Optimization of Ultrasound-Assisted Extraction on Antioxidative Activity of Malus toringoides Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. High-Performance Liquid Chromatography (HPLC) Analysis of M.toringoides
2.4. Protection Rate of HUVECs
2.5. Single Factor Experiments and Response Surface Design
2.6. Extraction Method
2.7. Experimental Design of Response Surface Methods
3. Results and Discussion
3.1. The HPLC Analysis of M.toringoides Extracts
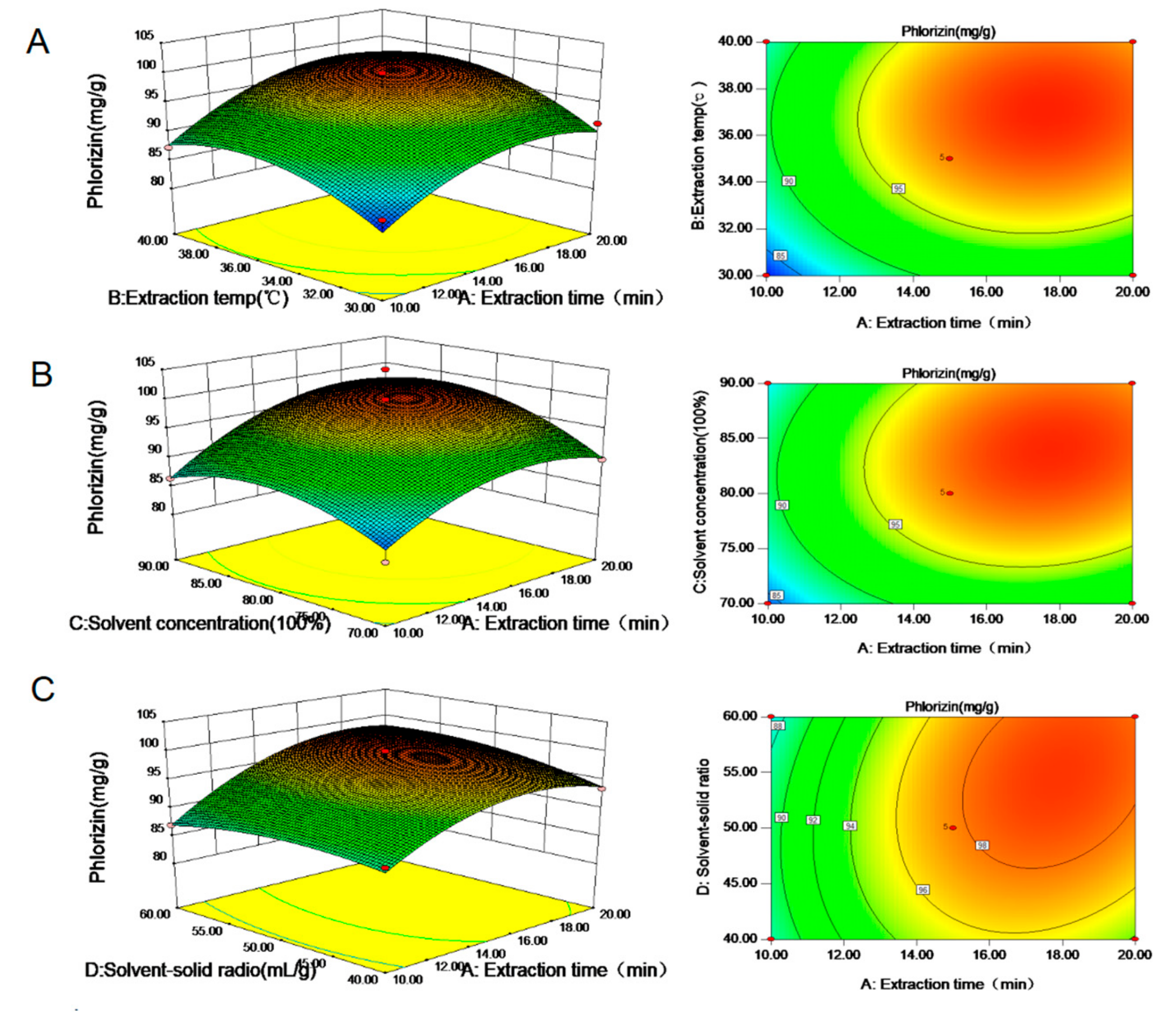
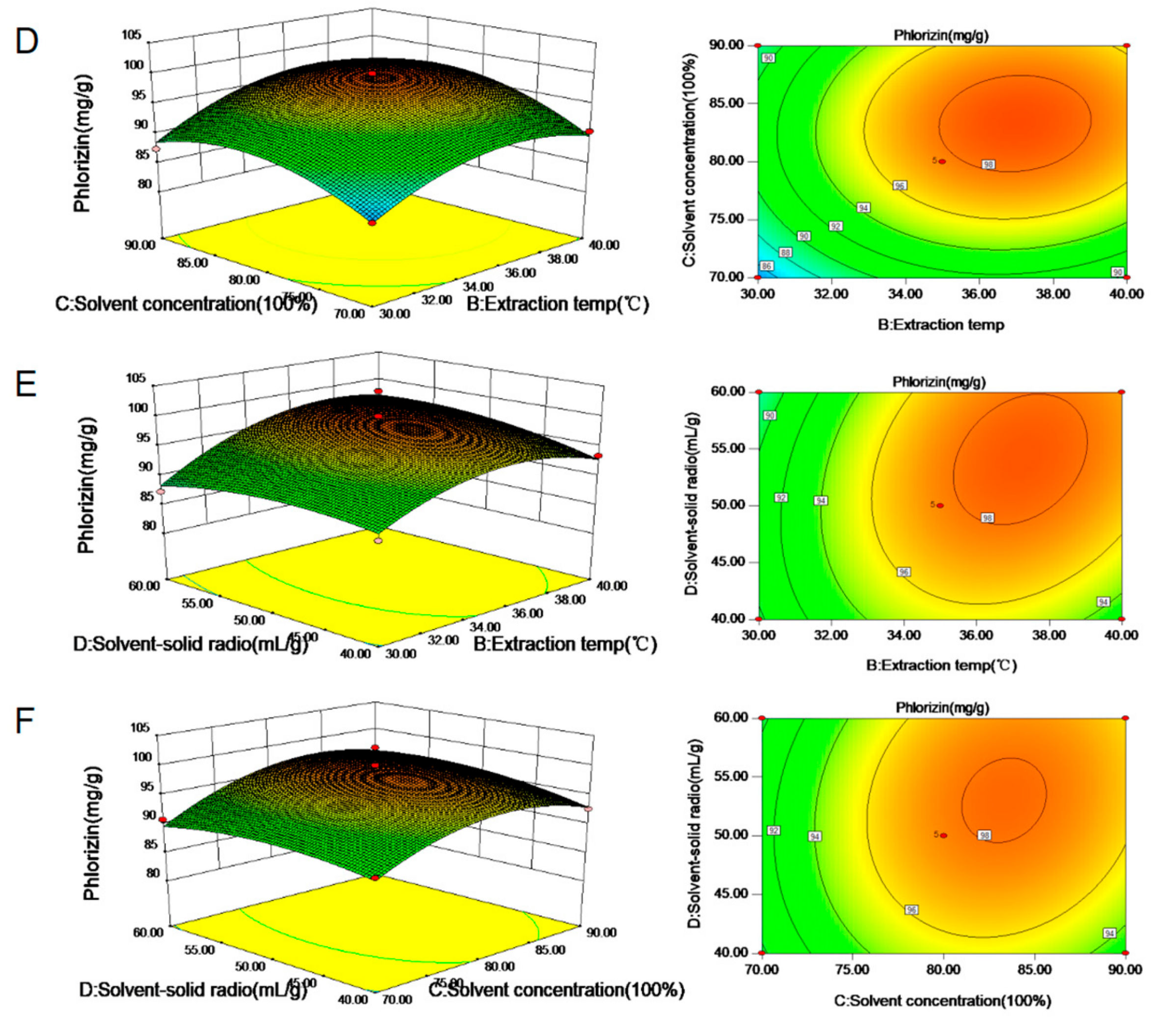
3.2. Analysis of Response Surface Model
3.3. Effects of Extraction Conditions on Phlorizin Extracted from M.toringoides
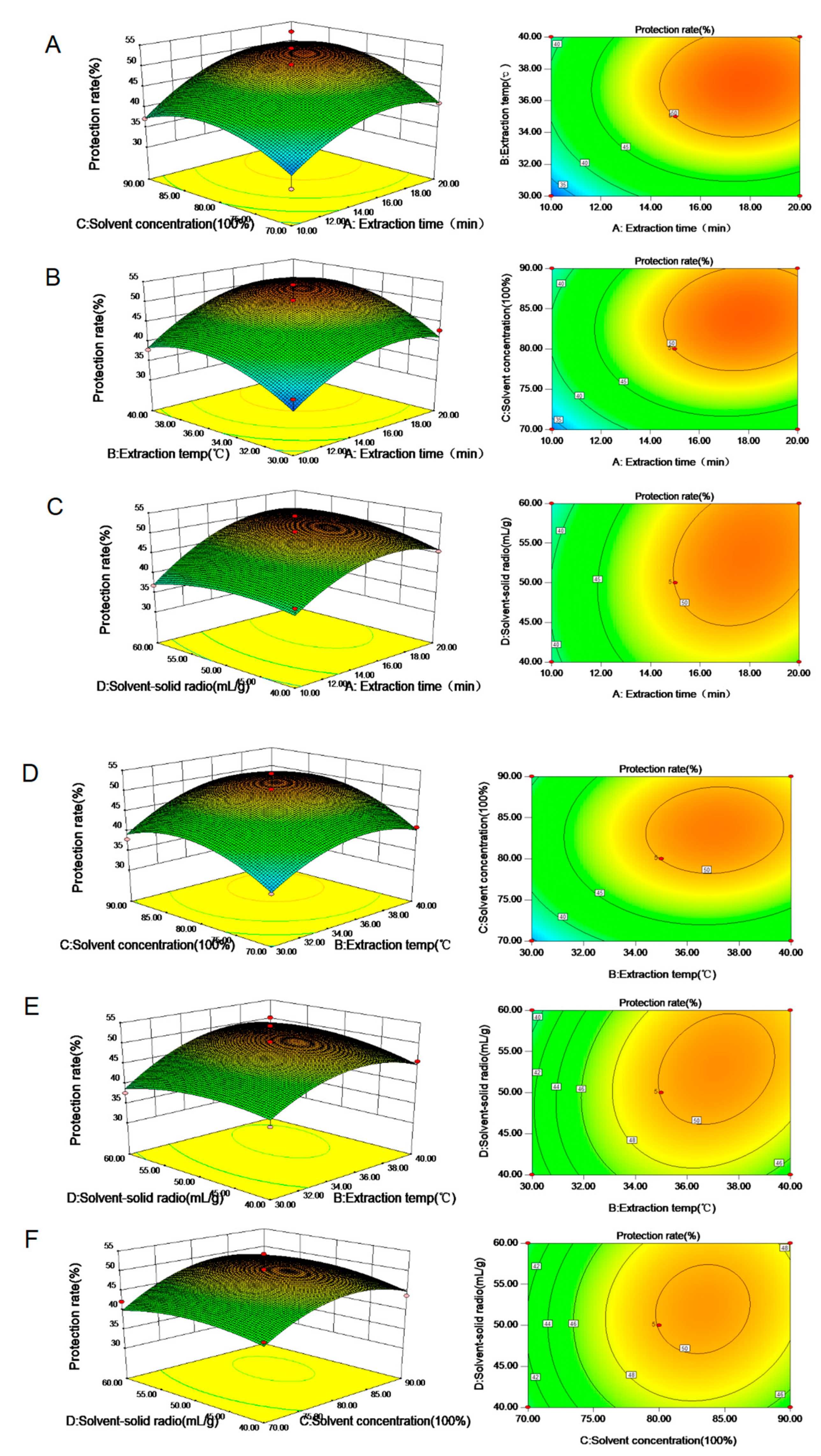
3.4. Determination of HUVEC Protection
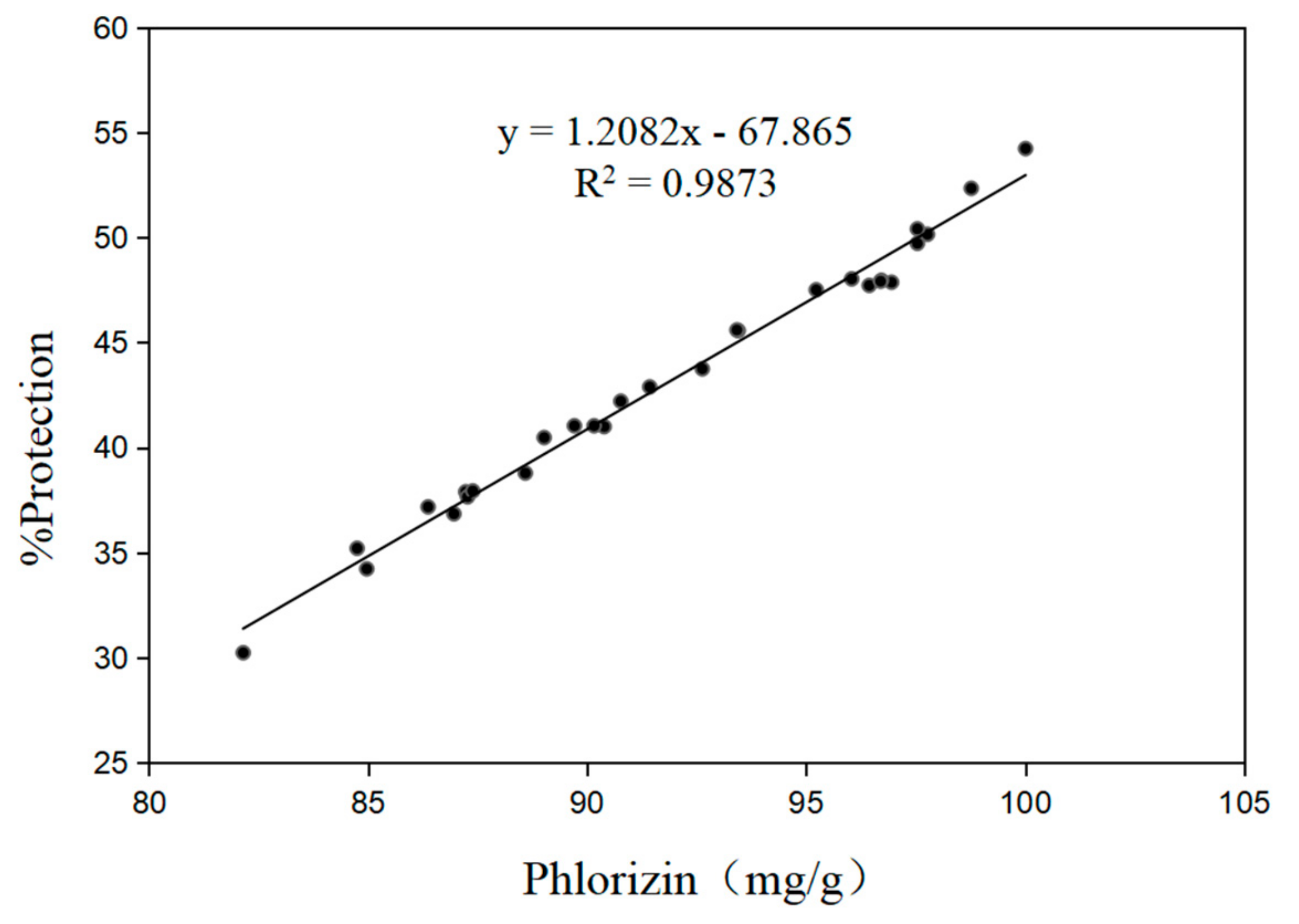
3.5. Analysis of the Relationship between Protection Rate and Percentage of Phlorizin
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Huang, S.; Liu, H.F.; Meng, N.; Li, B.; Wang, J.L. Hypolipidemic and Antioxidant Effects of Malus toringoides (Rehd.) Hughes Leaves in High-Fat-Diet-Induced Hyperlipidemic Rats. J. Med. Food 2017, 20, 258–264. [Google Scholar] [CrossRef]
- Li, D.; Peng, C.; Xie, X.F. Antidiabetic effect of flavonoids from Malus toringoides (Rehd.) Hughes leaves in diabetic mice and rats. J. Ethnopharmacol. 2014, 153, 561–567. [Google Scholar] [CrossRef]
- Wen, X.; Tian, T.; Shen, Y.; Zhang, T.; Diao, Z.W.; Zhang, Z.Q. Optimization of Ultrasonic-assisted Extraction and Antioxidant Activity of Flavonoids from Malus toringoides. Nat. Prod. Res. Dev. 2016, 28, 452–456. [Google Scholar]
- Arvindekar, A.U.; Laddha, K.S. An efficient microwave-assisted extraction of anthraquinones from Rheum emodi: Optimisation using RSM, UV and HPLC analysis and antioxidant studies. Ind. Crops Prod. 2016, 83, 587–595. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, X.; Xiang, C.R.; Liu, J.; Zhou, L.J.; Li, T.; Yang, Z.S.; Ding, C.B. Comparative evaluation of maceration and ultrasonic-assisted extraction of phenolic compounds from fresh olives. Ultrason. Sonochem. 2017, 37, 328–334. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Neves, V.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers infusions and decoctions: A comparison with green tea (Camellia sinensis). Food Chem. 2016, 200, 322–329. [Google Scholar] [CrossRef]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Valentina, R.N.; Kelvin, C. Optimisation of Pueraria isoflavonoids by response surface methodology using ultrasonic-assisted extraction. Food Chem. 2017, 231, 231–237. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, L.Z.; Zhao, L.; Shu, G.; Yuan, Z.X.; Fu, H.L.; Lv, C.; Lin, J.-C. Optimization of the ultrasound-assisted extraction of antioxidant phloridzin from Lithocarpus polystachyus Rehd. using response surface methodology. J. Sep. Sci. 2017, 10, 4329–4337. [Google Scholar] [CrossRef]
- Mehmet, H.; Elif, M.I. Optimization of ultrasound-assisted antioxidant compounds extraction from germinated chickpea using response surface methodology. LWT-Food Sci. Technol. 2017, 77, 208–216. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef]
- Florina, D.; Mircea, O. Optimization of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melongena L.) peel. Ultrason. Sonochem. 2016, 31, 637–646. [Google Scholar] [CrossRef]
- Xu, D.P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef]
- Tarun, B.; Praveen, D.; Indra, D.; Bhatt Ranbeer, S.R.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef]
- Ameer, K.; Bae, S.W.; Jo, Y.; Lee, H.G.; Ameer, A.; Kwon, J.H. Optimization of microwave-assisted extraction of total extract, stevioside and rebaudioside-A from Stevia rebaudiana (Bertoni) leaves, using response surface methodology (RSM) and artificial neural network (ANN) modelling. Food Chem. 2017, 229, 198–207. [Google Scholar] [CrossRef]
- Chemat, F.; Tomao, V.; Virot, M. Towards the industrial production of antioxidants from food processing by-products with ultrasound-assisted extraction. Ultrason. Sonochem. 2010, 17, 1066–1074. [Google Scholar]
- Chemat, F.; Zille, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2016, 18, 813–835. [Google Scholar] [CrossRef]
- Chen, F.; Sun, Y.; Zhao, G.; Liao, X.; Hu, X.; Wu, J. Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatographyemass spectrometry. Ultrason. Sonochem. 2016, 14, 767–778. [Google Scholar] [CrossRef]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguieres, A. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef]
- Hossain, M.B.; Brunton, N.P.; Patras, A.; Tiwari, B.; O’Donnell, C.P.; Martin-Diana, A.B. Optimization of ultrasound assisted extraction ofantioxidant compounds from marjoram (Origanum majorana L.) using responsesurface methodology. Ultrason. Sonochem. 2012, 19, 582–590. [Google Scholar] [CrossRef]
- Liao, P.; Liu, Y.; Zhao, M.; Yang, Y.; Cui, X. The Development of Panax notoginseng Medicinal Liquor Processing Technology Using the Response Surface Method and a Study of its Antioxidant activity and Its Effects on Mouse Melanoma B16 Cells. Food Funct. 2017. [Google Scholar] [CrossRef]
- Norziah, M.H.; Amir, E.T.; Abidin, S.Z.; Mahmood, W.A.K.; Juliano, P. The effects of ultrasound assisted extraction on antioxidative activity of polyphenolics obtained from Momordica charantia fruit using response surface approach. Food Biosci. 2017, 17, 7–16. [Google Scholar] [CrossRef]
- Thiruvikraman, S.V.; Guha, A.; Roboz, J.; Taubman, M.B.; Nemerson, Y.; Fallon, J.T. In situ localization of tissue factor in human atherosclerotic plaques by binding of digoxigenin-labeled factors VIIa and X. Lab. Investig. 1996, 75, 451–461. [Google Scholar] [CrossRef]
- Wilcox, J.N.; Smith, K.M.; Schwartz, S.M.; Gordon, D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc. Natl. Acad. Sci. USA 1989, 86, 2839–2843. [Google Scholar] [CrossRef]
- Westrick, R.J.; Bodary, P.F.; Xu, Z.; Shen, Y.C.; Broze, G.J.; Eitzman, D.T. Deficiency of tissue factor pathway inhibitor promotes atherosclerosis and thrombosis in mice. Cell. Physiol. Biochem. 2015, 28, 765–783. [Google Scholar] [CrossRef]
- Kam, A.; Li, K.M.; Razmovski-Naumovski, V.; Nammi, S.; Chan, K.; Li, G.Q. Combination of TNF—A, homocyteine and afenosine exacerbated cytotoxicity in human cardiovascular and cerebrovascular endothelial cells. Cell. Physiol. Biochem. 2012, 30, 805–814. [Google Scholar]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabetes Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef]
- Wong, K.H.; Razmovski-Naumovski, V.; Li, K.M.; Li, G.Q.; Chan, K. Differentiation of Pueraria lobata and Pueraria thomsonii using partial least square discriminant analysis (PLS-DA). J. Pharm. Biomed. Anal. 2013, 84, 5–13. [Google Scholar] [CrossRef]
- Wong, K.H.; Razmovski-Naumovski, V.; Li, K.M.; Li, G.Q.; Chan, K. Differentiating Puerariae Lobatae Radix and Puerariae Thomsonii Radix using HPTLC coupled with multivariate classification analyses. J. Pharm. Biomed. Anal. 2014, 95, 11–19. [Google Scholar] [CrossRef]
- Wong, K.H.; Razmovski-Naumovski, V.; Li, K.M.; Li, G.Q.; Chan, K. Comparing morphological, chemical and anti-diabetic characteristics of Puerariae lobatae Radix and Puerariae thomsonii Radix. J. Ethnopharmacol. 2015, 164, 53–63. [Google Scholar]
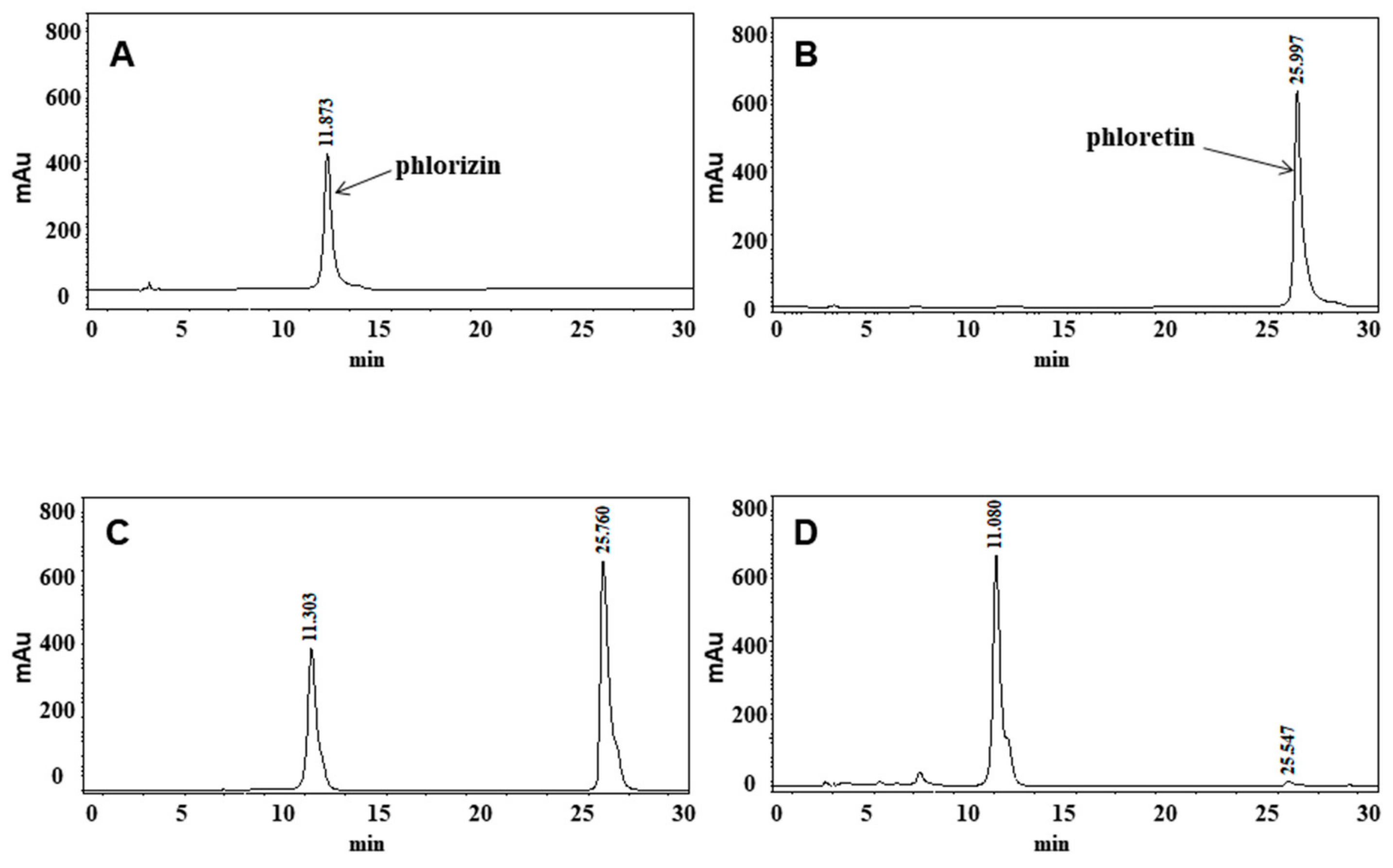
| Independent Variable | Factor Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1: Extraction time (min) | 10 | 15 | 20 |
| X2: Extraction Temp (°C) | 25 | 30 | 35 |
| X3: Solvent Concentration (100%) | 70 | 80 | 90 |
| X4: Solvent-Solid Ratio (mL/g) | 40 | 50 | 60 |
| Run | X1: Extraction Time (min) | X2: Extraction Temp (°C) | X3: Solvent Concentration | X4: Solvent-Solid Ratio (mL/g) | Y1: Phlorizin (mg/g) | Y2: Protection Rate (%) |
|---|---|---|---|---|---|---|
| 1 | 15 | 35 | 70 | 60 | 90.77 | 42.21 |
| 2 | 20 | 35 | 70 | 50 | 89.71 | 41.04 |
| 3 | 15 | 35 | 90 | 60 | 96.44 | 47.72 |
| 4 | 15 | 35 | 80 | 50 | 100.01 | 54.23 |
| 5 | 20 | 30 | 80 | 50 | 91.43 | 42.89 |
| 6 | 10 | 35 | 90 | 50 | 86.37 | 37.18 |
| 7 | 10 | 40 | 80 | 50 | 87.23 | 37.90 |
| 8 | 20 | 35 | 80 | 60 | 96.95 | 47.87 |
| 9 | 15 | 40 | 90 | 50 | 95.23 | 47.51 |
| 10 | 15 | 40 | 70 | 50 | 90.39 | 41.00 |
| 11 | 15 | 30 | 80 | 40 | 88.59 | 38.79 |
| 12 | 15 | 35 | 80 | 50 | 96.72 | 47.96 |
| 13 | 10 | 35 | 70 | 50 | 82.15 | 30.24 |
| 14 | 15 | 35 | 90 | 40 | 92.63 | 43.75 |
| 15 | 10 | 35 | 80 | 60 | 86.96 | 36.85 |
| 16 | 15 | 30 | 80 | 60 | 87.27 | 37.66 |
| 17 | 10 | 30 | 80 | 50 | 84.75 | 35.21 |
| 18 | 15 | 30 | 90 | 50 | 87.39 | 37.94 |
| 19 | 15 | 30 | 70 | 50 | 84.97 | 34.23 |
| 20 | 15 | 35 | 70 | 40 | 90.16 | 41.04 |
| 21 | 20 | 35 | 80 | 40 | 93.45 | 45.57 |
| 22 | 15 | 40 | 80 | 60 | 97.77 | 50.15 |
| 23 | 15 | 35 | 80 | 50 | 97.54 | 49.72 |
| 24 | 15 | 35 | 80 | 50 | 96.70 | 47.92 |
| 25 | 10 | 35 | 80 | 40 | 89.02 | 40.48 |
| 26 | 15 | 40 | 80 | 40 | 93.42 | 45.60 |
| 27 | 15 | 35 | 80 | 50 | 97.54 | 50.41 |
| 28 | 20 | 40 | 80 | 50 | 96.04 | 48.03 |
| 29 | 20 | 35 | 90 | 50 | 98.77 | 52.34 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 651.06 | 14 | 46.5 | 22.05 | <0.0001 | significant |
| X1-Extraction time (min) | 207.29 | 1 | 207.29 | 98.3 | <0.0001 | |
| X2-Extraction temp (°C) | 106.1 | 1 | 106.1 | 50.32 | <0.0001 | |
| X3-solvent concentration (100%) | 68.53 | 1 | 68.53 | 32.5 | <0.0001 | |
| X4-Solvent-solid ratio (mL/g) | 6.59 | 1 | 6.59 | 3.13 | 0.0988 | |
| X1X2 | 1.15 | 1 | 1.15 | 0.54 | 0.4732 | |
| X1X3 | 5.87 | 1 | 5.87 | 2.78 | 0.1176 | |
| X1X4 | 7.75 | 1 | 7.75 | 3.68 | 0.0758 | |
| X2X3 | 1.45 | 1 | 1.45 | 0.69 | 0.4209 | |
| X2X4 | 8.02 | 1 | 8.02 | 3.81 | 0.0714 | |
| X3X4 | 2.56 | 1 | 2.56 | 1.21 | 0.2893 | |
| X12 | 116.8 | 1 | 116.8 | 55.39 | <0.0001 | |
| X22 | 105.59 | 1 | 105.59 | 50.07 | <0.0001 | |
| X32 | 102.37 | 1 | 102.37 | 48.55 | <0.0001 | |
| X42 | 17.97 | 1 | 17.97 | 8.52 | 0.0112 | |
| Residual | 29.52 | 14 | 2.11 | |||
| Lack of Fit | 22.21 | 10 | 2.22 | 1.22 | 0.4603 | not significant |
| Pure Error | 7.31 | 4 | 1.83 | |||
| Cor Total | 680.58 | 28 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value, Prob > F | |
|---|---|---|---|---|---|---|
| Model | 924.53 | 14 | 66.04 | 11.33 | <0.0001 | significant |
| X1: Extraction time (min) | 298.8 | 1 | 298.8 | 51.28 | <0.0001 | |
| X2: Extraction temp (℃) | 157.47 | 1 | 157.47 | 27.02 | 0.0001 | |
| X3: Solvent concentration (100%) | 112.12 | 1 | 112.12 | 19.24 | 0.0006 | |
| X4: Solvent-solid ratio (mL/g) | 4.36 | 1 | 4.36 | 0.75 | 0.4018 | |
| X1X2 | 1.5 | 1 | 1.5 | 0.26 | 0.6197 | |
| X1X3 | 4.75 | 1 | 4.75 | 0.82 | 0.3818 | |
| X1X4 | 8.79 | 1 | 8.79 | 1.51 | 0.2396 | |
| X2X3 | 1.96 | 1 | 1.96 | 0.34 | 0.5712 | |
| X2X4 | 8.07 | 1 | 8.07 | 1.38 | 0.259 | |
| X3X4 | 1.96 | 1 | 1.96 | 0.34 | 0.5712 | |
| X12 | 154.09 | 1 | 154.09 | 26.44 | 0.0001 | |
| X22 | 143.91 | 1 | 143.91 | 24.7 | 0.0002 | |
| X32 | 149.39 | 1 | 149.39 | 25.64 | 0.0002 | |
| X42 | 28.95 | 1 | 28.95 | 4.97 | 0.0427 | |
| Residual | 81.58 | 14 | 5.83 | |||
| Lack of Fit | 54.96 | 10 | 5.5 | 0.83 | 0.6347 | not significant |
| Pure Error | 26.62 | 4 | 6.65 | |||
| Cor Total | 1006.11 | 28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Q.; Guo, L.; Liu, H.; Liu, Y.; Zhang, C.; Peng, C.; Liu, Z.; Huang, S.; Li, B. Optimization of Ultrasound-Assisted Extraction on Antioxidative Activity of Malus toringoides Using Response Surface Methodology. Processes 2019, 7, 270. https://doi.org/10.3390/pr7050270
Ye Q, Guo L, Liu H, Liu Y, Zhang C, Peng C, Liu Z, Huang S, Li B. Optimization of Ultrasound-Assisted Extraction on Antioxidative Activity of Malus toringoides Using Response Surface Methodology. Processes. 2019; 7(5):270. https://doi.org/10.3390/pr7050270
Chicago/Turabian StyleYe, Qiang, Li Guo, Hongmei Liu, Yushi Liu, Cunyan Zhang, Cheng Peng, Zhiming Liu, Shan Huang, and Bin Li. 2019. "Optimization of Ultrasound-Assisted Extraction on Antioxidative Activity of Malus toringoides Using Response Surface Methodology" Processes 7, no. 5: 270. https://doi.org/10.3390/pr7050270
APA StyleYe, Q., Guo, L., Liu, H., Liu, Y., Zhang, C., Peng, C., Liu, Z., Huang, S., & Li, B. (2019). Optimization of Ultrasound-Assisted Extraction on Antioxidative Activity of Malus toringoides Using Response Surface Methodology. Processes, 7(5), 270. https://doi.org/10.3390/pr7050270





