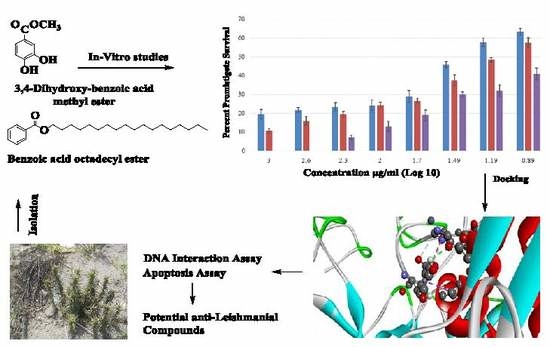
Benzoic Acid Derivatives of Ifloga spicata (Forssk.) Sch.Bip. as Potential Anti-Leishmanial against Leishmania tropica
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental
2.2. Plant Material, Extraction, and Fractionation
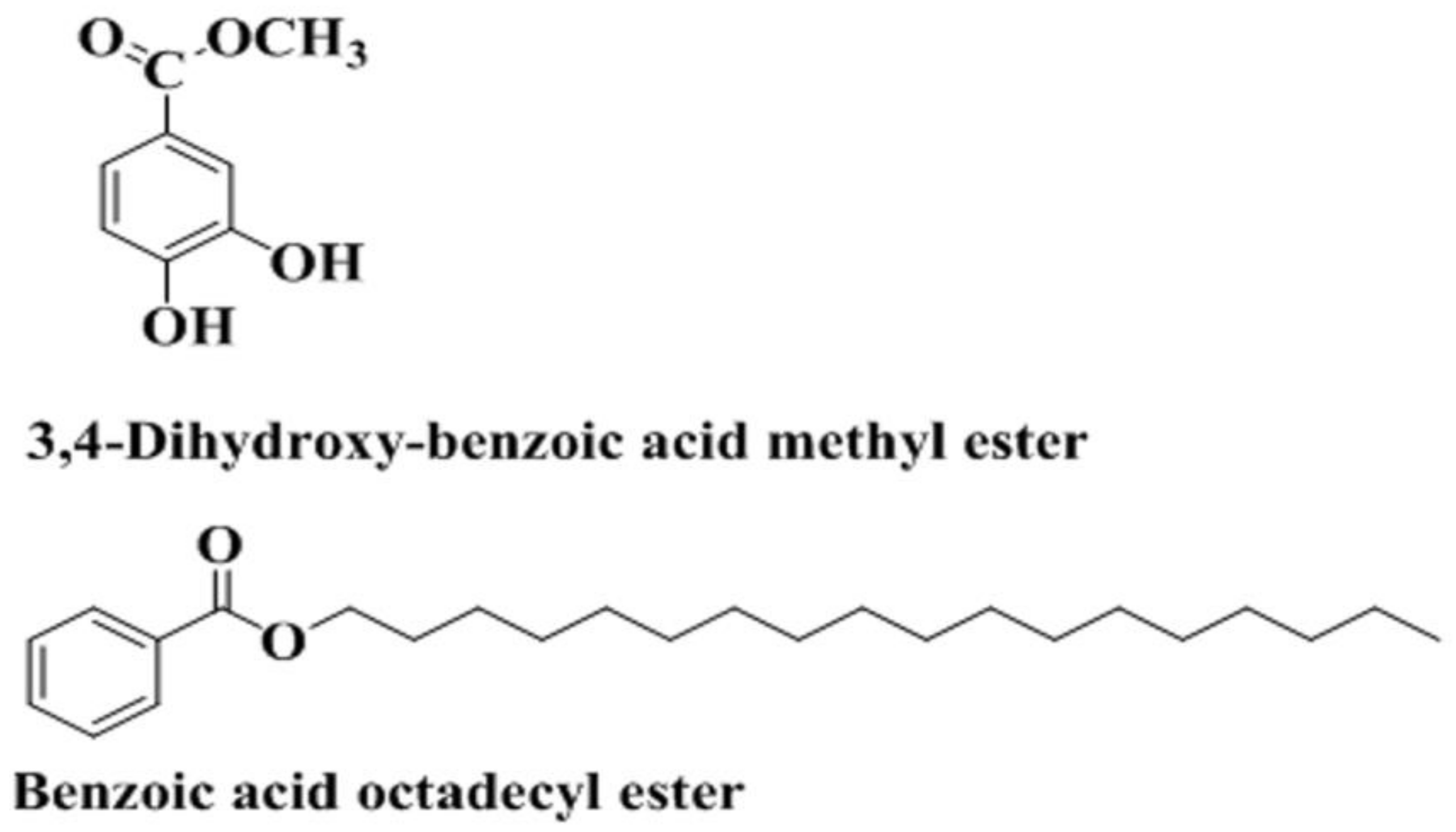
2.3. Bioguided Isolation and Characterization
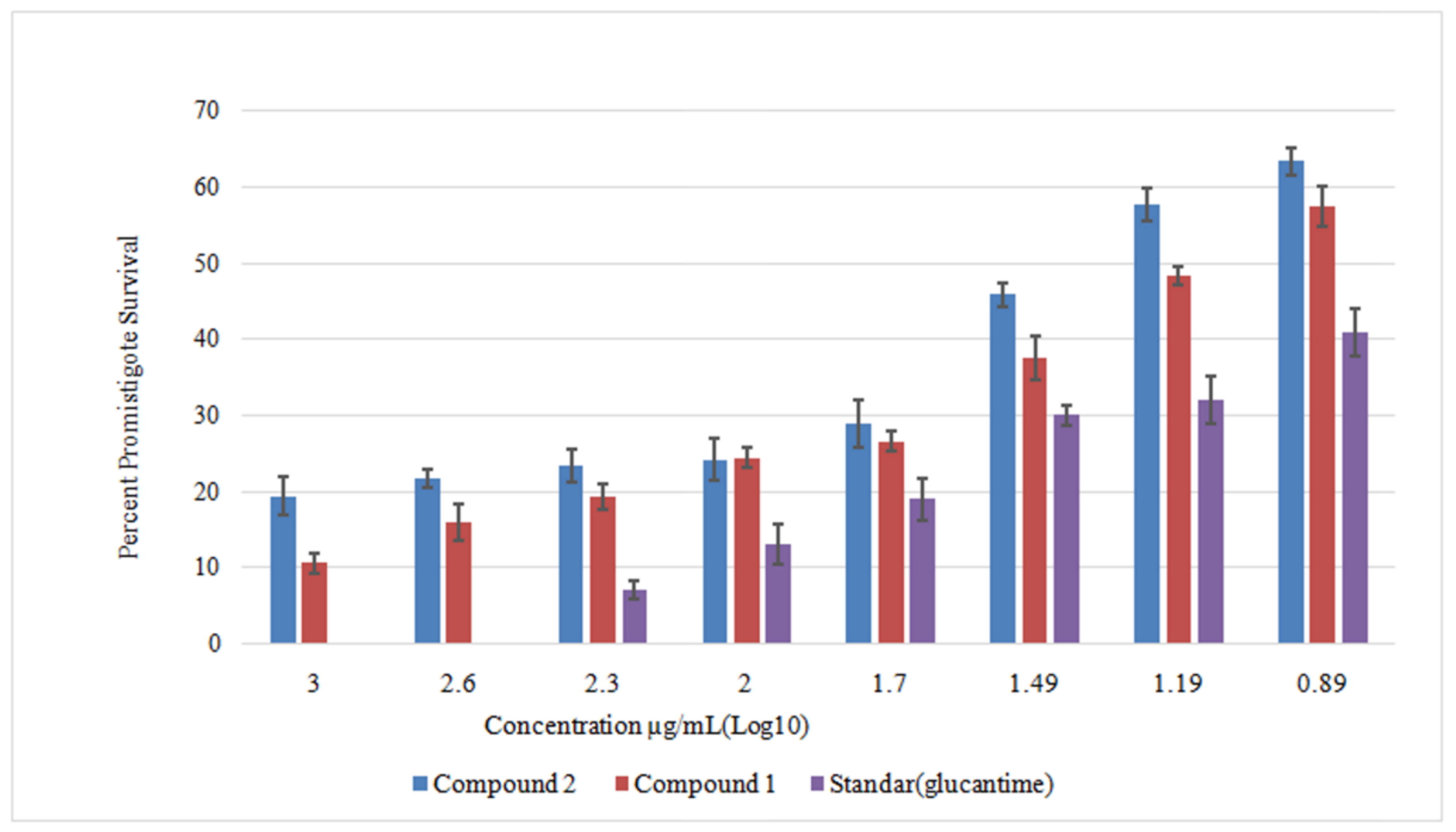
2.4. Anti-Leishmanial Activity against Leishmania Promastigotes
2.5. Molecular Docking of Leishmanolysin (gp63)
2.6. Mechanistic Anti-Leishmanial Studies
2.6.1. SYTOX Assay
2.6.2. DNA Extraction
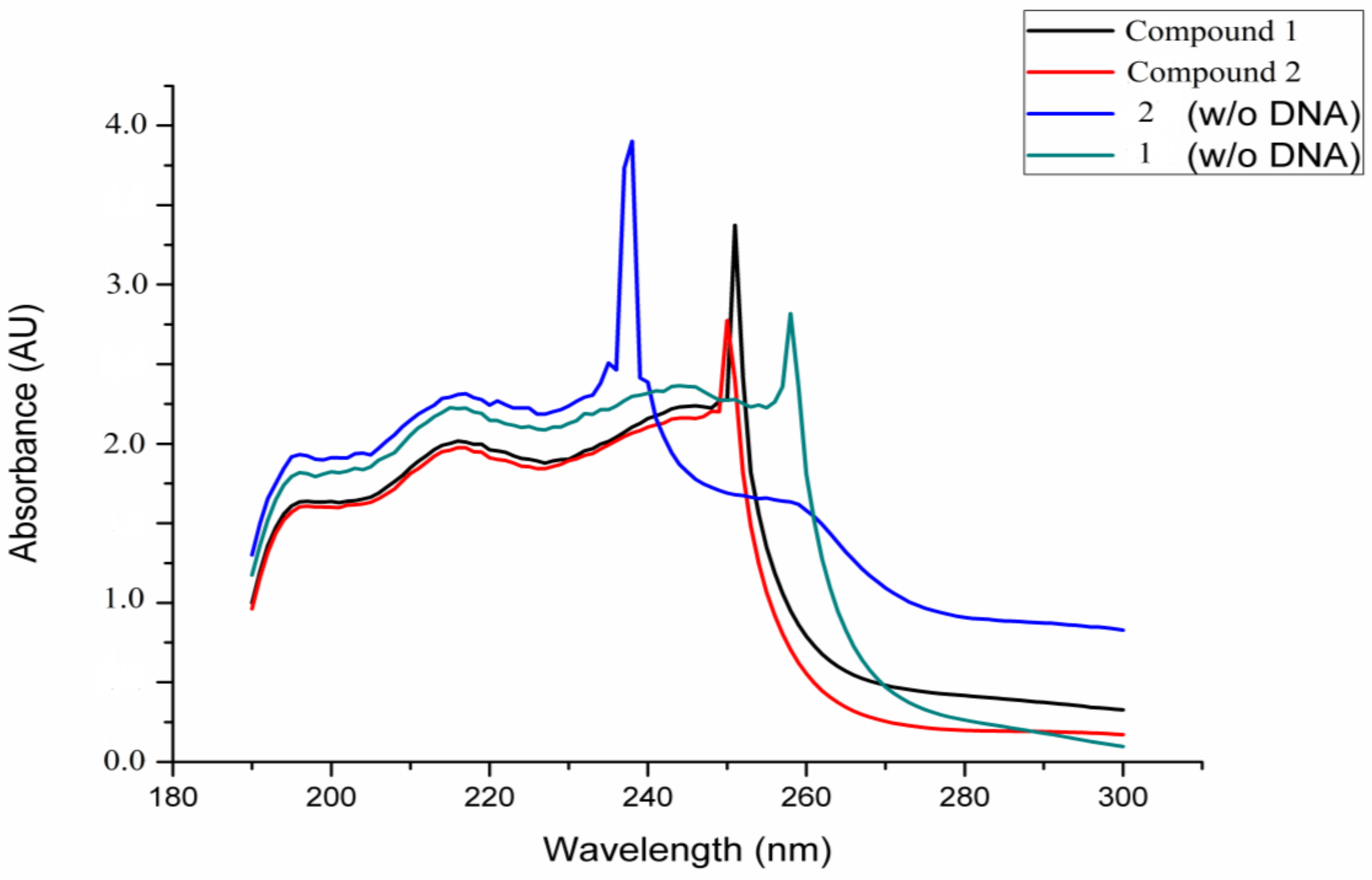
2.6.3. DNA Interaction Assay
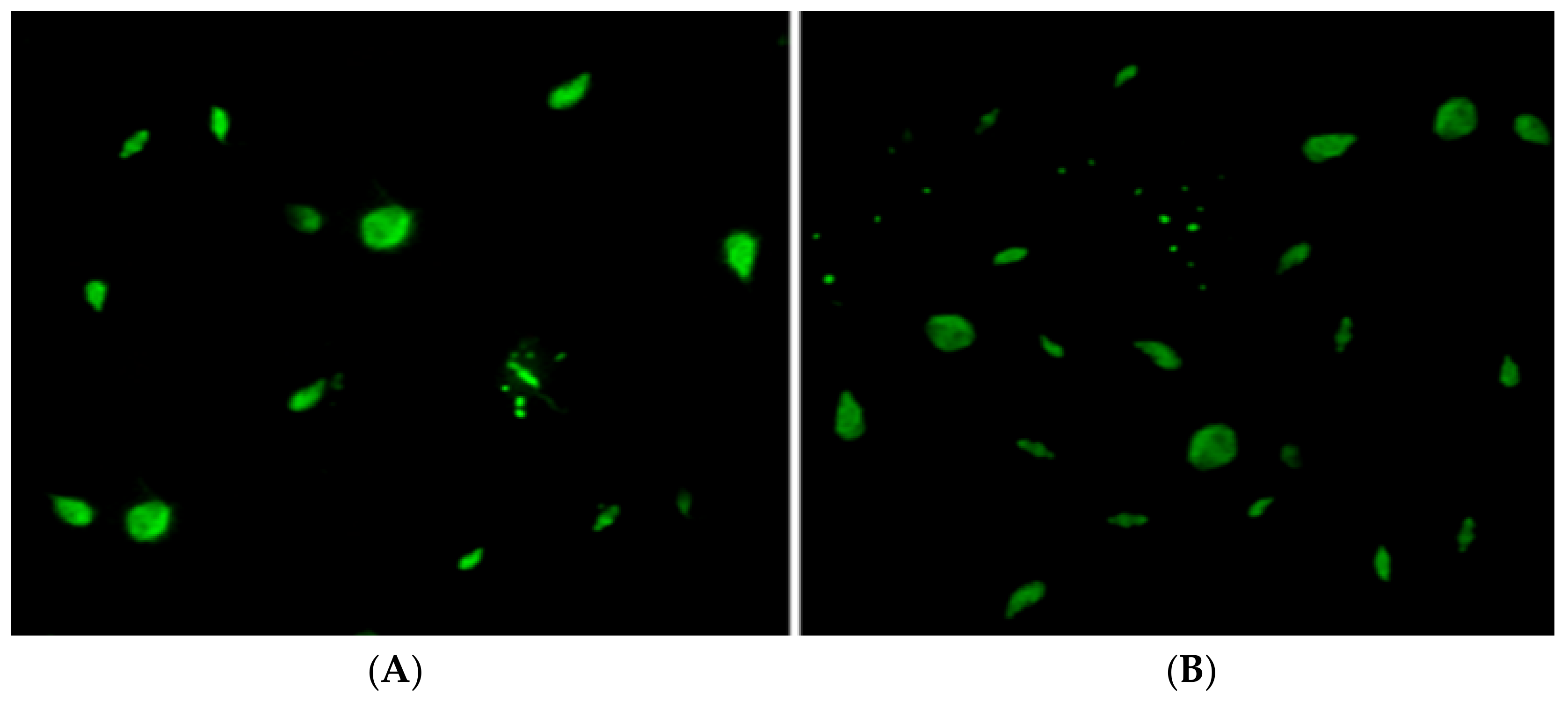
2.6.4. Apoptosis Analysis Studies
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oliveira, F.; Jochim, R.C.; Valenzuela, J.G.; Kamhawi, S. Sand flies, Leishmania, and transcriptome-borne solutions. Parasitol. Int. 2009, 58, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Feasey, N.; Wansbrough-Jones, M.; Mabey, D.C.; Solomon, A.W. Neglected tropical diseases. Br. Med. Bull. 2009, 93, 179–200. [Google Scholar] [CrossRef] [Green Version]
- Reithinger, R.; Dujardin, J.-C.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef]
- Santos, D.; Silva, I.B.; Paz, D.; Ribeiro, F.A.C.; Garcia, C.D.M.; Teixeira, A.K.; Maugéri, D.; Katz, L.M.; Barbiéri, S.; Lúcia, C. Leishmanicidal and Immunomodulatory Activities of the Palladacycle Complex DPPE 1.1, a Potential Candidate for Treatment of Cutaneous Leishmaniasis. Front. Microbiol. 2018, 9, 1427. [Google Scholar] [CrossRef]
- Sharma, U.; Singh, S. Immunobiology of leishmaniasis. Indian J. Exp. Biol. 2009, 47, 412–423. [Google Scholar] [PubMed]
- Shah, N.A.; Khan, M.R.; Nadhman, A. Antileishmanial, toxicity, and phytochemical evaluation of medicinal plants collected from Pakistan. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Jamal, Q.; Shah, A.; Ali, N.; Ashraf, M.; Awan, M.M.; Lee, C.M. Prevalence and comparative analysis of cutaneous leishmaniasis in Dargai Region in Pakistan. Pak. J Zool. 2013, 45, 537–541. [Google Scholar]
- Ullah, N.; Nadhman, A.; Siddiq, S.; Mehwish, S.; Islam, A.; Jafri, L.; Hamayun, M. Plants as antileishmanial agents: Current scenario. Phytother. Res. 2016, 30, 1905–1925. [Google Scholar] [CrossRef] [PubMed]
- De Sarkar, S.; Sarkar, D.; Sarkar, A.; Dighal, A.; Staniek, K.; Gille, L.; Chatterjee, M. Berberine chloride mediates its antileishmanial activity by inhibiting Leishmania mitochondria. Parasitol. Res. 2019, 118, 335–345. [Google Scholar] [CrossRef]
- Ahmad, S.; Ullah, F.; Sadiq, A.; Ayaz, M.; Imran, M.; Ali, I.; Zeb, A.; Ullah, F.; Shah, M.R. Chemical composition, antioxidant and anticholinesterase potentials of essential oil of Rumex hastatus D. Don collected from the North West of Pakistan. BMC Complement. Altern. Med. 2016, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.; Ayaz, M.; Khan, A.-U.; Ullah, F.; Farhan; Shah, A.-U.-H.A.; Iqbal, H.; Hussain, S. 1,1-Diphenyl, 2-picrylhydrazyl free radical scavenging, bactericidal, fungicidal and leishmanicidal properties of Teucrium stocksianum. Toxicol. Ind. Health 2015, 31, 1037–1043. [Google Scholar]
- Domingues Passero, L.F.; Laurenti, M.D.; Santos-Gomes, G.; Soares Campos, B.L.; Sartorelli, P.; Lago, G.; Henrique, J. Plants used in traditional medicine: Extracts and secondary metabolites exhibiting antileishmanial activity. Curr. Clin. Pharmacol. 2014, 9, 187–204. [Google Scholar] [CrossRef]
- Hazra, S.; Ghosh, S.; Hazra, B. Phytochemicals With Antileishmanial Activity: Prospective Drug Targets. In Studies in Natural Products Chemistry; Elsevier: New York, NY, USA, 2017; Volume 52, pp. 303–336. [Google Scholar]
- Cabanillas, B.J.; Le Lamer, A.-C.; Castillo, D.; Arevalo, J.; Estevez, Y.; Rojas, R.; Valadeau, C.; Bourdy, G.; Sauvain, M.; Fabre, N. Dihydrochalcones and benzoic acid derivatives from Piper dennisii. Planta Med. 2012, 78, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.M.; Elokely, K.M.; Atef, A.; Mohammad, A.E.I.; Jacob, M.; Cutler, S.J.; Doerksen, R.J.; Ross, S.A. Isolation and characterization of new secondary metabolites from Asphodelus microcarpus. Med. Chem. Res. 2014, 23, 3510–3515. [Google Scholar] [CrossRef] [Green Version]
- Flores, N.; Jiménez, I.A.; Giménez, A.; Ruiz, G.; Gutiérrez, D.; Bourdy, G.; Bazzocchi, I.L. Benzoic acid derivatives from Piper species and their antiparasitic activity. J. Nat. Prod. 2008, 71, 1538–1543. [Google Scholar] [CrossRef]
- Verma, R.K.; Prajapati, V.K.; Verma, G.K.; Chakraborty, D.; Sundar, S.; Rai, M.; Dubey, V.K.; Singh, M.S. Molecular docking and in vitro antileishmanial evaluation of chromene-2-thione analogues. ACS Med. Chem. Lett. 2012, 3, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Lieke, T.; Nylen, S.; Eidsmo, L.; McMaster, W.; Mohammadi, A.; Khamesipour, A.; Berg, L.; Akuffo, H. Leishmania surface protein gp63 binds directly to human natural killer cells and inhibits proliferation. Clin. Exp. Immunol. 2008, 153, 221–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadhman, A.; Nazir, S.; Khan, M.I.; Arooj, S.; Bakhtiar, M.; Shahnaz, G.; Yasinzai, M. PEGylated silver doped zinc oxide nanoparticles as novel photosensitizers for photodynamic therapy against Leishmania. Free Radic. Biol. Med. 2014, 77, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Nadhman, A.; Sirajuddin, M.; Nazir, S.; Yasinzai, M. Photo-inducedLeishmaniaDNA degradation by silver-doped zinc oxide nanoparticle: Anin-vitroapproach. IET Nanobiotech. 2016, 10, 129–133. [Google Scholar] [CrossRef]
- Lee, N.; Bertholet, S.; Debrabant, A.; Muller, J.; Duncan, R.; Nakhasi, H. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ. 2002, 9, 53. [Google Scholar] [CrossRef]
- Wanderley, J.L.M.; Barcinski, M.A. Apoptosis and apoptotic mimicry: The Leishmania connection. Cell. Mol. Life Sci. 2010, 67, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.; Loiseau, P.M.; Bories, C.; Bréard, J. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2004, 48, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowski, S.; Sajid, M.; Reece, S.E. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasit. Vect. 2011, 4, 44. [Google Scholar] [CrossRef]
- Abouri, M.; El Mousadik, A.; Msanda, F.; Boubaker, H.; Saadi, B.; Cherifi, K. An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. Int. J. Med. Plants Res. 2012, 1, 99–123. [Google Scholar]
- Osman, A.K.; Al-Ghamdi, F.; Bawadekji, A. Floristic diversity and vegetation analysis of Wadi Arar: A typical desert Wadi of the Northern Border region of Saudi Arabia. Saudi J. Biol. Sci. 2014, 21, 554–565. [Google Scholar] [CrossRef] [Green Version]
- Hammiche, V.; Maiza, K. Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367. [Google Scholar] [CrossRef]
- García, M.; Perera, W.H.; Scull, R.; Monzote, L. Antileishmanial assessment of leaf extracts from Pluchea carolinensis, Pluchea odorata and Pluchea rosea. Asian Pac. J. Trop. Med. 2011, 4, 836–840. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.M.; Ullah, F.; Hussain, S.; Khan, S.B.; Asiri, A.M.; Ahmad, S.; Khan, H.; Farooq, U. A new trypsin inhibitory phthalic acid ester from Heliotropium strigosum. Med. Chem. Res. 2014, 23, 2712–2714. [Google Scholar] [CrossRef]
- Ovais, M.; Ayaz, M.; Khalil, A.T.; Shah, S.A.; Jan, M.S.; Raza, A.; Shahid, M.; Shinwari, Z.K. HPLC-DAD finger printing, antioxidant, cholinesterase, and α-glucosidase inhibitory potentials of a novel plant Olax nana. BMC Complement. Altern. Med. 2018, 18, 1. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Shahid, M.; Ahmad, W.; Ullah, I.; Ahmad, A.; Syed, N.i.H. GC-MS analysis and gastroprotective evaluations of crude extracts, isolated saponins, and essential oil from Polygonum hydropiper L. Front. Chem. 2017, 5, 58. [Google Scholar] [CrossRef]
- Zohra, T.; Ovais, M.; Khalil, A.T.; Qasim, M.; Ayaz, M.; Shinwari, Z.K. Extraction optimization, total phenolic, flavonoid contents, HPLC-DAD analysis and diverse pharmacological evaluations of Dysphania ambrosioides (L.) Mosyakin & Clemants. Nat. Prod. Res. 2019, 33, 136–142. [Google Scholar]
- Ali, M.; Muhammad, S.; Shah, M.R.; Khan, A.; Rashid, U.; Farooq, U.; Ullah, F.; Sadiq, A.; Ayaz, M.; Ali, M. Neurologically potent molecules from Crataegus oxyacantha; isolation, anticholinesterase inhibition, and molecular docking. Front. Pharmacol. 2017, 8, 327. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Wadood, A.; Junaid, M.; Ullah, F.; Khan, N.Z. Cytotoxicity and molecular docking studies on phytosterols isolated from Polygonum hydropiper L. Steroids 2019, 141, 30–35. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A. Anti-Alzheimer’s Studies on β-Sitosterol Isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Shinwari, Z.K.; Khalil, A.T. Investigation of the cytotoxic and antileishmanial effects of Fagonia indica L. extract and extract mediated silver nanoparticles (AgNPs). Pak. J. Bot. 2017, 49, 1561–1568. [Google Scholar]
- Khalid, W.; Badshah, A.; Khan, A.-u.; Nadeem, H.; Ahmed, S. Synthesis, characterization, molecular docking evaluation, antiplatelet and anticoagulant actions of 1, 2, 4 triazole hydrazone and sulphonamide novel derivatives. Chem. Cent. J. 2018, 12, 11. [Google Scholar] [CrossRef]
- Khan, H.; Nadhman, A.; Azam, S.S.; Anees, M.; Khan, I.; Ullah, I.; Sohail, M.F.; Shahnaz, G.; Yasinzai, M. In-vitro antileishmanial potential of peptide drug hirudin. Chem. Biol. Drug Des. 2017, 89, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.K.; Shaheen, U.; Asghar, F.; Badshah, A.; Nadhman, A.; Azam, S.; Ali, M.I.; Shahnaz, G.; Yasinzai, M. Antileishmanial, DNA Interaction, and Docking Studies of Some Ferrocene-Based Heteroleptic Pentavalent Antimonials. Arch. Pharm. 2016, 349, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Zia, N.; Ahmad, I.; Khalil, A.T.; Raza, A.; Ayaz, M.; Sadiq, A.; Ullah, F.; Shinwari, Z.K. Phyto-Therapeutic and Nanomedicinal Approach to Cure Alzheimer Disease: Present Status and Future Opportunities. Front. Aging Neurosci. 2018, 10, 284. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef]
- Ayaz, M.; Subhan, F.; Sadiq, A.; Ullah, F.; Ahmed, J.; Sewell, R. Cellular efflux transporters and the potential role of natural products in combating efflux mediated drug resistance. Front. Biosci. (Landmark Ed.) 2017, 22, 732–756. [Google Scholar] [CrossRef] [Green Version]
- Ovais, M.; Ahmad, I.; Khalil, A.T.; Mukherjee, S.; Javed, R.; Ayaz, M.; Raza, A.; Shinwari, Z.K. Wound healing applications of biogenic colloidal silver and gold nanoparticles: Recent trends and future prospects. Appl. Microbiol. Biotechnol. 2018, 102, 4305–4318. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Junaid, M.; Ullah, F.; Sadiq, A.; Subhan, F.; Khan, M.A.; Ahmad, W.; Ali, G.; Imran, M.; Ahmad, S. Molecularly characterized solvent extracts and saponins from Polygonum hydropiper L. show high anti-angiogenic, anti-tumor, brine shrimp, and fibroblast NIH/3T3 cell line cytotoxicity. Front. Pharmacol. 2016, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ullah, F.; Zeb, A.; Ayaz, M.; Ullah, F.; Sadiq, A. Evaluation of Rumex hastatus D. Don for cytotoxic potential against HeLa and NIH/3T3 cell lines: Chemical characterization of chloroform fraction and identification of bioactive compounds. BMC Complement. Altern. Med. 2016, 16, 308. [Google Scholar] [CrossRef]
- Seo, D.-J.; Kim, K.-Y.; Park, R.-D.; Kim, D.-H.; Han, Y.-S.; Kim, T.-H.; Jung, W.-J. Nematicidal activity of 3, 4-dihydroxybenzoic acid purified from Terminalia nigrovenulosa bark against Meloidogyne incognita. Microb. Pathog. 2013, 59, 52–59. [Google Scholar]
- Del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; Gregory, D.J.; Forget, G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: A signaling point of view. Clin. Microbiol. Rev. 2005, 18, 293–305. [Google Scholar] [CrossRef]
- Kakarsulemankhel, J.K. Leishmaniasis in Pak-Afghan Region. Int. J. Agric. Biol. 2011, 13, 611–620. [Google Scholar]
- Wulsten, I.F.; Costa-Silva, T.A.; Mesquita, J.T.; Lima, M.L.; Galuppo, M.K.; Taniwaki, N.N.; Borborema, S.E.; Da Costa, F.B.; Schmidt, T.J.; Tempone, A.G. Investigation of the anti-Leishmania (Leishmania) infantum activity of some natural sesquiterpene lactones. Molecules 2017, 22, 685. [Google Scholar] [CrossRef]
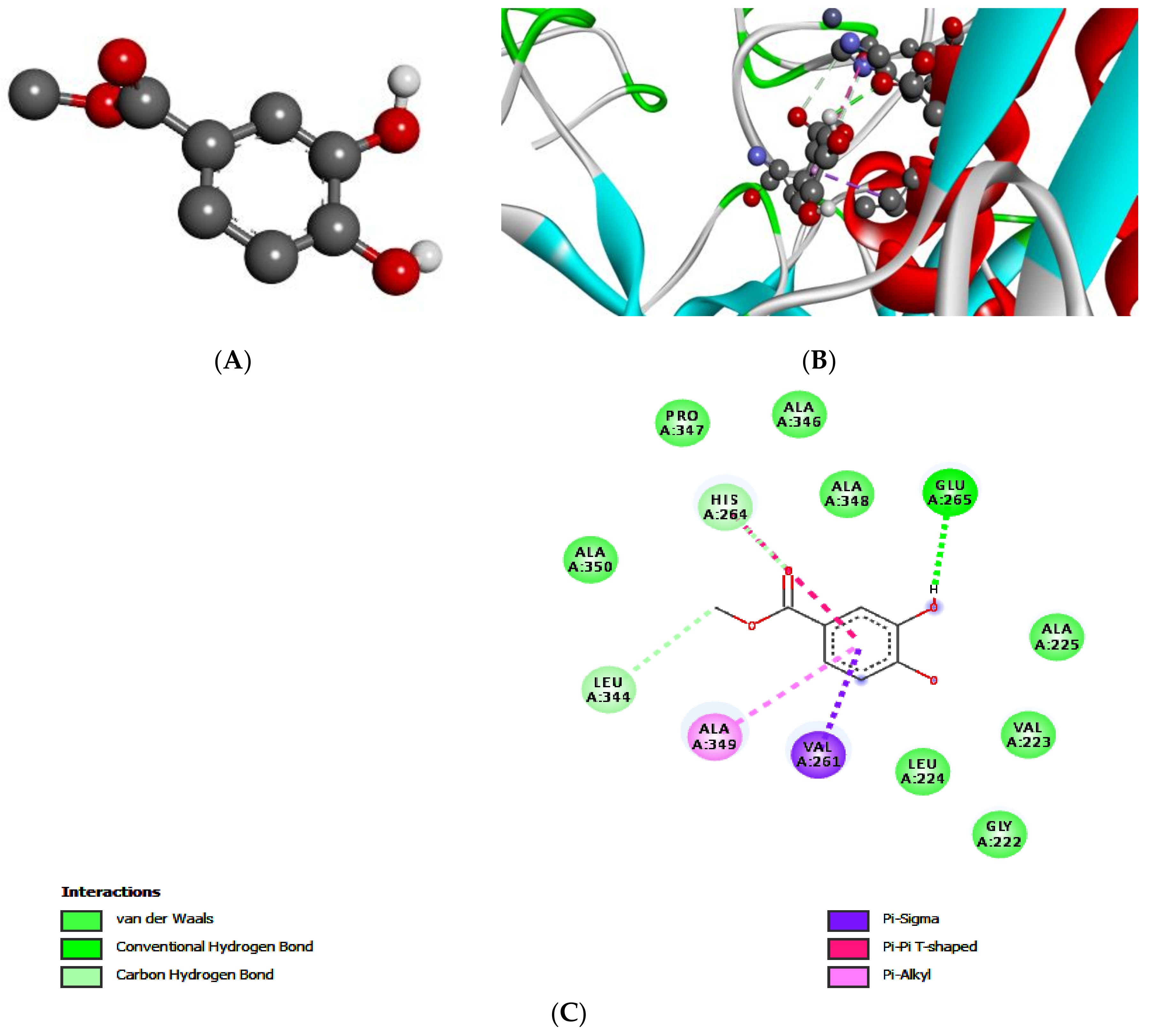
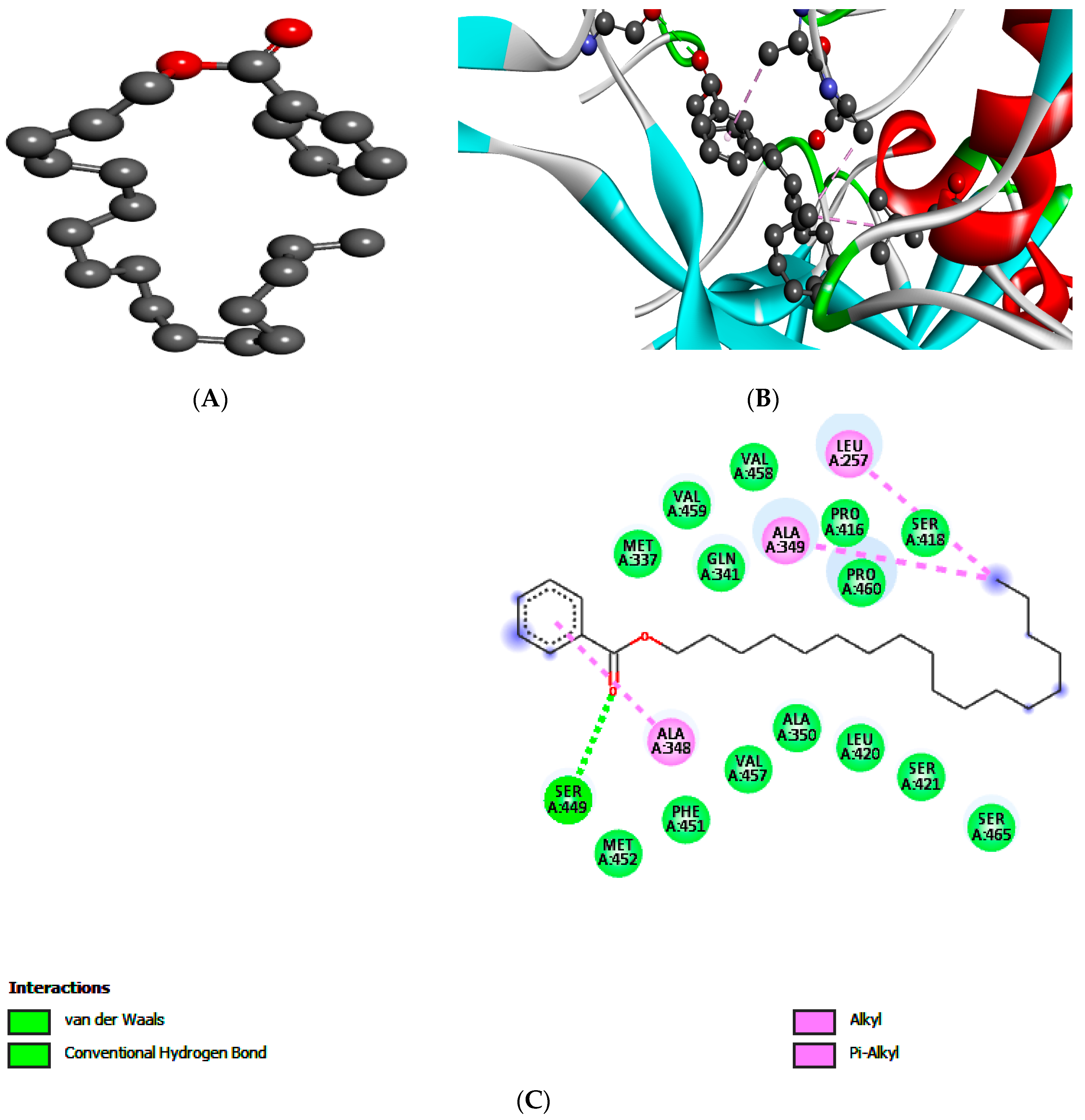
| Targets | Compound 1 | Compound 2 | ||||
|---|---|---|---|---|---|---|
| gp63 | E-Value | H-Bonds | Bonding Residue | E-Value | H-Bonds | Bonding Residue |
| −5.3 | 2 | Glu A:265 | −5.6 | 1 | Ser A:449 | |
| Leu A:344 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.M.; Ullah, F.; Ayaz, M.; Sadiq, A.; Hussain, S.; Ali Shah, A.-u.-H.; Shah, S.A.A.; Ullah, N.; Ullah, F.; Ullah, I.; et al. Benzoic Acid Derivatives of Ifloga spicata (Forssk.) Sch.Bip. as Potential Anti-Leishmanial against Leishmania tropica. Processes 2019, 7, 208. https://doi.org/10.3390/pr7040208
Shah SM, Ullah F, Ayaz M, Sadiq A, Hussain S, Ali Shah A-u-H, Shah SAA, Ullah N, Ullah F, Ullah I, et al. Benzoic Acid Derivatives of Ifloga spicata (Forssk.) Sch.Bip. as Potential Anti-Leishmanial against Leishmania tropica. Processes. 2019; 7(4):208. https://doi.org/10.3390/pr7040208
Chicago/Turabian StyleShah, Syed Majid, Farhat Ullah, Muhammad Ayaz, Abdul Sadiq, Sajid Hussain, Azhar-ul-Haq Ali Shah, Syed Adnan Ali Shah, Nazif Ullah, Farman Ullah, Ikram Ullah, and et al. 2019. "Benzoic Acid Derivatives of Ifloga spicata (Forssk.) Sch.Bip. as Potential Anti-Leishmanial against Leishmania tropica" Processes 7, no. 4: 208. https://doi.org/10.3390/pr7040208
APA StyleShah, S. M., Ullah, F., Ayaz, M., Sadiq, A., Hussain, S., Ali Shah, A.-u.-H., Shah, S. A. A., Ullah, N., Ullah, F., Ullah, I., & Nadhman, A. (2019). Benzoic Acid Derivatives of Ifloga spicata (Forssk.) Sch.Bip. as Potential Anti-Leishmanial against Leishmania tropica. Processes, 7(4), 208. https://doi.org/10.3390/pr7040208