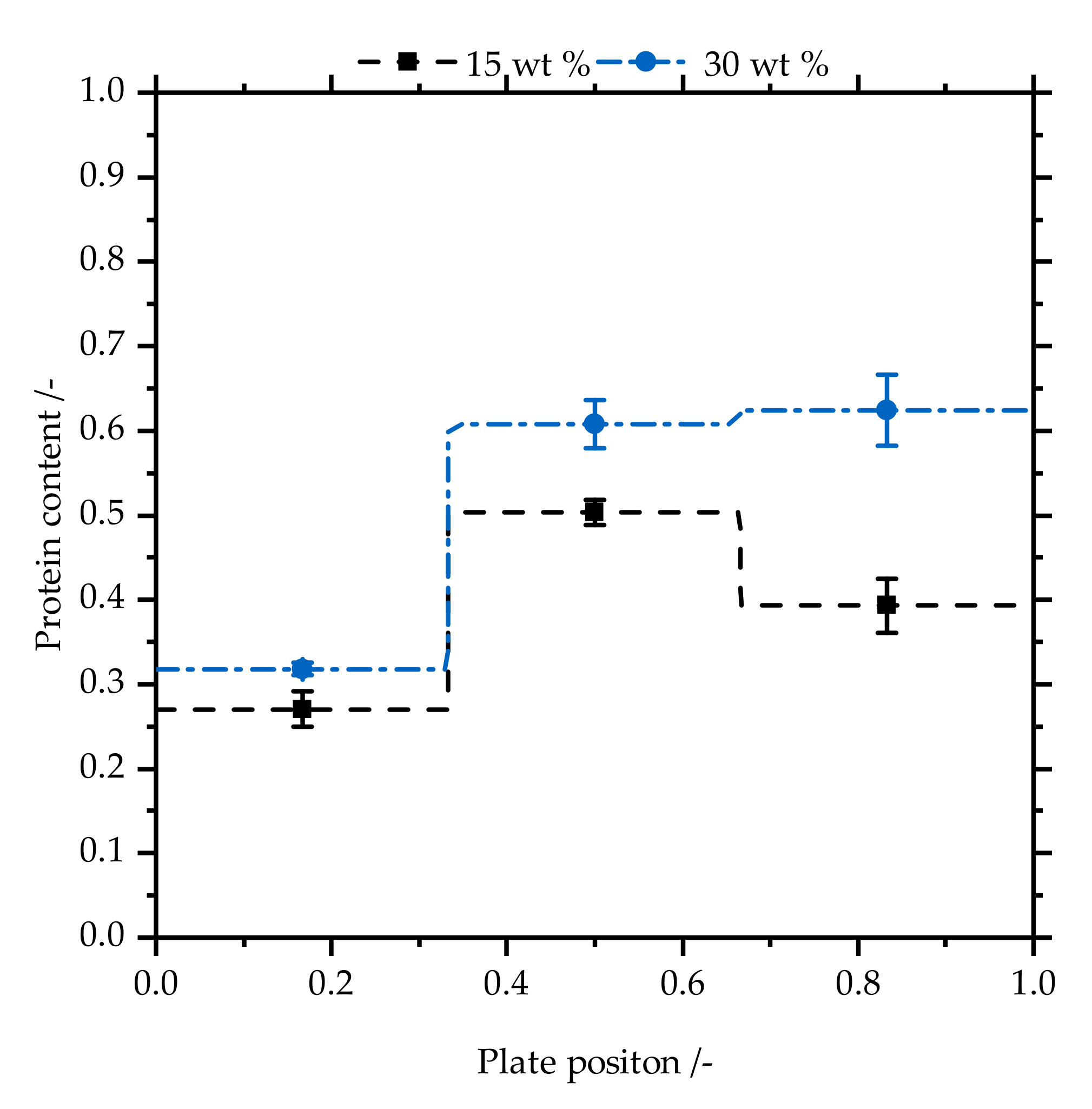
3.2. Powder on the Electrodes
3.2.1. Powder Height
3.2.2. Protein Content
3.3. Estimation of the Charge Correlated with the In Situ Particle Size Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iversen, P.; Lacks, D.J. A life of its own: The tenuous connection between Thales of Miletus and the study of electrostatic charging. J. Electrost. 2012, 70, 309–311. [Google Scholar] [CrossRef]
- O’Grady, P.F. Thales of Miletus. The Beginnings of Western Science and Philosophy; Ashgate: Aldershot, UK, 2002; ISBN 0754605337. [Google Scholar]
- Harper, W.R. The Volta Effect as a Cause of Static Electrification. Proc. R. Soc. A Math. Phys. Eng. Sci. 1951, 205, 83–103. [Google Scholar] [CrossRef]
- Harper, W.R. Contact and Frictional Electrification. Monographs on the Physics and Chemistry of Materials; Clarendon Press: Oxford, UK, 1967. [Google Scholar]
- Davies, D.K. Charge generation on dielectric surfaces. J. Phys. D Appl. Phys. 1969, 2, 1533. [Google Scholar] [CrossRef]
- Lowell, J.; Rose-Innes, A.C. Contact electrification. Adv. Phys. 1980, 29, 947–1023. [Google Scholar] [CrossRef]
- Liu, C.; Bard, A.J. Electrostatic electrochemistry at insulators. Nat. Mater. 2008, 7, 505–509. [Google Scholar] [CrossRef]
- McCarty, L.S.; Whitesides, G.M. Electrostatic charging due to separation of ions at interfaces: Contact electrification of ionic electrets. Angew. Chem. Int. Ed. Engl. 2008, 47, 2188–2207. [Google Scholar] [CrossRef]
- Ducati, T.R.D.; Simoes, L.H.; Galembeck, F. Charge partitioning at gas-solid interfaces: Humidity causes electricity buildup on metals. Langmuir 2010, 26, 13763–13766. [Google Scholar] [CrossRef]
- Baytekin, H.T.; Patashinski, A.Z.; Branicki, M.; Baytekin, B.; Soh, S.; Grzybowski, B.A. The mosaic of surface charge in contact electrification. Science 2011, 333, 308–312. [Google Scholar] [CrossRef]
- Salaneck, W.R. Double mass transfer during polymer-polymer contacts. J. Appl. Phys. 1976, 47, 144–147. [Google Scholar] [CrossRef]
- Schella, A.; Herminghaus, S.; Schröter, M. Influence of humidity on tribo-electric charging and segregation in shaken granular media. Soft Matter 2017, 13, 394–401. [Google Scholar] [CrossRef]
- Németh, E.; Albrecht, V.; Schubert, G.; Simon, F. Polymer tribo-electric charging: Dependence on thermodynamic surface properties and relative humidity. J. Electrost. 2003, 58, 3–16. [Google Scholar] [CrossRef]
- Baytekin, H.T.; Baytekin, B.; Soh, S.; Grzybowski, B.A. Is water necessary for contact electrification? Angew. Chem. Int. Ed. Engl. 2011, 50, 6766–6770. [Google Scholar] [CrossRef]
- Matsusyama, T.; Yamamoto, H. Impact charging of particulate materials. Chem. Eng. Sci. 2006, 61, 2230–2238. [Google Scholar] [CrossRef]
- Matsuyama, T.; Yamamoto, H. Electrification of single polymer particles by successive impacts with metal targets. IEEE Trans. Ind. Appl. 1995, 31, 1441–1445. [Google Scholar] [CrossRef]
- Watanabe, H.; Ghadiri, M.; Matsuyama, T.; Maruyama, H.; Matsusaka, S.; Ghadiri, M.; Matsuyama, T.; Ding, Y.L.; Pitt, K.G.; Maruyama, H.; et al. Triboelectrification of pharmaceutical powders by particle impact. Int. J. Pharm. 2007, 334, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Grosshans, H.; Papalexandris, M.V. Large Eddy simulation of triboelectric charging in pneumatic powder transport. Powder Technol. 2016, 301, 1008–1015. [Google Scholar] [CrossRef]
- Ireland, P.M. Triboelectrification of particulate flows on surfaces: Part I—Experiments. Powder Technol. 2010, 198, 189–198. [Google Scholar] [CrossRef]
- Fotovat, F.; Bi, X.T.; Grace, J.R. A perspective on electrostatics in gas-solid fluidized beds: Challenges and future research needs. Powder Technol. 2018, 329, 65–75. [Google Scholar] [CrossRef]
- Mehrani, P.; Murtomaa, M.; Lacks, D.J. An overview of advances in understanding electrostatic charge buildup in gas-solid fluidized beds. J. Electrost. 2017, 87, 64–78. [Google Scholar] [CrossRef]
- Engers, D.A.; Fricke, M.N.; Storey, R.P.; Newman, A.W.; Morris, K.R. Triboelectrification of pharmaceutically relevant powders during low-shear tumble blending. J. Electrost. 2006, 64, 826–835. [Google Scholar] [CrossRef]
- Karner, S.; Urbanetz, N.A. Arising of electrostatic charge in the mixing process and its influencing factors. Powder Technol. 2012, 226, 261–268. [Google Scholar] [CrossRef]
- Ghori, M.U.; Supuk, E.; Conway, B.R. Tribo-electric charging and adhesion of cellulose ethers and their mixtures with flurbiprofen. Eur. J. Pharm. Sci. 2014, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Forward, K.M.; Lacks, D.J.; Sankaran, R.M. Particle-size dependent bipolar charging of Martian regolith simulant. Geophys. Res. Lett. 2009, 36, 139. [Google Scholar] [CrossRef]
- Waitukaitis, S.R.; Lee, V.; Pierson, J.M.; Forman, S.L.; Jaeger, H.M. Size-Dependent Same-Material Tribocharging in Insulating Grains. Phys. Rev. Lett. 2014, 112. [Google Scholar] [CrossRef]
- Lacks, D.J.; Levandovsky, A. Effect of particle size distribution on the polarity of triboelectric charging in granular insulator systems. J. Electrost. 2007, 65, 107–112. [Google Scholar] [CrossRef]
- Zhao, H.; Castle, G.S.P.; Inculet, I.I.; Bailey, A.G. Bipolar charging of poly-disperse polymer powders in fluidized beds. IEEE Trans. Ind. Appl. 2003, 39, 612–618. [Google Scholar] [CrossRef]
- Mukherjee, R.; Gupta, V.; Naik, S.; Sarkar, S.; Sharma, V.; Peri, P.; Chaudhuri, B. Effects of particle size on the triboelectrification phenomenon in pharmaceutical excipients: Experiments and multi-scale modeling. Asian J. Pharm. Sci. 2016, 11, 603–617. [Google Scholar] [CrossRef]
- Trigwell, S.; Grable, N.; Yurteri, C.U.; Sharma, R.; Mazumder, M.K. Effects of surface properties on the tribocharging characteristics of polymer powder as applied to industrial processes. IEEE Trans. Ind. Appl. 2003, 39, 79–86. [Google Scholar] [CrossRef]
- Karner, S.; Maier, M.; Littringer, E.; Urbanetz, N.A. Surface roughness effects on the tribo-charging and mixing homogeneity of adhesive mixtures used in dry powder inhalers. Powder Technol. 2014, 264, 544–549. [Google Scholar] [CrossRef]
- Lacks, D.J.; Duff, N.; Kumar, S.K. Nonequilibrium accumulation of surface species and triboelectric charging in single component particulate systems. Phys. Rev. Lett. 2008, 100, 188305. [Google Scholar] [CrossRef]
- Burgo, T.A.L.; Silva, C.A.; Balestrin, L.B.S.; Galembeck, F. Friction coefficient dependence on electrostatic tribocharging. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.F.; Felix-Navarro, R.M. A semi-quantitative tribo-electric series for polymeric materials: The influence of chemical structure and properties. J. Electrost. 2004, 62, 277–290. [Google Scholar] [CrossRef]
- Grosshans, H.; Papalexandris, M.V. A model for the non-uniform contact charging of particles. Powder Technol. 2017, 305, 518–527. [Google Scholar] [CrossRef]
- Haeberle, J.; Schella, A.; Sperl, M.; Schröter, M.; Born, P. Double origin of stochastic granular tribocharging. Soft Matter 2018, 14, 4987–4995. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhou, Q.; Liang, C.; Chen, X.; Liu, D.; Zhao, C. Experimental investigation on electrostatic characteristics of a single grain in the sliding process. Powder Technol. 2018, 334, 132–142. [Google Scholar] [CrossRef]
- Ireland, P.M. Impact tribocharging of soft elastic spheres. Powder Technol. 2019, 348, 70–79. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; de Wit, M.; Boom, R.M.; Schutyser, M.A.I. Lupine protein enrichment by milling and electrostatic separation. Innov. Food Sci. Emerg. Technol. 2016, 33, 596–602. [Google Scholar] [CrossRef]
- Wang, J.; de Wit, M.; Boom, R.M.; Schutyser, M.A.I. Charging and separation behavior of gluten–starch mixtures assessed with a custom-built electrostatic separator. Sep. Purif. Technol. 2015, 152, 164–171. [Google Scholar] [CrossRef]
- Xing, Q.; de Wit, M.; Kyriakopoulou, K.; Boom, R.M.; Schutyser, M.A.I. Protein enrichment of defatted soybean flour by fine milling and electrostatic separation. Innov. Food Sci. Emerg. Technol. 2018, 50, 42–49. [Google Scholar] [CrossRef]
- Wang, J.; de Wit, M.; Schutyser, M.A.I.; Boom, R.M. Analysis of electrostatic powder charging for fractionation of foods. Innov. Food Sci. Emerg. Technol. 2014, 26, 360–365. [Google Scholar] [CrossRef]
- Wang, J.; Suo, G.; de Wit, M.; Boom, R.M.; Schutyser, M.A.I. Dietary fibre enrichment from defatted rice bran by dry fractionation. J. Food Eng. 2016, 186, 50–57. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Jafari, M.; Rajabzadeh, A.R.; Legge, R.L. Development and optimization of a triboelectrification bioseparation process for dry fractionation of legume flours. Sep. Purif. Technol. 2016, 163, 48–58. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Jafari, M.; Rajabzadeh, A.R.; Legge, R.L. Solvent-free production of protein-enriched fractions from navy bean flour using a triboelectrification-based approach. J. Food Eng. 2016, 174, 21–28. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, F.; Wang, L.; Li, Y.; Wang, R.; Chen, Z. Tribocharging properties of wheat bran fragments in air–solid pipe flow. Food Res. Int. 2014, 62, 262–271. [Google Scholar] [CrossRef]
- Landauer, J.; Aigner, F.; Kuhn, M.; Foerst, P. Effect of particle-wall interaction on triboelectric separation of fine particles in a turbulent flow. Adv. Powder Technol. 2019, 30, 1099–1107. [Google Scholar] [CrossRef]
- Landauer, J.; Foerst, P. Triboelectric separation of a starch-protein mixture—Impact of electric field strength and flow rate. Adv. Powder Technol. 2018, 29, 117–123. [Google Scholar] [CrossRef]
- Landauer, J.; Tauwald, S.M.; Foerst, P. A Simple μ-PTV Setup to Estimate Single-Particle Charge of Triboelectrically Charged Particles. Front. Chem. 2019, 7, 1. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landauer, J.; Foerst, P. Influence of Particle Charge and Size Distribution on Triboelectric Separation—New Evidence Revealed by In Situ Particle Size Measurements. Processes 2019, 7, 381. https://doi.org/10.3390/pr7060381
Landauer J, Foerst P. Influence of Particle Charge and Size Distribution on Triboelectric Separation—New Evidence Revealed by In Situ Particle Size Measurements. Processes. 2019; 7(6):381. https://doi.org/10.3390/pr7060381
Chicago/Turabian StyleLandauer, Johann, and Petra Foerst. 2019. "Influence of Particle Charge and Size Distribution on Triboelectric Separation—New Evidence Revealed by In Situ Particle Size Measurements" Processes 7, no. 6: 381. https://doi.org/10.3390/pr7060381
APA StyleLandauer, J., & Foerst, P. (2019). Influence of Particle Charge and Size Distribution on Triboelectric Separation—New Evidence Revealed by In Situ Particle Size Measurements. Processes, 7(6), 381. https://doi.org/10.3390/pr7060381