Antioxidant Activity and Selenium and Polyphenols Content from Selected Medicinal Plants Natives from Various Areas Abundant in Selenium (Poland, Lithuania, and Western Ukraine)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Assessment of Selenium Concentration
2.3. Sample Preparation for Total Polyphenol Content and Antioxidant Potential
2.4. Total Polyphenol (TP) Content Assay
2.5. Free Radical ABTS Scavenging Ability Assay (ABTS)
2.6. Ferric Reducing Antioxidant Power Assay (FRAP)
2.7. Statistical Analysis
3. Results
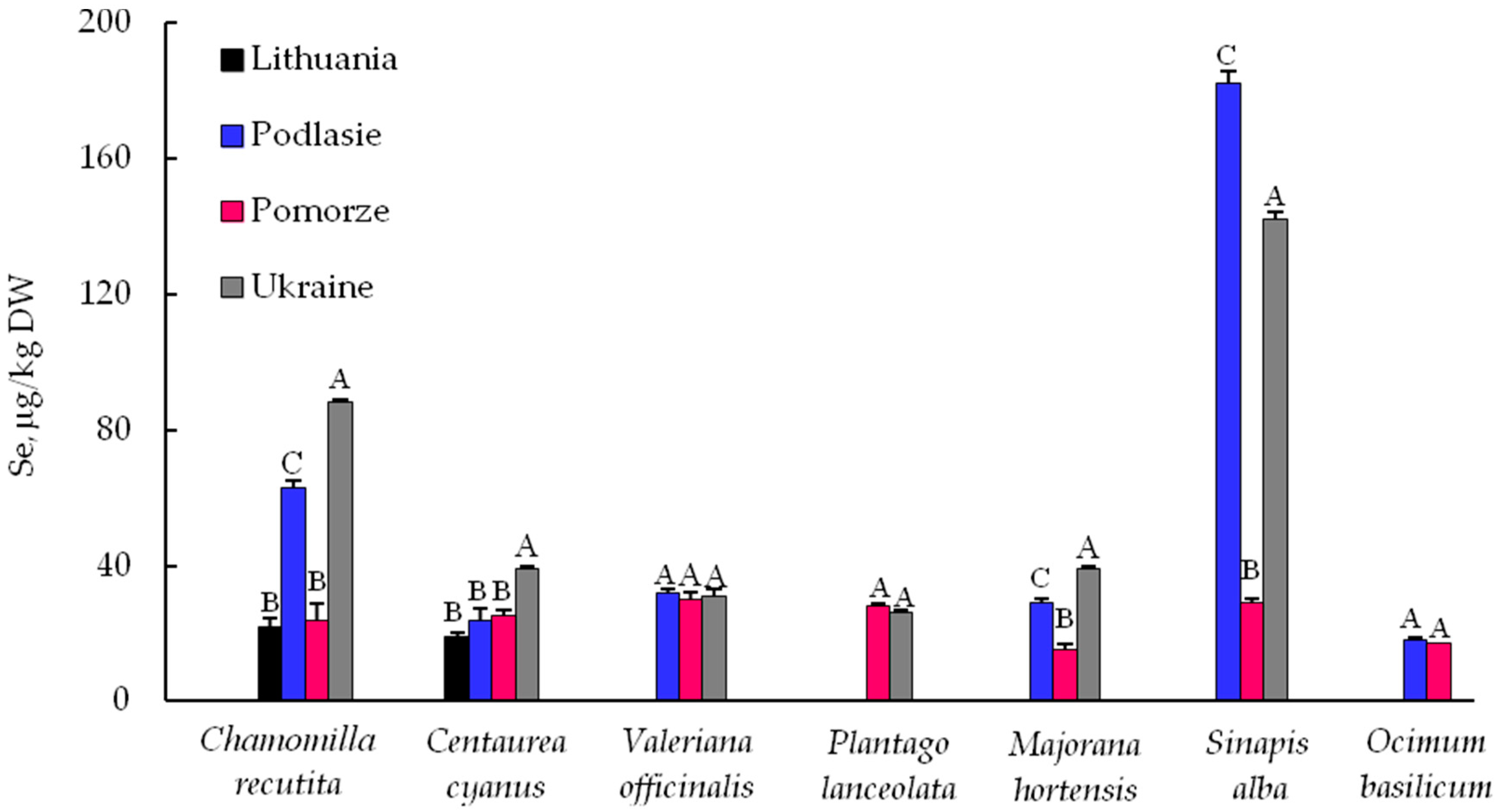
3.1. Selenium (Se)
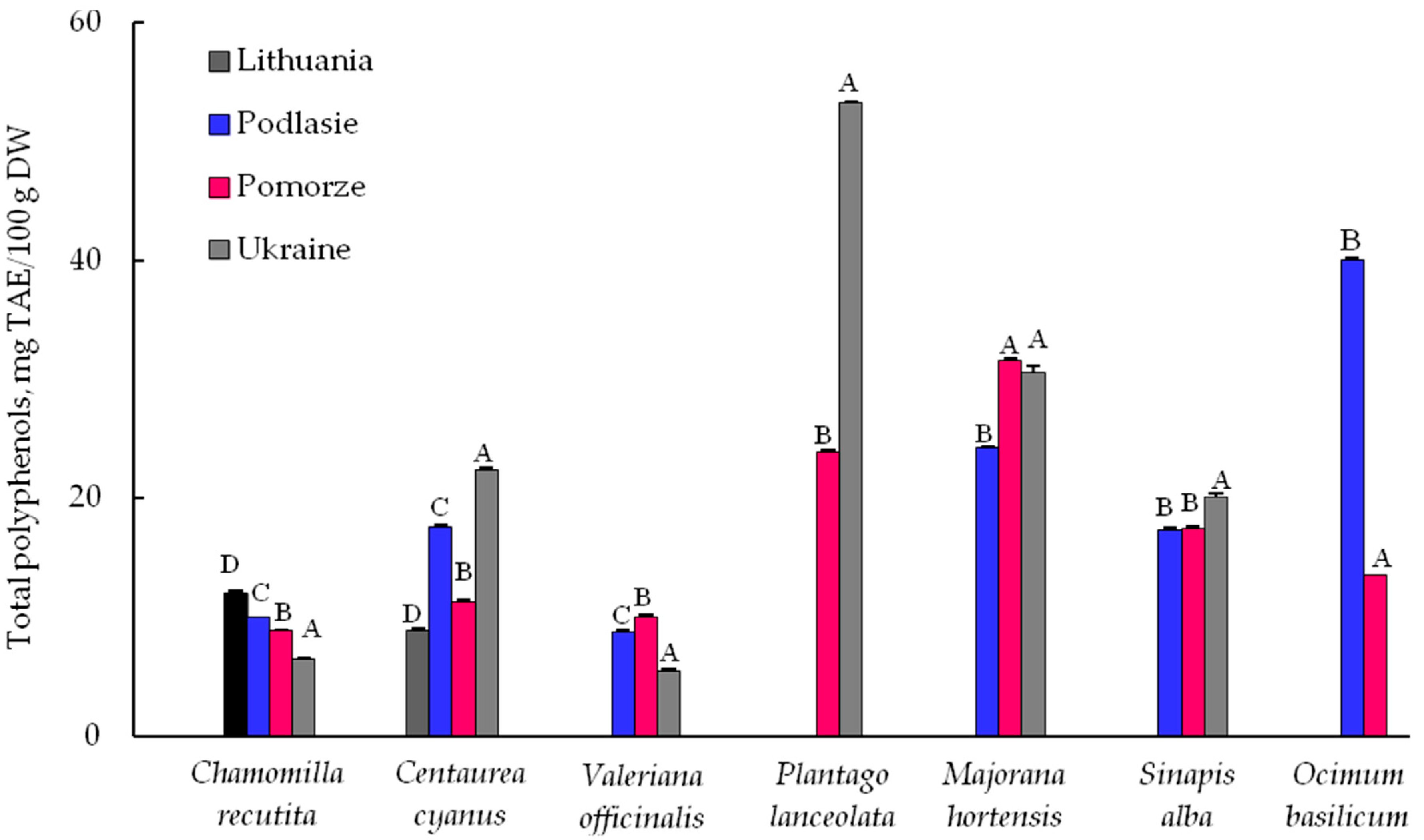
3.2. Total Polyphenols
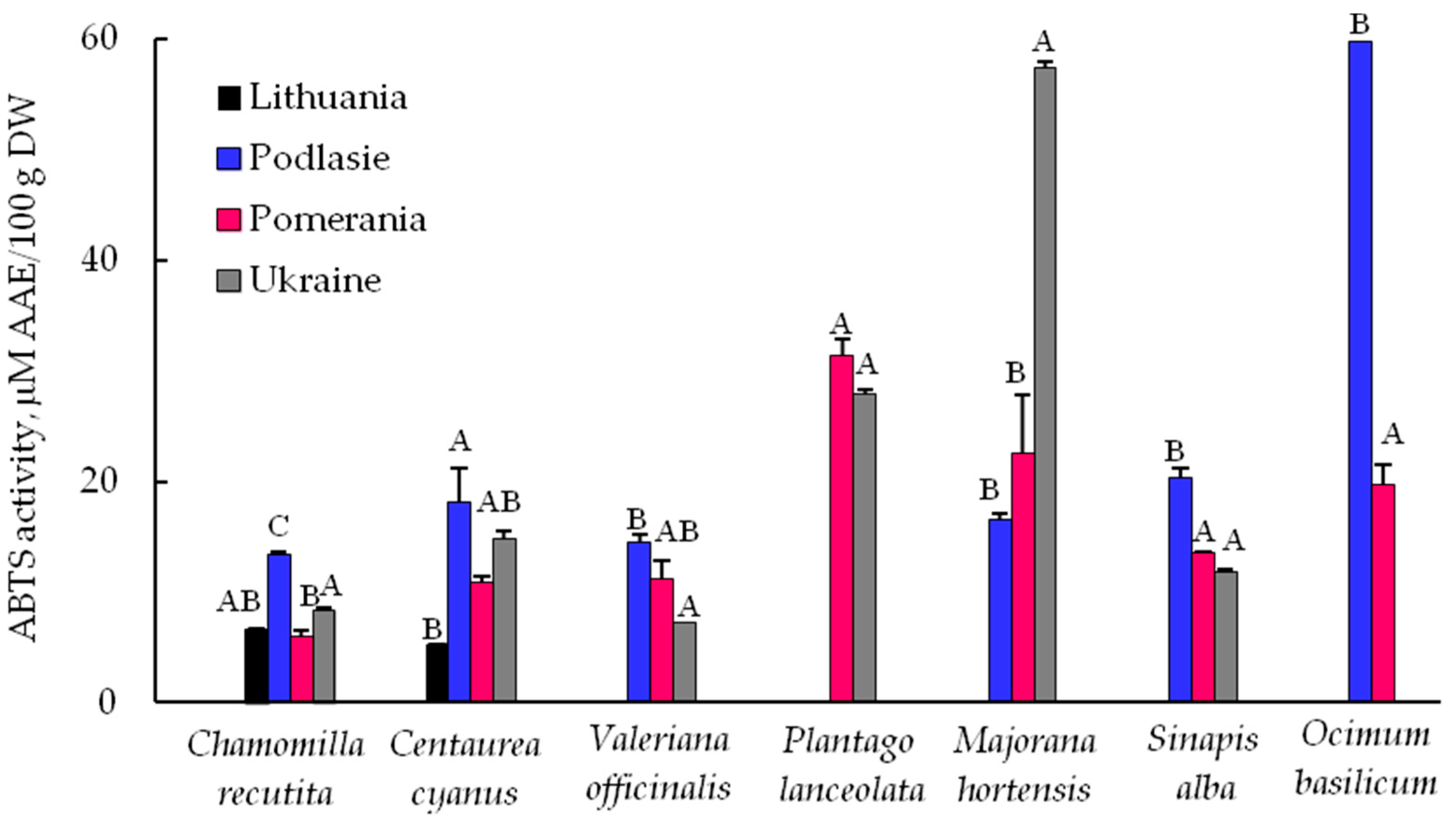
3.3. ABTS Radicals Scavenging Ability of Plant Extracts
3.4. FRAP Activity
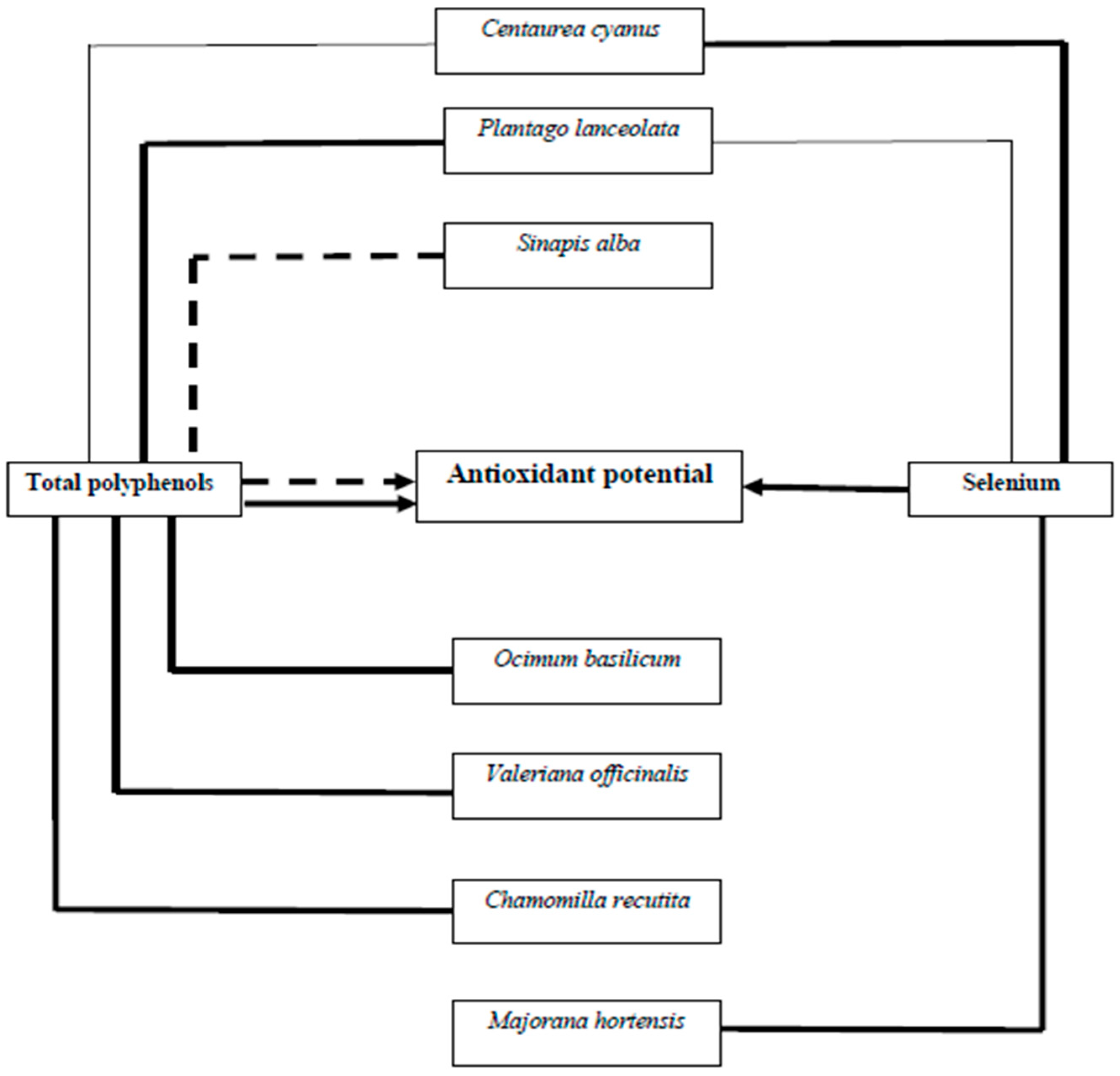
3.5. Correlations Between Selenium, Polyphenol, and ABTS Radicals Scavenging Ability and FRAP Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Selenium and selenium-antagonistic elements in nutritional cancer prevention. Crit. Rev. Biotechnol. 2009, 29, 10–17. [Google Scholar] [CrossRef]
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. 2015, 32, 195–199. [Google Scholar] [CrossRef]
- Oldfield, J.E. Selenium World Atlas: Updated Edition; Selenium-Tellurium Development Association: Grimbergen, Belgium, 2002; pp. 1–59. [Google Scholar]
- White, P.J.; Bawen, H.C.; Parmaguru, P.; Fritz, M.; Spracklen, W.P.; Spiby, R.E.; Meacham, M.C.; Meam, A.; Harriman, M.; Trueman, L.J.; et al. Interactions between selenium an sulfur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1927–1937. [Google Scholar] [CrossRef]
- Piotrowska, M. Zawartość selenu w uprawnych glebach Polski. Rocz. Glebozn. 1984, 35, 23–31. [Google Scholar]
- Terry, N.; Zayed, A.M.; De Souza, M.P.; Tarun, A.S. Selenium in higher plants. Annu. Rev. Plant Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Sridhar, C.; Reddy, Y.S.R.; De, B. Free radicals, antioxidants, diseases and phytomedicines: Current status and future prospect. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 91–100. [Google Scholar]
- Iuss Working Group Wrb. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Report; FAO: Rome, Italy, 2014. [Google Scholar]
- Pustelnikovas, O. Geoekologiczna ocena składu chemicznego gleb i osadów rzecznych na tle jednostek geomorfologicznych Litwy. Landf. Anal. 2008, 9, 96–103. [Google Scholar]
- Miklaszewski, S. Mapa gleb Litwy 1:1500 000. Dosw. Rol. 1927, 3–4, 1–33. [Google Scholar]
- Białousz, S. Mapa gleb Polski 1: 1 500 00. Gleby—Klasyfikacja genetyczna. In Atlas Rzeczypospolitej Polskiej; PAN: Warszawa, Poland, 1994. [Google Scholar]
- Voronina, A.V. Soil Map of Ukraine 1:2 500 000; General Directorate of Surveying and Cartography of the Soviet Ministry, GUGK: Moscow, USSR, 1977. [Google Scholar]
- Reimann, C.; Birke, M.; Demetriades, A.; Filzmoser, P.; O’Connor, P. Chemistry of Europe’s Agricultural Soils; Geologisches Jahrbuch (Reihe B); Schweizerbart Science Publishers: Stuttgart, Germany, 2014; Volume 102, pp. 1–523. [Google Scholar]
- Birke, M.; Reimann, C.; Demetriades, A.; Dinelli, E.; Rauch, U.; The GEMAS Project Team. Geochemical mapping at European scale—the GEMAS-project. In Proceedings of the International Workshop on Groundwater Systems in Europe, Berlin, Germany, 22–23 August 2013. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; Mcmahon, T. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 4, 439–473. [Google Scholar] [CrossRef]
- Watkinson, J.H. Fluorometric determination of selenium in biological material with 2,3-Diaminonaphthalene. Anal. Chem. 1966, 38, 92–97. [Google Scholar] [CrossRef]
- Grzebuła, S.; Witkowski, P. The determination of selenium trace levels in biological materials with fluorometric method. Selenium determination in tissues and bodily fluids. Pol. Arch. Weter 1977, 20, 125–138. (In Polish) [Google Scholar]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Bioactive non-coloured polyphenols content of grapes, wines and vinification by-products: Evaluation of the antioxidant activities of their extracts. Food Res. Int. 2010, 4, 805–813. [Google Scholar] [CrossRef]
- Shi, F.; Jia, X.; Zhao, C.; Chen, Y. Antioxidant activities of various extracts from Artemisisa selengensis Turcz (LuHao). Molecules 2010, 15, 4934–4946. [Google Scholar] [CrossRef]
- Benzi, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2001; pp. 1–403. [Google Scholar]
- Zabłocki, Z. Selen w Glebach i Roślinach Pomorza Zachodniego. Ph.D. Thesis, Agricultural Academy of Szczecin, Szczecin, Poland, 1990. [Google Scholar]
- Sotek, Z.; Białecka, B.; Pilarczyk, B.; Kruzhel, B.; Drozd, R.; Pilarczyk, R.; Tomza-Marciniak, A.; Lysak, H.; Bąkowska, M.; Vovk, S. The content of selenium, polyphenols and antioxidative activity in selected medicinal plants from Poland and Western Ukraine. Acta Pol. Pharm. Drug Res. 2018, 75, 1107–1116. [Google Scholar]
- Čuvardić, M.S. Selenium in soil. Matica Srp. Proc. Nat. Sci. 2003, 104, 23–37. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Petruzzelli, G.; Petacco, F.; Malorgio, F.; Ferri, T. Absorption of selenium by Lactuca sativa as affected by carboxymethylcellulose. Chemosphere 2007, 67, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Bitterli, C.; Bañuelos, G.S.; Schulin, R. Use of transfer factors to characterize uptake of selenium by plants. J. Geochem. Explor. 2010, 107, 206–216. [Google Scholar] [CrossRef]
- Ulewicz-Magulska, B.; Wesołowski, M. A chemometric approach to distribution of selenium in medicinal plants cultivated in Poland. J. Med. Food 2013, 16, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, G.S.; Ajwa, H.A.; Mackey, B.; Wu, L.; Cook, C.; Akohoue, S.; Zambruzuski, S. Evaluation of different plant species used for phytoremediation of high soil selenium. J. Environ. Qual. 1997, 26, 639–646. [Google Scholar] [CrossRef]
- Galeas, M.L.; Zhang, L.H.; Freeman, J.L.; Wegner, M.; Pilon-Smits, E.A.H. Seasonal fluctuations of selenium and sulfur accumulation in selenium hyperaccumulators and related non-accumulators. New Phytol. 2007, 173, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.W.; Elliott, T.L.; Davies, T.J. Community restructuring can maintain diversity across a severity gradient in the absence of foundation species. Ecosphere 2014, 5, 1–16. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Bykova, N.V. Role of organic acids in the integration of cellular redox metabolism and mediation of redox signalling in photosynthetic tissues of higher plants. Free Radic. Biol. Med. 2018, 122, 74–85. [Google Scholar] [CrossRef]
- Lopez-Bucio, J.; Nieto-Jacobo, M.F.; Ramırez-Rodrıguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Chrpova, D.; Kourimska, L.; Gordon, M.H.; Hermanova, V.; Roubickova, I.; Panek, J. Antioxidant activity of selected phenols and herbs used in diets for medical conditions. Czech J. Food Sci. 2010, 28, 317–325. [Google Scholar] [CrossRef]
- El-Lateef Gharib, A.; Teixeira da Silva, J.A. Composition, total phenolic content and antioxidant activity of the essential oil of four Lamiaceae herbs. Med. Aromat. Plant Sci. Biotechnol. 2013, 7, 19–27. [Google Scholar]
- Benedec, D.; Vlase, L.; Hanganu, D.; Oniga, I. Antioxidant potential and polyphenolic content of Romanian Ocimum basilicum. Dig. J. Nanomater. Biostruct. 2012, 7, 1263–1270. [Google Scholar]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Pilerood, S.A.; Prakash, J. Evaluation of nutritional composition and antioxidant activity of Borage (Echium amoenum) and Valerian (Valerian officinalis). J. Food Sci. Technol. 2014, 51, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Elmastas, M.; Cinkilic, S.; Aboul-Enein, H.Y. Antioxidant capacity and determination of total phenolic compounds in daisy (Matricaria chamomilla, Fam. Asteraceae). World J. Anal. Chem. 2015, 3, 9–14. [Google Scholar] [CrossRef]
- Nichita, C.; Neagu, G.; Cucu, A.; Vulturescu, V.; Vifor, Ş.; Berteşteanu, G. Antioxidative properties of Plantago lanceolata L. extracts evaluated by chemiluminescence method. AgroLife Sci. J. 2016, 5, 95–102. [Google Scholar]
- Erol-Dayi, Ö.; Pekmez, M.; Bona, M.; Aras-Perk, A.; Arda, N. Total phenolic contents, antioxidant activities and cytotoxicity of three Centaurea species: C. calcitrapa subsp. calcitrapa, C. ptosimopappa and C. spicata. Free Rad Antiox 2011, 1, 31–36. [Google Scholar] [CrossRef]
- Thangi, J.; Shashitha, K.N.; Ashwini, H.A.; Schlini, P. A correlation study of antioxidant potential’s from Synapis alba. IAJPS 2016, 3, 234–239. [Google Scholar]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef]
- Robbins, R.J.; Keck, A.S.; Banuelos, G.; Finley, J.W. Cultivation conditions and selenium fertilization alter the phenolic profile, glucosinolate, and sulforaphane content of broccoli. J. Med. Food 2005, 8, 204–214. [Google Scholar] [CrossRef]
- Schiavon, M.; dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.H.; Sambo, P.; Masi, A.; Malagoli, M. Impact of selenium fertilization on chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef]
- Dutta, R.K.; Maharia, R.S. Antioxidant responses of some common medicinal plants grown in copper mining areas. Food Chem. 2012, 131, 259–265. [Google Scholar] [CrossRef]

| Climatic Factors | Lithuania | Poland | Ukraine | |
|---|---|---|---|---|
| Olita Region Lazdijai | Pomorze Region Połczyn Zdrój | Podlasie Region Koryciny | Lviv Region Brody 2 | |
| Mean annual precipitation (mm) | 593 | 649 | 560 | 602 |
| The highest mean rainfalls (mm)—month | 81 July | 82 July | 75 July | 87 July |
| The lowest mean rainfalls (mm)—month | 22 February | 30 February | 28 February | 31 January |
| Mean annual temperature (°C) | 6.3 | 7.7 | 6.9 | 7.6 |
| The warmest month, mean temperature (°C) | 16.9 July | 17.7 July | 18.0 July | 18.5 July |
| The coldest month, mean temperature (°C) | January −5.3 | January −3.4 | December −2.3 | January −4.5 |
| Type of soil | Luvisols | Luvisols | Luvisols | Chernozems |
| Parameter | Herbs | Spices | |||||
|---|---|---|---|---|---|---|---|
| Centaurea cyanus | Chamomilla recutita | Plantago lanceolata | Valeriana officinalis | Majorana hortensis | Ocimum basilicum | Sinapis alba | |
| Se & ABTS | 0.656 *** | 0.429 * | 0.689 * | ns | 0.691 ** | ns | 0.551 * |
| Se & FRAP | 0.607 *** | -0.413 * | ns | ns | 0.821 *** | ns | –0.557 * |
| Se & TP | 0.626 *** | –0.619 *** | ns | ns | ns | ns | ns |
| TP & ABTS | 0.537 ** | ns | ns | 0.519 * | ns | 0.990 *** | –0.671 ** |
| TP & FRAP | 0.527 ** | 0.915 *** | 0.818 ** | 0.901 *** | ns | 0.999 *** | –0.880 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotek, Z.; Białecka, B.; Pilarczyk, B.; Drozd, R.; Pilarczyk, R.; Tomza-Marciniak, A.; Kruzhel, B.; Lysak, H.; Bąkowska, M.; Vovk, S. Antioxidant Activity and Selenium and Polyphenols Content from Selected Medicinal Plants Natives from Various Areas Abundant in Selenium (Poland, Lithuania, and Western Ukraine). Processes 2019, 7, 878. https://doi.org/10.3390/pr7120878
Sotek Z, Białecka B, Pilarczyk B, Drozd R, Pilarczyk R, Tomza-Marciniak A, Kruzhel B, Lysak H, Bąkowska M, Vovk S. Antioxidant Activity and Selenium and Polyphenols Content from Selected Medicinal Plants Natives from Various Areas Abundant in Selenium (Poland, Lithuania, and Western Ukraine). Processes. 2019; 7(12):878. https://doi.org/10.3390/pr7120878
Chicago/Turabian StyleSotek, Zofia, Bożenna Białecka, Bogumiła Pilarczyk, Radosław Drozd, Renata Pilarczyk, Agnieszka Tomza-Marciniak, Barna Kruzhel, Halyna Lysak, Małgorzata Bąkowska, and Stakh Vovk. 2019. "Antioxidant Activity and Selenium and Polyphenols Content from Selected Medicinal Plants Natives from Various Areas Abundant in Selenium (Poland, Lithuania, and Western Ukraine)" Processes 7, no. 12: 878. https://doi.org/10.3390/pr7120878
APA StyleSotek, Z., Białecka, B., Pilarczyk, B., Drozd, R., Pilarczyk, R., Tomza-Marciniak, A., Kruzhel, B., Lysak, H., Bąkowska, M., & Vovk, S. (2019). Antioxidant Activity and Selenium and Polyphenols Content from Selected Medicinal Plants Natives from Various Areas Abundant in Selenium (Poland, Lithuania, and Western Ukraine). Processes, 7(12), 878. https://doi.org/10.3390/pr7120878






