1. Introduction
Separation of colloidal particles such as microorganisms, cells, and ceramic particles has been studied. The sophisticated separation of colloids depends on their different characteristics. For example, a porous membrane combined with chromatography has been used to separate a microorganism suspension via pH-gradient flow through a particle-packed column [
1], and microfiltration with a cross flow has been performed to sweep the filtered residue from the membrane pores [
2,
3]. Glass beads packed in a column and microchannels have been used for separation based on the different mobilities of the colloidal particles [
4,
5]. At present, the pores in a membrane, the gaps between glass beads, and the microchannel diameter used for separation need to be uniform. If the pores used for filtration were able to be dynamically change, separation performance could be greatly enhanced in a wide range of applications.
In chromatography, spherical gel particles are packed in a column, and then water is permeated through the column to increase the pressure drop by narrowing the gaps between the gel particles through the deformation of the packed gel. The gel particles at the bottom of the column feel more pressure and thus deform to a greater extent than the gel particles at the top of the column. Through such deformation, the gaps between the gel particles become smaller at the bottom of the column compared with those at the top. Because the flow of water through deformable gel particles changes the pores in the flow direction, injected colloids are dynamically separated depending on their size and morphology [
6]. Silica particle suspensions have been separated using gel particle deformation in columns. The spherical gel particles prepared by radial polymerization were packed in a column to separate an injected silica particle suspension with particle sizes ranging from 100 nm to 10 µm It was found that silica particles with a diameter of 100 nm passed through the column, whereas those with a diameter of 10 µm were captured at the top of the gel layer. The dynamic filtering efficiency of silica particles with a diameter of 1 µm depended on the gel size, gel elasticity, and flow rate of water. It may be possible to recover a filtered colloid at the top of the gel layer by floating the filtered colloid particles using the elasticity of the gel layer and then applying a cross flow across the column [
7]. However, an appropriate technique to recover the filtered colloid particles in a gel layer has not been proposed yet.
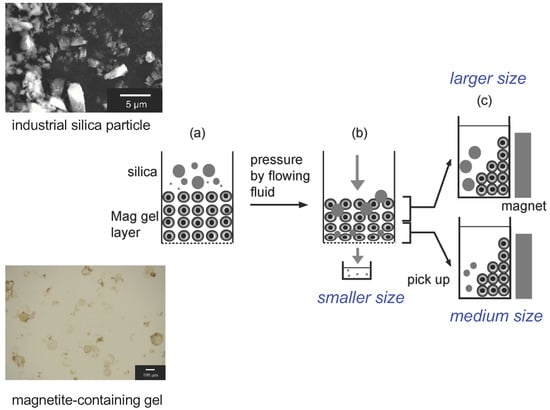
In this study, particle separation using a magnetite-containing gel layer in a column is proposed (
Figure 1). The magnetite-containing gel should be easily moved by the application of a magnetic field to allow the separation of the filtered particles in the gel layer from the gel particles. Materials including magnetite have been studied from a chemistry perspective for their preparation [
8,
9] and applications [
10]. A macroscopic magnetite assembly has been used as a filter medium for the separation of several particles and subsequent recovery by the application of a magnetic field [
11,
12]. Here, a column packed with a magnetite-containing gel was used to separate industrial silica particles prepared by a dry process. Large volumes of silica particles are industrially produced by dry processes; that is, silica ore is cracked to obtain electronics materials such as fillers to control heat transfer. The regulation of the size and morphology of the cracked silica particles is crucial for potential high-performance applications. This study had three main objectives: (1) the preparation of a magnetite-containing gel, (2) the individual filtration of silica particles with diameters of 1 and 10 µm, and (3) the filtration of industrial silica particle suspensions using the magnetite-containing gel and the recovery of the filtered silica particles from the gel layer by using a magnet. The sizes of the silica particles obtained after separation were analyzed by dynamic light scattering (DLS).
2. Materials and Methods
2.1. Materials
Magnetite was prepared from iron (III) chloride hexahydrate (095-00875), iron (II) chloride tetrahydrate (099-00915), and sodium hydroxide (192-15985) purchased from Wako Pure Chemical Industries, Japan. D-Glucose (07-0690-6-5) was obtained from Sigma-Aldrich, MI, USA. The monomer, crosslinker, and initiator used to prepare the gel particles were N,N-dimethylacrylamide (049-19185), N,N′-methylenebisacrylamide (M0506), and ammonium persulfate (018-03282) purchased from Wako Pure Chemical Industries, Japan. Span 80 (S0060) and Tween 80 (T2533) were obtained from Tokyo Chemical Industry Co., Japan. Ethylenediaminetetraacetic acid (EDTA; 09-1320) was purchased from Katayama Chemical Ltd., Japan. Glass beads (105–125 µm, BZ-01) were purchased from AS-ONE, Japan. Black silica particles (size: 10 µm) and white silica particles (size: 1.0 μm) were purchased from Micromod Partikeltechnologie Gmbh, Germany. Industrial silica particles were donated by Nitchitsu Co., Ltd., Japan. Other chemicals were of analytical grade or higher.
2.2. Preparation of Magnetite
Solution 1 was first prepared by dissolving sodium hydroxide in distilled water to give a concentration of 10 mol/L. Iron(III) chloride hexahydrate (11 g, 0.041 mol) and iron(II) chloride tetrahydrate (4.07 g, 0.020 mol) were dissolved in water at 303 K in a water bath (Eyela, SB-350) while stirring at 500 rpm by a stirrer (Eyela, Mazel, ZZ-1200) to give solution 2. Glucose (8.75 g, 0.049 mol) was dissolved in solution 1, and then solution 1 was added dropwise to solution 2 at a rate of one drop every two seconds. The obtained solution was stirred at 303 K and 500 rpm for 1 h. The precipitate present after this time responded to a neodymium magnet (0.10 T, 50 × 20 × 2 mm, Sangyo Supply Co., Ltd., Sendai, Japan). The magnetite particles were observed by optical microscopy (VH-S5, Keyence Corporation, Osaka, Japan), and their size distribution was determined by measuring the diameters of more than 200 particles. The mean size of the magnetite particles was 670 nm.
2.3. Polymerization of Spherical Gel Particles Including Magnetite
To prepare the oil phase, Span 80 (9.0 g) and Tween 80 (3.0 g) were dissolved in hexane (200 g). N,N-Dimethylacrylamide (9.33 g), N,N′-methylenebisacrylamide (0.16 g), EDTA (0.15 g), and water (25 g) were mixed, and then ammonium persulfate (0.33 g) was added. The prepared magnetite suspension (10 (w/w)%, 10 mL) was dispersed in the oil phase. The water phase was dropped into the oil phase over 30 s and then stirred at 500 rpm and 333 K for 4 h. Under these conditions, the added magnetite particles moved into the water phase. After the polymerization, the reactor was cooled to room temperature. The obtained magnetite-containing gel suspension was filtered under vacuum and then washed with hexane (250 mL) and ethanol (1 L) twice for 2 h. The obtained suspension was finally washed with distilled water, filtered through a nylon mesh (161 µm, NBC Meshtec Inc., N-N0110S 115, P1604A09925-03, Tokyo, Japan), and then filtered again through a finer nylon mesh (42 µm, NBC Meshtec Inc., N-N0330T 115, E1802A00255-01, Tokyo, Japan) to remove the residual polymer, magnetite, and larger-size gel particles. The obtained magnetite-containing gel is referred to as “Mag gel”. The concentration of Mag gel in water was set to 10 wt %. The obtained Mag gel was observed by optical microscopy (VH-S5, Keyence Corporation, Osaka, Japan), and its particle size distribution was determined by measuring the diameters of more than 200 particles. For comparison, polymerized gel spheres without magnetite were also fabricated.
The magnetite concentration in the Mag gel was quantitatively determined. Mag gel (1 mL) was dried in a drying oven (DX-31, Yamato Scientific Co., Ltd., Tokyo, Japan). Nitric acid solution (3.0 M, 25 mL) was added to the dried gel at 333 K, and then the mixture was agitated using a shaking bath (SB-20, As One, Japan). The time dependence of iron concentration was determined by atomic absorption spectroscopy (AA-6800, Shimadzu, Corporation, Kyoto, Japan) to determine the concentration of leaked iron ions at steady state in solution.
2.4. Water Permeation through the Mag Gel
Glass beads (105–125 µm) were first packed in a column (ID: 0.5 cm, OD: 0.9 cm, height: 20 cm, 7370522, Bio-Rad Laboratories, Inc., CA, USA) to a height of 1.0 cm. Mag gel suspension (10 (w/w)%, 1.4 mL) was added to the column and then allowed to settle via gravity for 15 h, so that the height of the gel layer was 1.0 cm. After filling the gel layer in the column with water, water was permeated through the column at 25 mL/h to measure the pressure drop of the Mag gel layer. The eluent was collected to check the concentrations of iron and gel that leaked from the column by ultraviolet–visible (UV–Vis) spectroscopy at 600 nm (V-630 BIO, JASCO, Tokyo, Japan).
2.5. Permeation of Silica Particle Suspensions through the Mag Gel Layer
Silica particle suspensions with particle sizes of 1.0 and 10 µm (1.0 g/L, 0.40 mL) were individually permeated through the Mag gel-packed column at a flow rate of 25 mL/h. The eluent from the column was collected to determine the concentration of silica particles by UV–Vis spectroscopy at 600 nm.
To separate the industrial silica particle suspension, the suspension (1.0 g/L, 0.4 mL) injected on the top of the Mag gel layer was permeated through the column at a flow rate of 10, 25, or 50 mL/h. The eluent was collected continuously, its absorbance was determined by UV–Vis spectroscopy at 600 nm, and the particle size was measured by DLS (ELZ-ZA PUS, Otsuka Electronics Co., Ltd., Osaka, Japan).
To recover the filtered particles in the magnetite-containing gel, the Mag gel and filtered particles in the upper half and lower half of the column were taken out by a pipette. A neodymium magnet was then applied to the bottom of each suspension to separate the filtered silica particles from the Mag gel by washing the suspension with water and ethanol. The particle size of the supernatant (5 mL) was determined by DLS.
4. Conclusions
Magnetite was introduced into spherical gel particles to give a magnetite-containing gel that had the ability to deform under pressure and respond to a magnetic field. Mag gel packed in a column was used to separate industrial silica particles with sizes of 300 nm, 800 nm, and 10 µm. A silica particle suspension was injected on the top of the Mag gel layer, and then water was permeated through the layer. The Mag gel layer responded to the pressure and shear stress of the fluid by deforming, causing the gaps between the Mag gel particles to change in the flow direction of the column. The pressure was higher at the bottom of the Mag gel layer than at the top, resulting in smaller gaps between the gel particles at the bottom of the column than at the top. Thus, larger silica particles were captured at the top of the column, and smaller silica particles were filtered to the bottom of the column or eluted. The sizes of the separated silica particles were determined by DLS. The filtered silica particles present in the Mag gel were recovered by the external application of a magnetic field. This separation technique has potential applications for the separation of different silica particles, cells, microorganisms, proteins, and polysaccharides. In engineering, the foundations of gel preparation and column design as well as the operating conditions should be considered in a mathematical manner. The interactions between gel and colloidal particles should also be considered because they affect elution and filtration.