Controlled Synthesis of Triangular Silver Nanoplates by Gelatin–Chitosan Mixture and the Influence of Their Shape on Antibacterial Activity
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Silver Seeds
2.3. Growth of Triangular Silver Nanoplates
2.4. Microorganism Preparation
2.5. Antibacterial Activity Test
2.6. Characterization
3. Results and Discussion
3.1. Effect of Silver Nitrate
3.2. Effect of Mixed Gelatin–Chitosan Solution
3.3. Effect of pH Condition
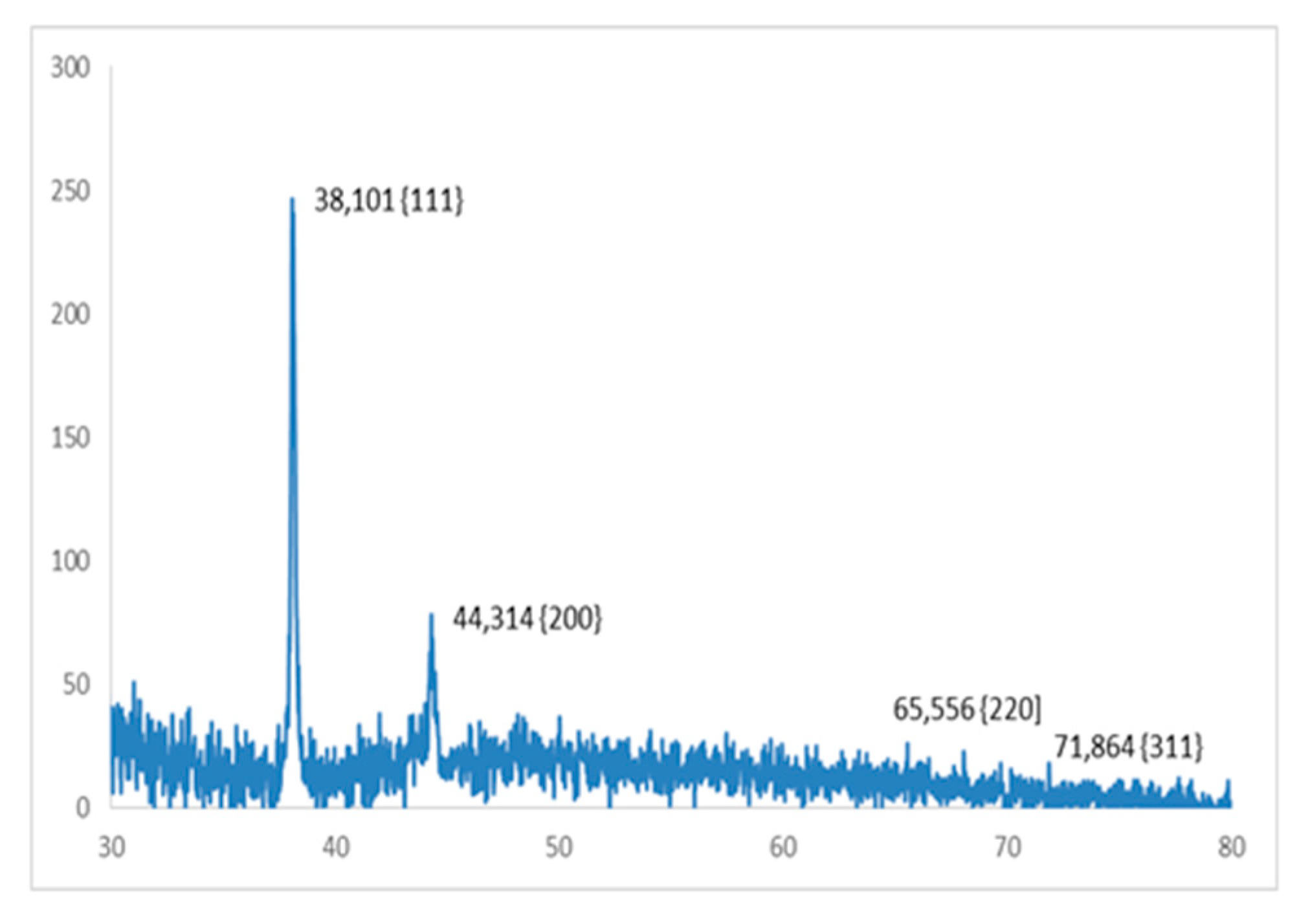
3.4. X-ray Diffraction Analysis of Silver Nanoparticles
3.5. FTIR Analysis of Gelatin–Chitosan-Triangular AgNPs Interaction
3.6. Proposed Mechanism of Triangular AgNPs
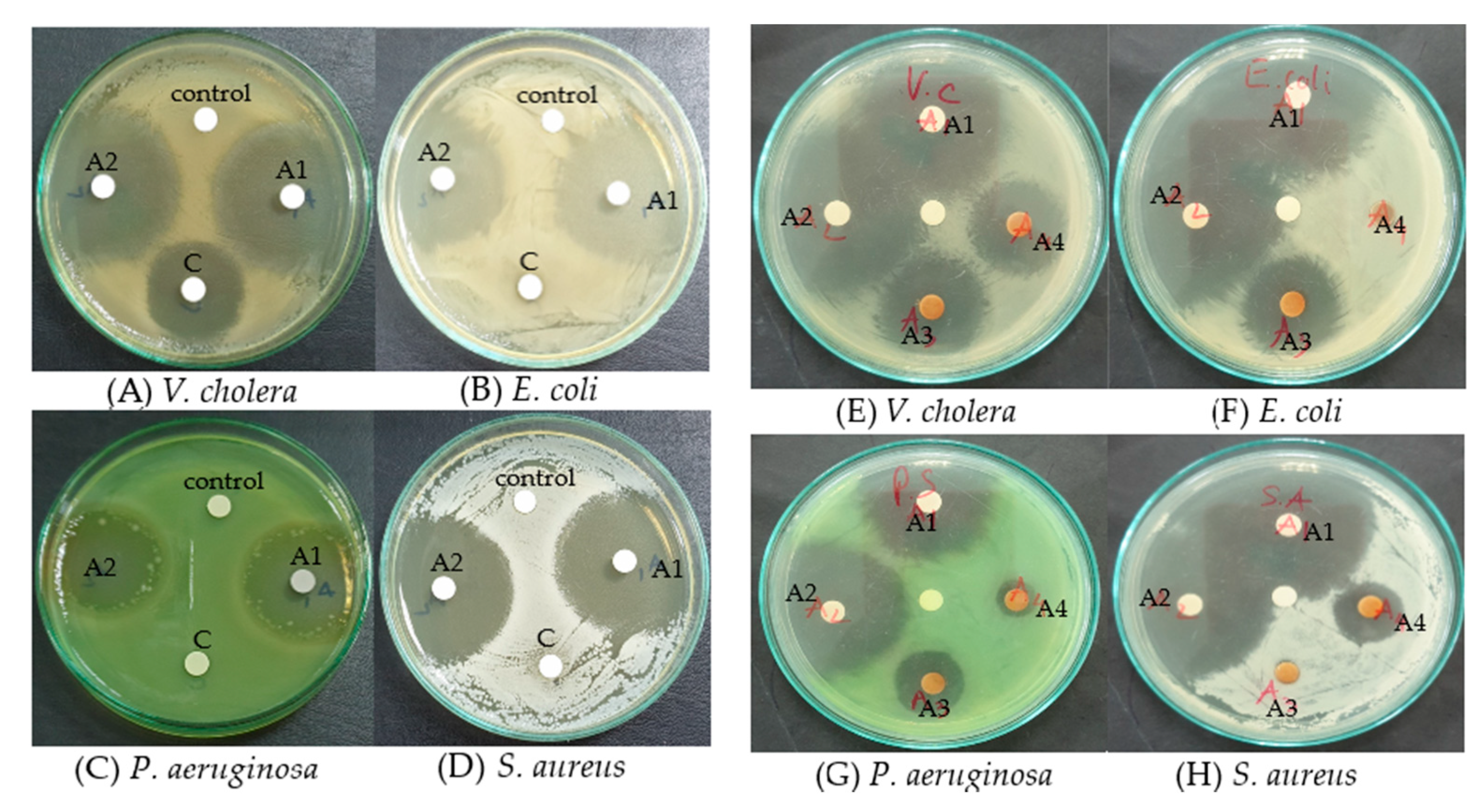
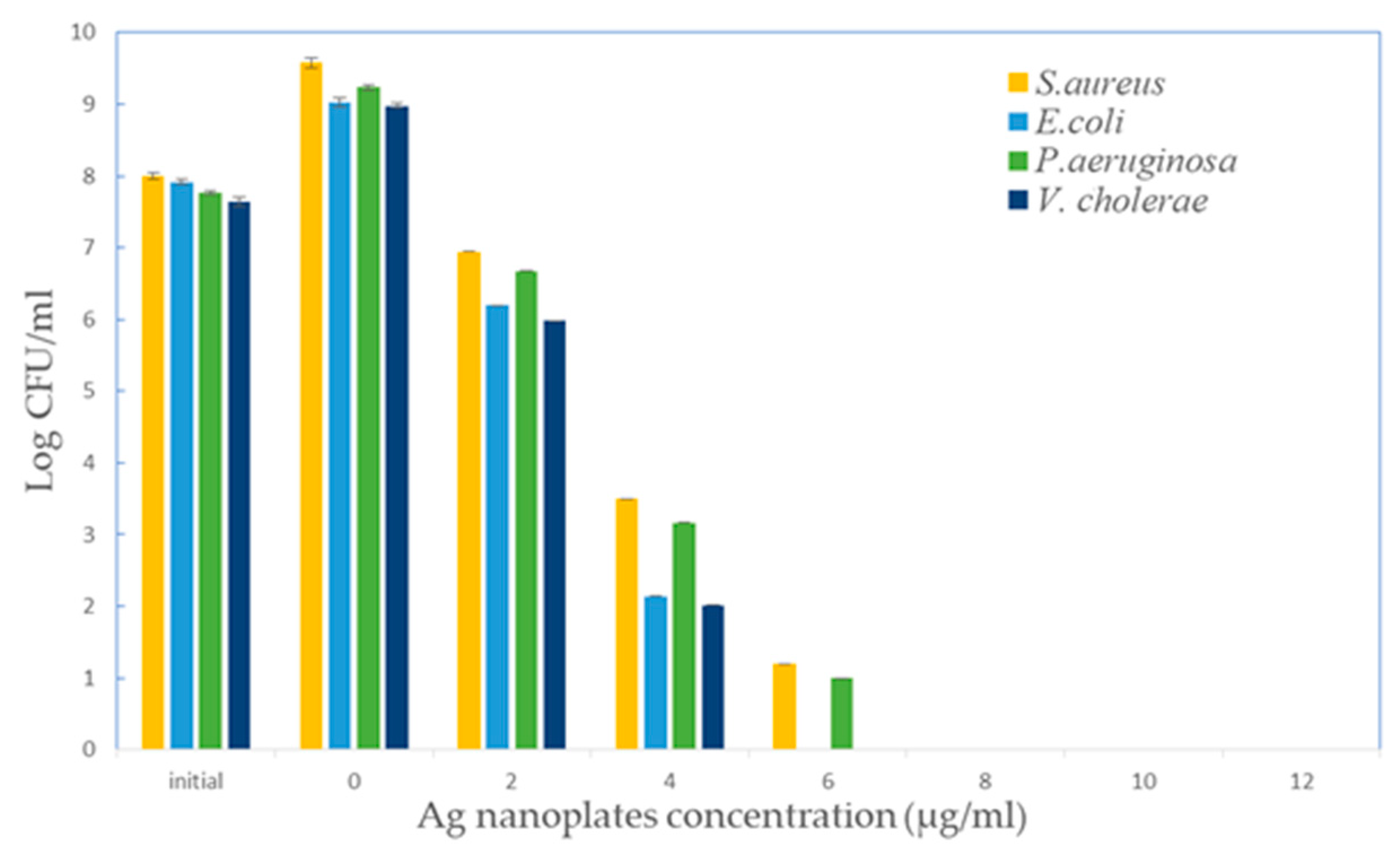
3.7. Antibacterial Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bach, L.G.; Islam, M.R.; Cao, X.T.; Park, J.M.; Lim, K.T. A novel photoluminescent nanohybrid of poly(ε-caprolactone) grafted Mg/Al layered double hydroxides and Tb3+ ions: Synthesis and characterization. J. Alloys Compd. 2014, 582, 22–28. [Google Scholar] [CrossRef]
- Tanasa, E.; Zaharia, C.; Radu, I.-C.; Surdu, V.-A.; Vasile, B.S.; Damian, C.-M.; Andronescu, E. Novel Nanocomposites Based on Functionalized Magnetic Nanoparticles and Polyacrylamide: Preparation and Complex Characterization. Nanomaterials 2019, 9, 1384. [Google Scholar] [CrossRef] [PubMed]
- Andronescu, E.; Predoi, D.; Neacsu, I.A.; Paduraru, A.V.; Musuc, A.M.; Trusca, R.; Oprea, O.; Tanasa, E.; Vasile, O.R.; Nicoara, A.I.; et al. Photoluminescent Hydroxylapatite: Eu3+ Doping Effect on Biological Behaviour. Nanomaterials 2019, 9, 1187. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-W.; Kim, Y.-H.; Du, W.X.; Kim, J.-Y. Stretchable and Low-Haze Ag-Nanowire-Network 2-D Films Embedded into a Cross-linked Polydimethylsiloxane Elastomer. Nanomaterials 2019, 9, 576. [Google Scholar] [CrossRef]
- Cernea, M.; Vasile, B.S.; Surdu, V.A.; Trusca, R.; Bartha, C.; Craciun, F.; Galassi, C. Probing the dielectric, piezoelectric and magnetic behavior of CoFe2O4/BNT-BT 0.08 composite thin film fabricated by sol-gel and spin-coating methods. Sci. Rep. 2018, 8, 17883. [Google Scholar] [CrossRef]
- Islam, M.R.; Bach, L.G.; Nga, T.T.; Lim, K.T. Covalent ligation of gold coated iron nanoparticles to the multi-walled carbon nanotubes employing click chemistry. J. Alloys Compd. 2013, 561, 201–205. [Google Scholar] [CrossRef]
- Tran, T.V.; Nguyen, D.T.C.; Le, H.T.N.; Tu, T.T.K.; Le, N.D.; Lim, K.T.; Bach, L.G.; Nguyen, T.D. MIL-53 (Fe)-directed synthesis of hierarchically mesoporous carbon and its utilization for ciprofloxacin antibiotic remediation. J. Environ. Chem. Eng. 2019, 7, 102881. [Google Scholar] [CrossRef]
- Islam, M.R.; Bach, L.G.; Jung, S.J.; Lim, K.T. Facile Synthesis, Characterization, and Optical Properties of Ag+Doped ZnS Nanocrystals via Co-precipitation Method using Thioglycerol as a Capping Agent. Mol. Cryst. Liq. Cryst. 2013, 583, 134–140. [Google Scholar] [CrossRef]
- Le, V.T.; Bach, L.G.; Pham, T.T.; Le, N.T.T.; Ngoc, U.T.P.; Tran, D.-H.N.; Nguyen, D.H. Synthesis and antifungal activity of chitosan-silver nanocomposite synergize fungicide against Phytophthora capsici. J. Macromol. Sci. Part A 2019, 56, 522–528. [Google Scholar] [CrossRef]
- Haynes, C.L.; Van Duyne, R.P. Plasmon-Sampled Surface-Enhanced Raman Excitation Spectroscopy. J. Phys. Chem. B 2003, 107, 7426–7433. [Google Scholar] [CrossRef]
- Zou, X.; Dong, S. Surface-Enhanced Raman Scattering Studies on Aggregated Silver Nanoplates in Aqueous Solution. J. Phys. Chem. B 2006, 110, 21545–21550. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yao, K.; Wang, J.; Yuan, J. Ionic liquids–water interfacial preparation of triangular Ag nanoplates and their shape-dependent antibacterial activity. J. Colloid Interface Sci. 2015, 437, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, B.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Seong, M.; Lee, D.G. Silver nanoparticles against Salmonella enterica serotype typhimurium: role of inner membrane dysfunction. Curr. Microbiol. 2017, 74, 661–670. [Google Scholar] [CrossRef]
- Khalandi, B.; Asadi, N.; Milani, M.; Davaran, S.; Abadi, A.J.N.; Abasi, E.; Akbarzadeh, A. A review on potential role of silver nanoparticles and possible mechanisms of their actions on bacteria. Drug Res. (Stuttg) 2017, 67, 70–76. [Google Scholar] [CrossRef]
- Zhao, R.; Lv, M.; Li, Y.; Sun, M.; Kong, W.; Wang, L.; Song, S.; Fan, C.; Jia, L.; Qiu, S.; et al. Stable nanocomposite based on PEGylated and silver nanoparticles loaded graphene oxide for long-term antibacte-rial activity. ACS Appl. Mater. Interfaces 2017, 9, 15328–15341. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Silver nanoparticles as an antimicrobial agent: a case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Siritongsuk, P.; Hongsing, N.; Thammawithan, S.; Daduang, S.; Klaynongsruang, S.; Tuanyok, A.; Patramanon, R. Two-phase bactericidal mechanism of silver nanoparticles against Burkholderia pseudomallei. PLoS ONE 2016, 11, e0168098. [Google Scholar]
- Kvítek, L.; Panáček, A.; Soukupová, J.; Kolář, M.; Večeřová, R.; Prucek, R.; Holecová, M.; Zbořil, R. Effect of Surfactants and Polymers on Stability and Antibacterial Activity of Silver Nanoparticles (NPs). J. Phys. Chem. C 2008, 112, 5825–5834. [Google Scholar] [CrossRef]
- Millstone, J.E.; Hurst, S.J.; Métraux, G.S.; Cutler, J.I.; Mirkin, C.A. Colloidal Gold and Silver Triangular Nanoprisms. Small 2009, 5, 646–664. [Google Scholar] [CrossRef]
- Chen, S.; Carroll, D.L. Synthesis and Characterization of Truncated Triangular Silver Nanoplates. Nano Lett. 2002, 2, 1003–1007. [Google Scholar] [CrossRef]
- Chen, S.; Fan, Z.; Carroll, D.L. Silver Nanodisks: Synthesis, Characterization, and Self-Assembly. J. Phys. Chem. B 2002, 106, 10777–10781. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Xia, Y. Transformation of Silver Nanospheres into Nanobelts and Triangular Nanoplates through a Thermal Process. Nano Lett. 2003, 3, 675–679. [Google Scholar] [CrossRef]
- Wang, C.-S.; Virgilio, N.; Wood-Adams, P.M.; Heuzey, M.-C. A gelation mechanism for gelatin/polysaccharide aqueous mixtures. Food Hydrocoll. 2018, 79, 462–472. [Google Scholar] [CrossRef]
- de Kruif, C.G.; Weinbreck, F.; de Vries, R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2004, 9, 340–349. [Google Scholar] [CrossRef]
- Suarasan, S.; Focsan, M.; Soritau, O.; Maniu, D.; Astilean, S. One-pot, green synthesis of gold nanoparticles by gelatin and investigation of their biological effects on Osteoblast cells. Colloids Surfaces B Biointerfaces 2015, 132, 122–131. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-C.; Hong, Y.-H. Coacervate formation of α-lactalbumin–chitosan and β-lactoglobulin–chitosan complexes. Food Res. Int. 2009, 42, 733–738. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Ledwith, D.M.; Whelan, A.M.; Kelly, J.M. A rapid, straight-forward method for controlling the morphology of stable silver nanoparticles. J. Mater. Chem. 2007, 17, 2459–2464. [Google Scholar] [CrossRef]
- Stevenson, K.; McVey, A.F.; Clark, I.B.N.; Swain, P.S.; Pilizota, T. General cabibration of microbial growth in microplate readers. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 36, 493–496. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol; American Society for microbiology: Washington, DC, USA, 2009; pp. 1–23. [Google Scholar]
- Lorian, V.; Atkinson, B.A. Determination of the range of antibacterial activity by use of viable counts. J. Clin. Microbiol. 1982, 16, 70–76. [Google Scholar]
- Sanders, E.R. Aseptic Laboratory Techniques: Plating Methods. J. Vis. Exp. 2012, 63, 3064. [Google Scholar] [CrossRef]
- Xue, C.; Mirkin, C.A. pH-Switchable Silver Nanoprism Growth Pathways. Angew. Chem. Int. Ed. 2007, 46, 2036–2038. [Google Scholar] [CrossRef]
- Shuford, K.L.; Ratner, M.A.; Schatz, G.C. Multipolar excitation in triangular nanoprisms. J. Chem. Phys. 2005, 123, 114713. [Google Scholar] [CrossRef] [PubMed]
- Lofton, C.; Sigmund, W. Mechanisms Controlling Crystal Habits of Gold and Silver Colloids. Adv. Funct. Mater. 2005, 15, 1197–1208. [Google Scholar] [CrossRef]
- Wang, C.-S.; Natale, G.; Virgilio, N.; Heuzey, M.-C. Synergistic gelation of gelatin B with xanthan gum. Food Hydrocoll. 2016, 60, 374–383. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, C.-S.; Heuzey, M.-C. Complexation of chitosan and gelatin: From soluble complexes to colloidal gel. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 96–104. [Google Scholar] [CrossRef]
- Pelc, D.; Marion, S.; Požek, M.; Basletić, M. Role of microscopic phase separation in gelation of aqueous gelatin solutions. Soft Matter 2014, 10, 348–356. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Y.; Wang, W.; Huang, C.; Wang, Y.; Yu, Z.; Zhang, H. Influence of pH of Gelatin Solution on Cycle Performance of the Sulfur Cathode. J. Electrochem. Soc. 2010, 157, A443–A446. [Google Scholar] [CrossRef]
- Torii, H. Theoretical Analyses of the Amide I Infrared Bands of Globular Proteins, Infrared Spectroscopy of Biomolecules; Tasumi, M., Mantsch, H.H., Chapman, D., Eds.; Wiley-Liss Inc.: Flatbush, NY, USA, 1996; Chapter 1. [Google Scholar]
- Venkatesham, M.; Ayodhya, D.; Madhusudhan, A.; Babu, .N.V.; Veerabhadram, G. A novel green one-step synthesis of silver nanoparticles using chitosan; catalytic activity and antimicrobial studies. Appl. Nanosci. 2012, 4, 113–119. [Google Scholar] [CrossRef]
- Antoniades, M.G.; Wey, J.S. The effect of coalescence on AgBr tabular grain formation. J. Imaging Sci. Technol. 1995, 39, 323–331. [Google Scholar]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Hwang, E.; Lee, J.; Chae, Y.; Kim, Y.; Kim, B.; Sang, B.; Gu, M. Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small 2008, 4, 746–750. [Google Scholar] [CrossRef]
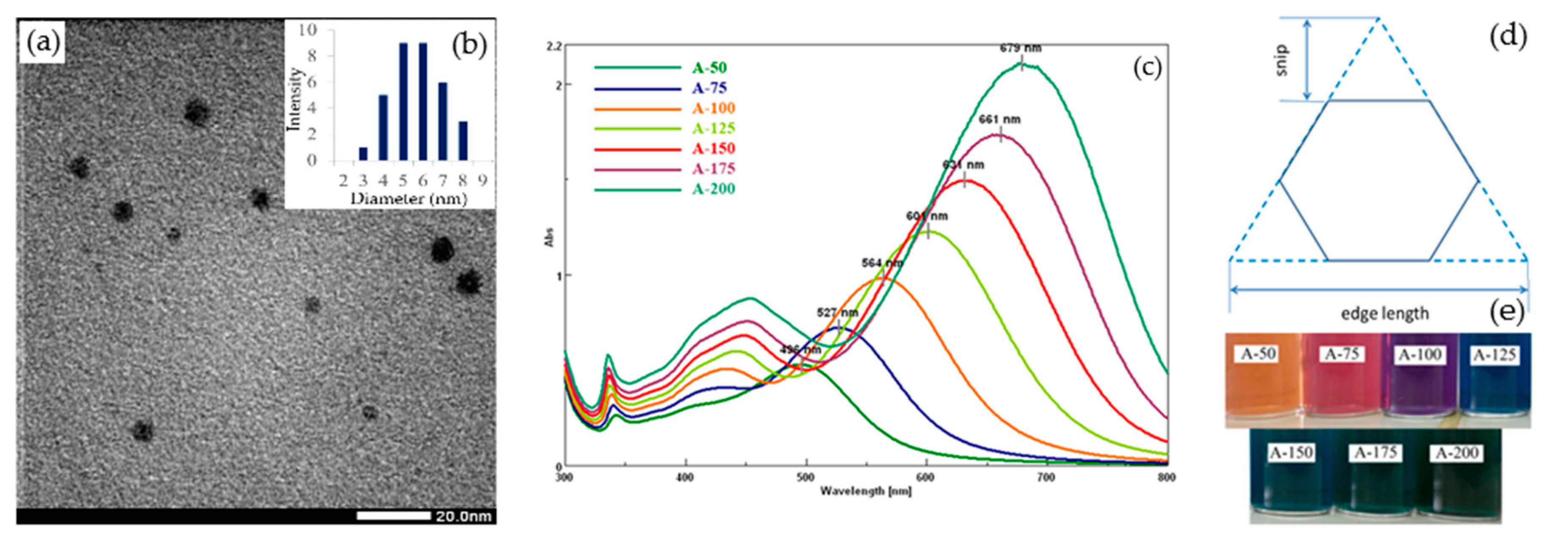
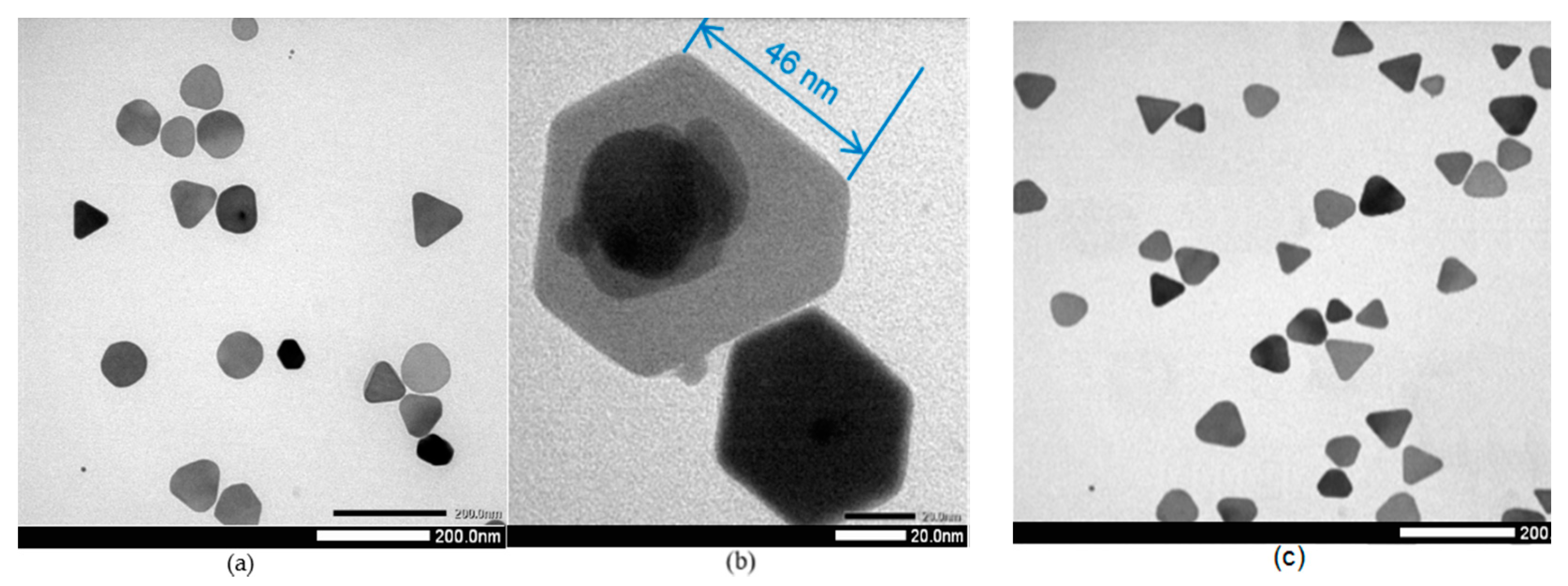
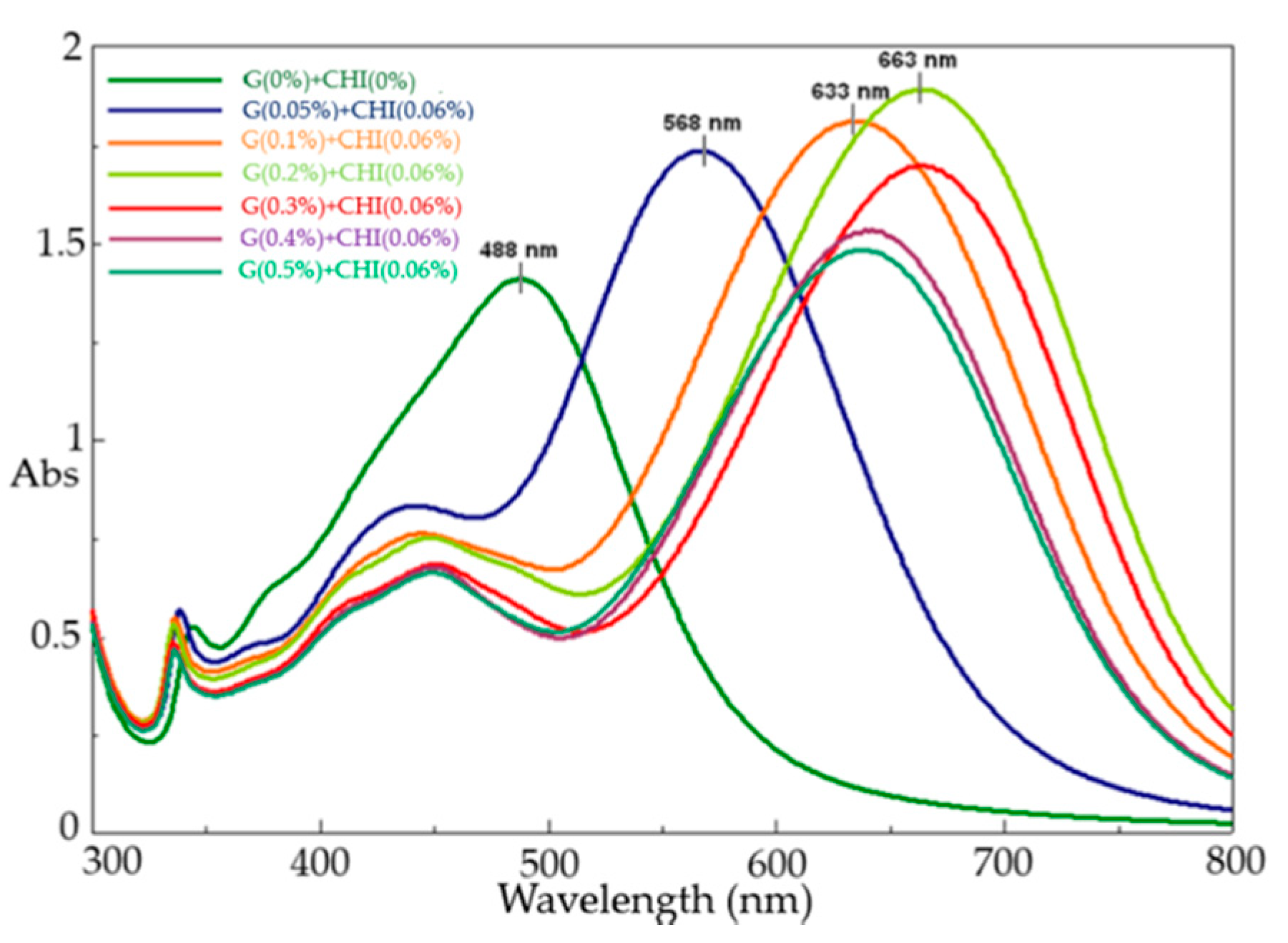


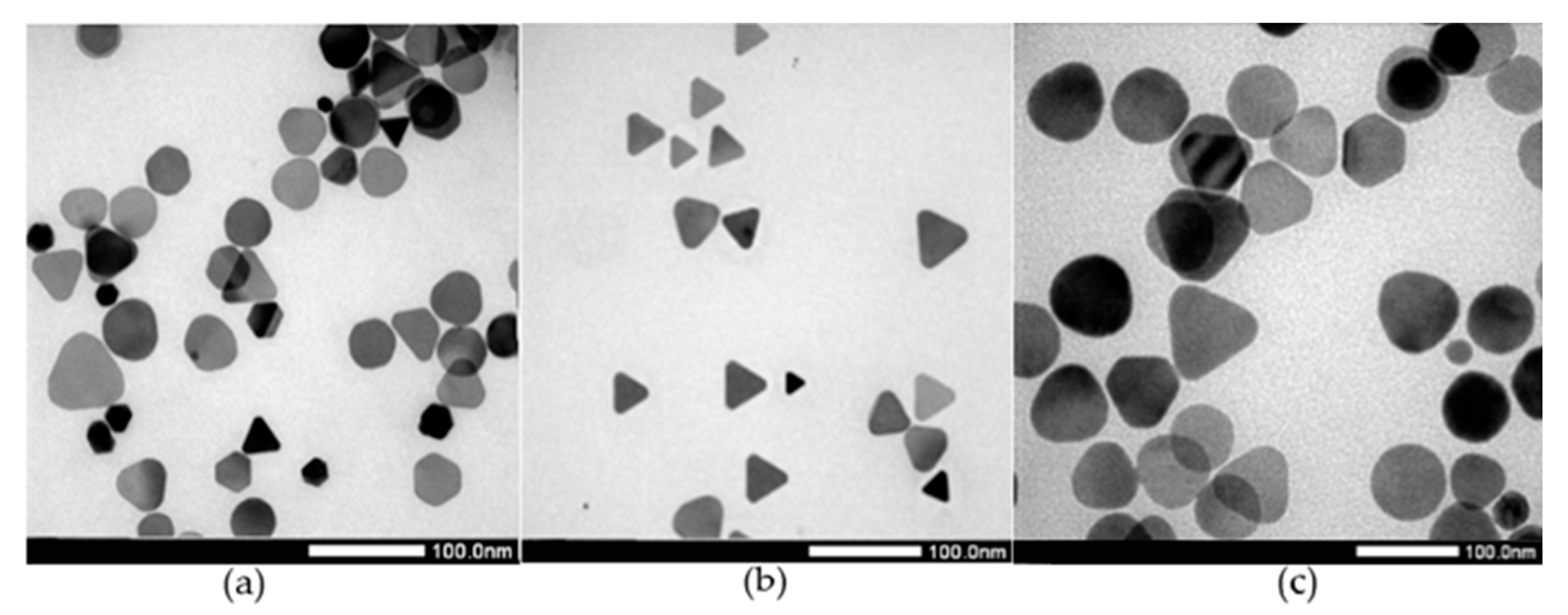


| Sample | Peak 1 | Peak 2 | Peak 3 | |||
|---|---|---|---|---|---|---|
| λmax (nm) | Abs | λmax (nm) | Abs | λmax (nm) | Abs | |
| G(0%) + CHI(0%) | 342 | 0.525 | - | - | 488 | 1.40 |
| G(0.05%) + CHI(0.06%) | 342 | 0.566 | 441 | 0.832 | 568 | 1.73 |
| G(0.1%) + CHI(0.06%) | 336 | 0.547 | 445 | 0.761 | 633 | 1.81 |
| G(0.2%) + CHI(0.06%) | 335 | 0.530 | 449 | 0.751 | 663 | 1.89 |
| G(0.3%) + CHI(0.06%) | 335 | 0.485 | 451 | 0.684 | 664 | 1.70 |
| G(0.4%) + CHI(0.06%) | 336 | 0.470 | 450 | 0.676 | 639 | 1.53 |
| G(0.5%) + CHI(0.06%) | 336 | 0.465 | 450 | 0.661 | 638 | 1.48 |
| Sample | Peak 1 | Peak 2 | Peak 3 | |||
|---|---|---|---|---|---|---|
| λmax (nm) | Abs | λmax (nm) | Abs | λmax (nm) | Abs | |
| pH 3.0 | 412 | 2.12 | - | - | - | - |
| pH 4.0 | 439 | 2.04 | - | - | - | - |
| pH 5.0 | 337 | 0.500 | 435 | 0.770 | 573 | 1.72 |
| pH 6.0 | 338 | 0.660 | 444 | 0.988 | 585 | 1.42 |
| pH 7.0 | 339 | 0.545 | 441 | 0.968 | 598 | 1.14 |
| pH 8.0 | 341 | 0.581 | - | - | 522 | 1.17 |
| pH 9.0 | 427 | 2.17 | - | - | - | - |
| Position (cm−1) Pure Chitosan (Curve b) | Position (cm−1) Pure Gelatin (Curve b) | Position (cm−1) Gelatin–chitosan-AgNPs (Curve c) | Assignment |
|---|---|---|---|
| 3447–2991 | 3447–2991 | –NH2, –OH, –CH2, –CH3 aliphatic group | |
| 3393 | 3304 | Hydrogen bonds of retain water | |
| 1657 | –CONH2 group of chitosan | ||
| 1650 | 1643 | Amide I (C=O stretching vibration) | |
| 1540 | 1547 | Amide II (N–H bending vibration) | |
| 1400–1500 | COO– symmetrical stretching vibration | ||
| 1422 | –OH group of the primary alcoholic group |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, Q.K.; Phung, D.D.; Vo Nguyen, Q.N.; Hoang Thi, H.; Nguyen Thi, N.H.; Nguyen Thi, P.P.; Bach, L.G.; Van Tan, L. Controlled Synthesis of Triangular Silver Nanoplates by Gelatin–Chitosan Mixture and the Influence of Their Shape on Antibacterial Activity. Processes 2019, 7, 873. https://doi.org/10.3390/pr7120873
Vo QK, Phung DD, Vo Nguyen QN, Hoang Thi H, Nguyen Thi NH, Nguyen Thi PP, Bach LG, Van Tan L. Controlled Synthesis of Triangular Silver Nanoplates by Gelatin–Chitosan Mixture and the Influence of Their Shape on Antibacterial Activity. Processes. 2019; 7(12):873. https://doi.org/10.3390/pr7120873
Chicago/Turabian StyleVo, Quoc Khuong, Duc Duy Phung, Quynh Nhu Vo Nguyen, Hong Hoang Thi, Nhat Hang Nguyen Thi, Phuong Phong Nguyen Thi, Long Giang Bach, and Lam Van Tan. 2019. "Controlled Synthesis of Triangular Silver Nanoplates by Gelatin–Chitosan Mixture and the Influence of Their Shape on Antibacterial Activity" Processes 7, no. 12: 873. https://doi.org/10.3390/pr7120873
APA StyleVo, Q. K., Phung, D. D., Vo Nguyen, Q. N., Hoang Thi, H., Nguyen Thi, N. H., Nguyen Thi, P. P., Bach, L. G., & Van Tan, L. (2019). Controlled Synthesis of Triangular Silver Nanoplates by Gelatin–Chitosan Mixture and the Influence of Their Shape on Antibacterial Activity. Processes, 7(12), 873. https://doi.org/10.3390/pr7120873




