Energetic Analysis of Different Configurations of Power Plants Connected to Liquid Chemical Looping Gasification
Abstract
1. Introduction
2. Materials and Methods
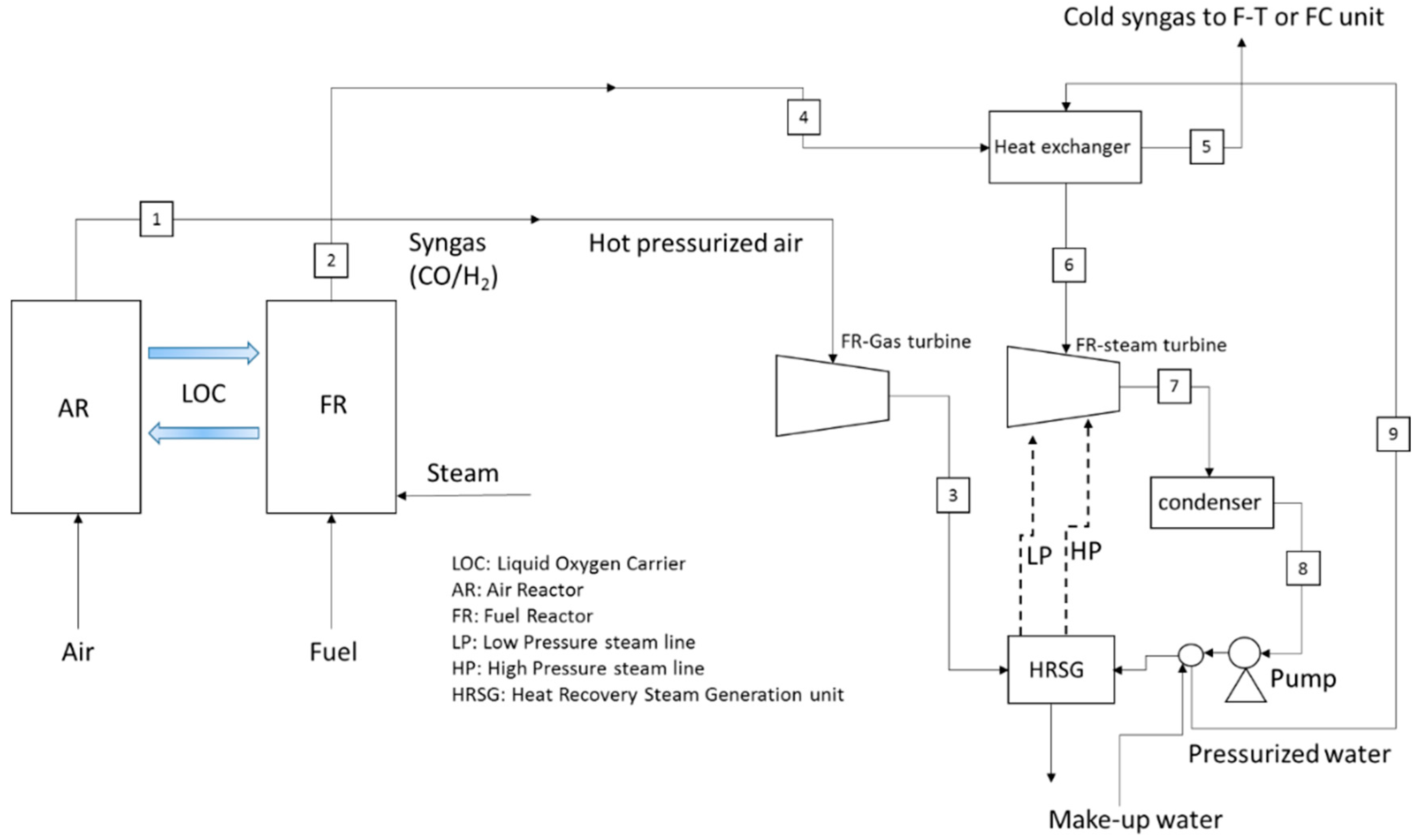
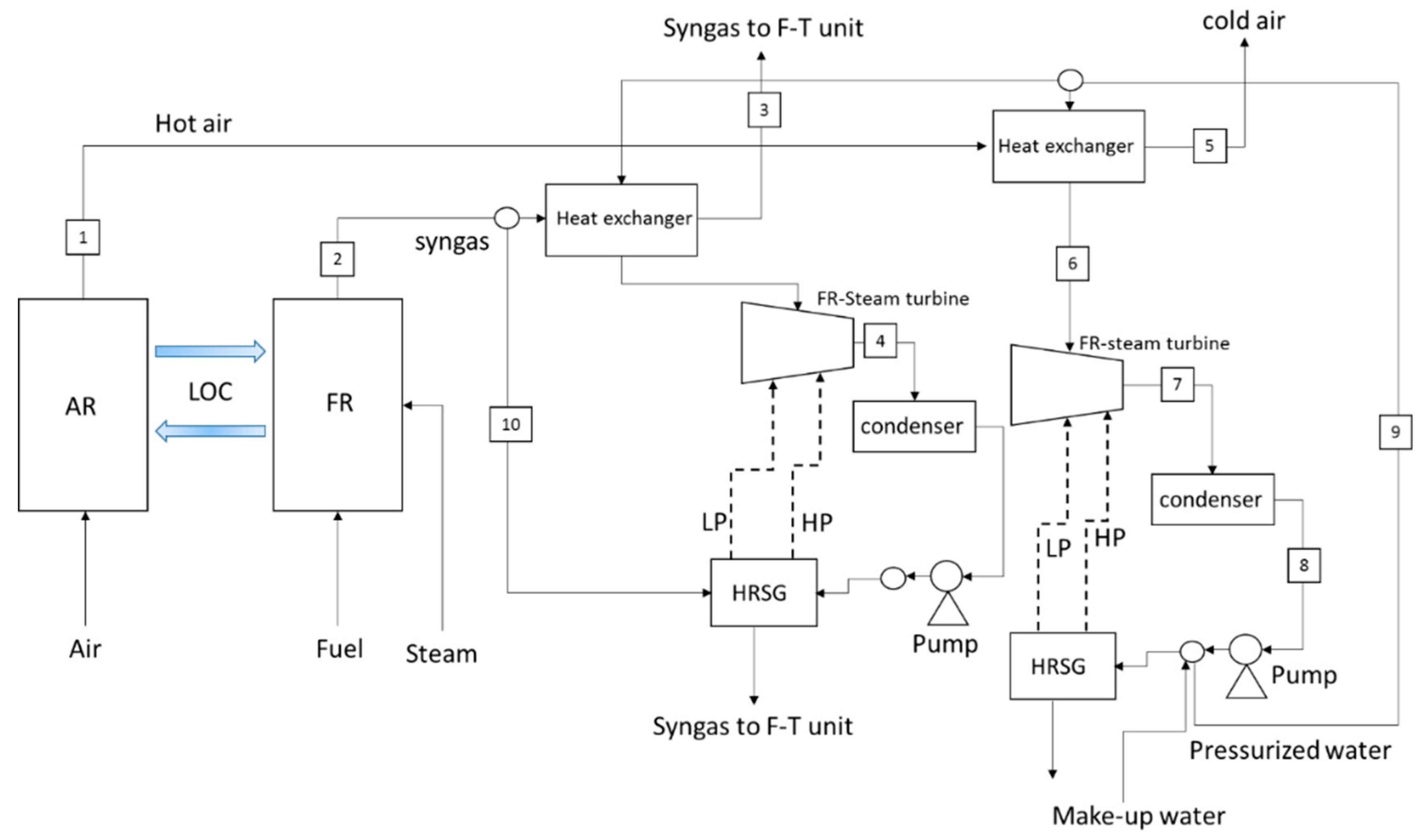
2.1. LCLG Syngas Production Plant and Power Blocks
2.2. Simulation of the LCLG and Power Plants
2.3. Operating Regime of the LCLG
3. Results
3.1. Operating Temperature in the Air Reactor
3.2. Operating Pressure in the Air Reactor
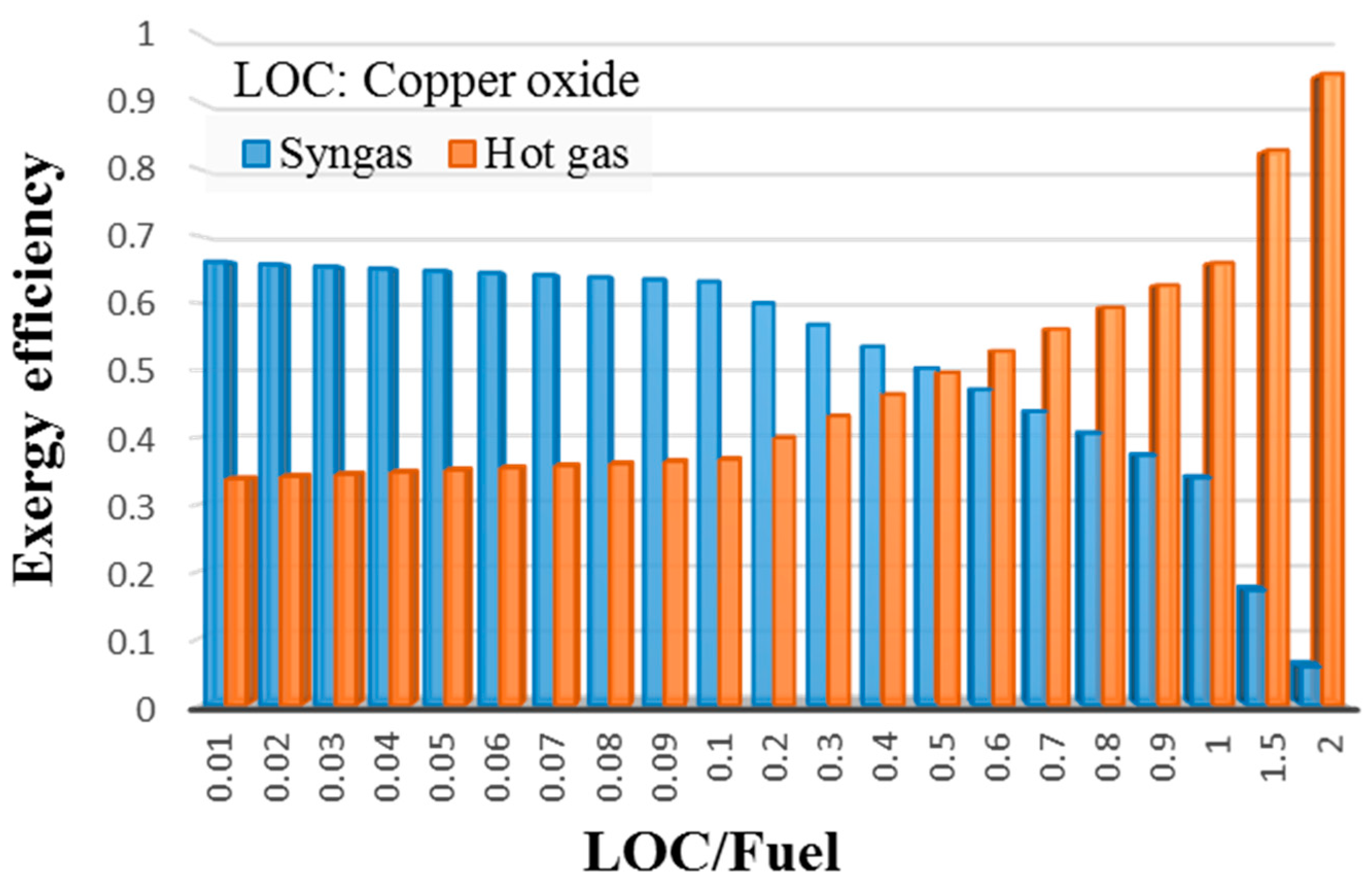
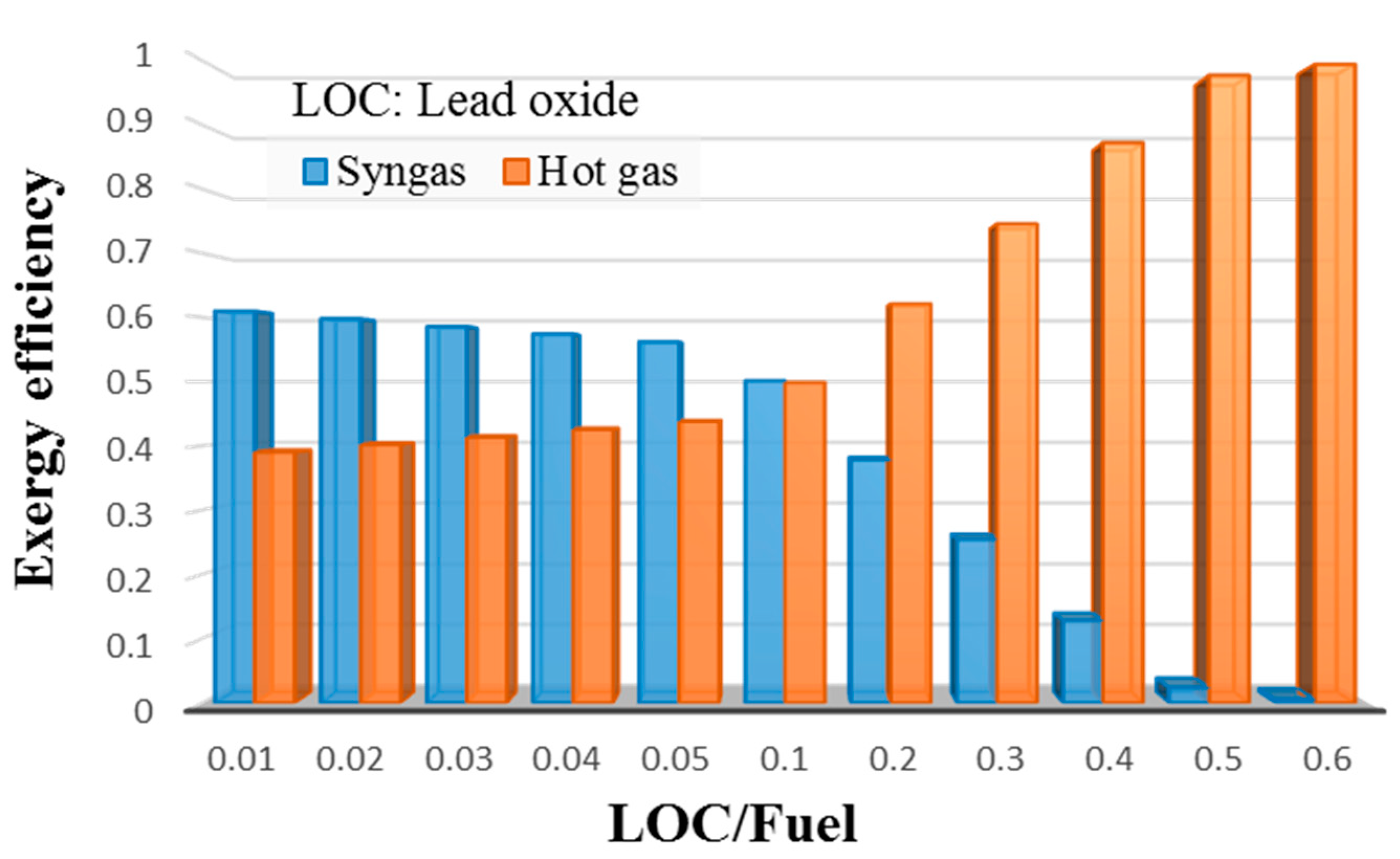
3.3. Exergy Analysis of LCLG
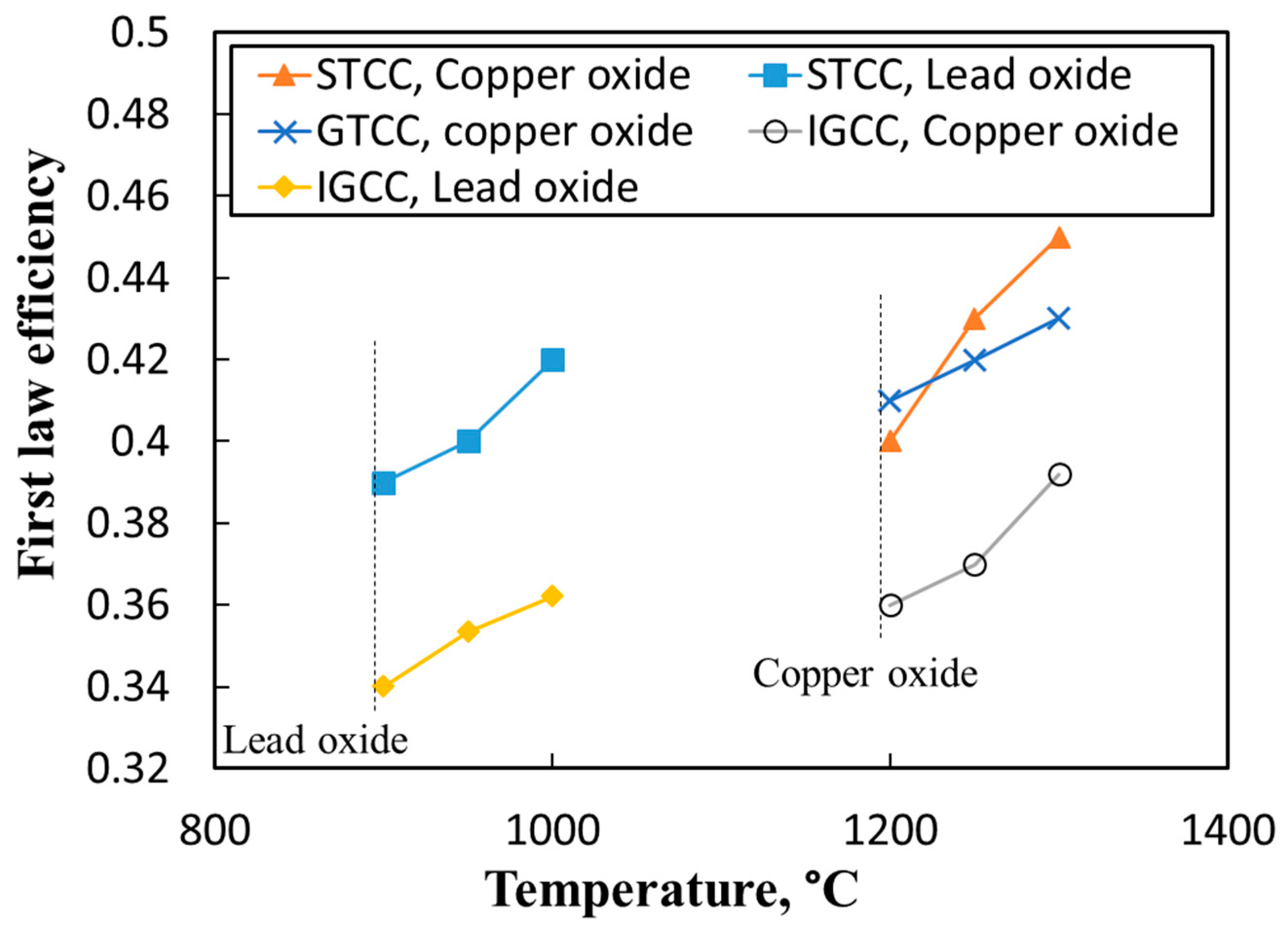
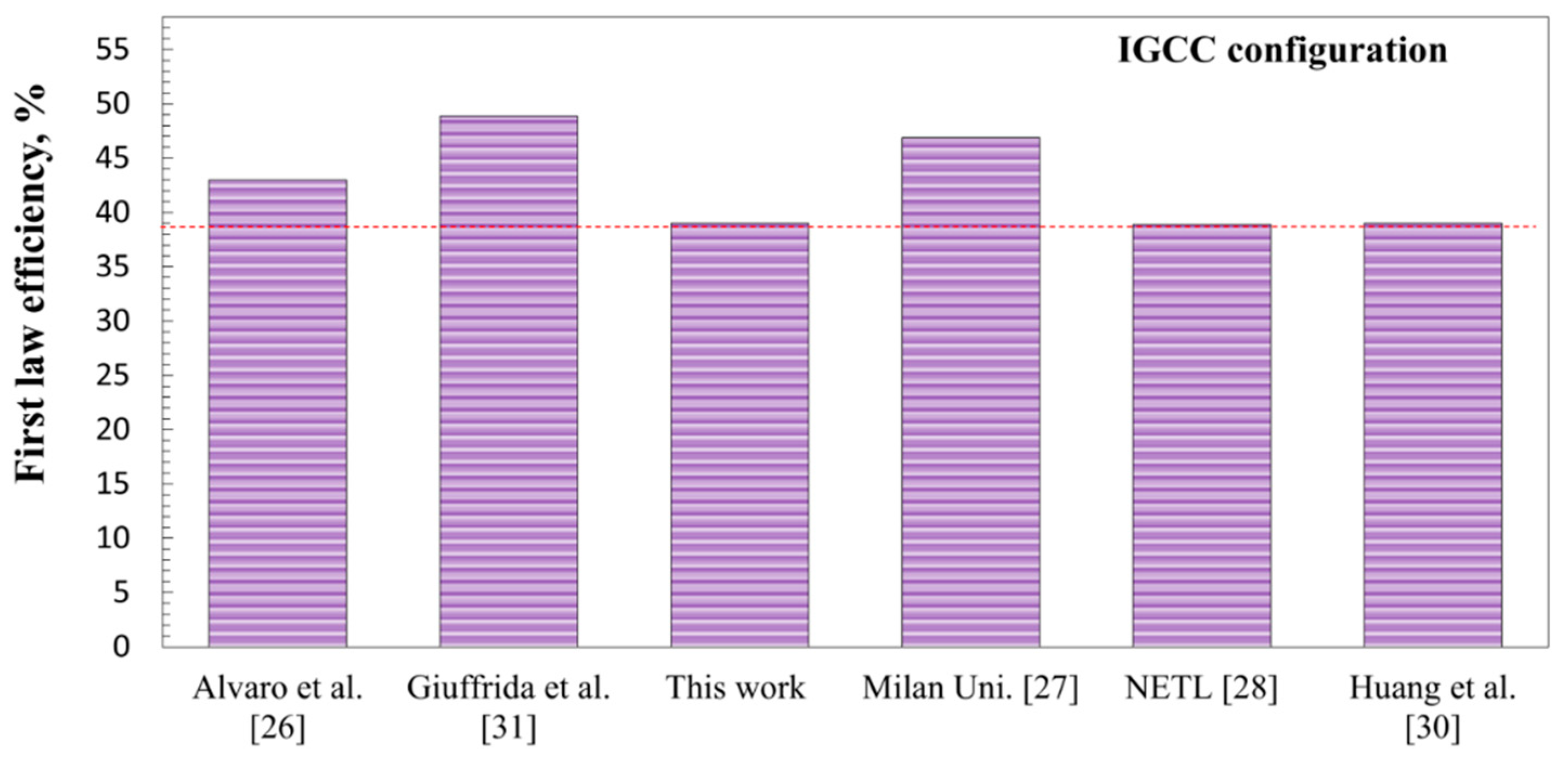
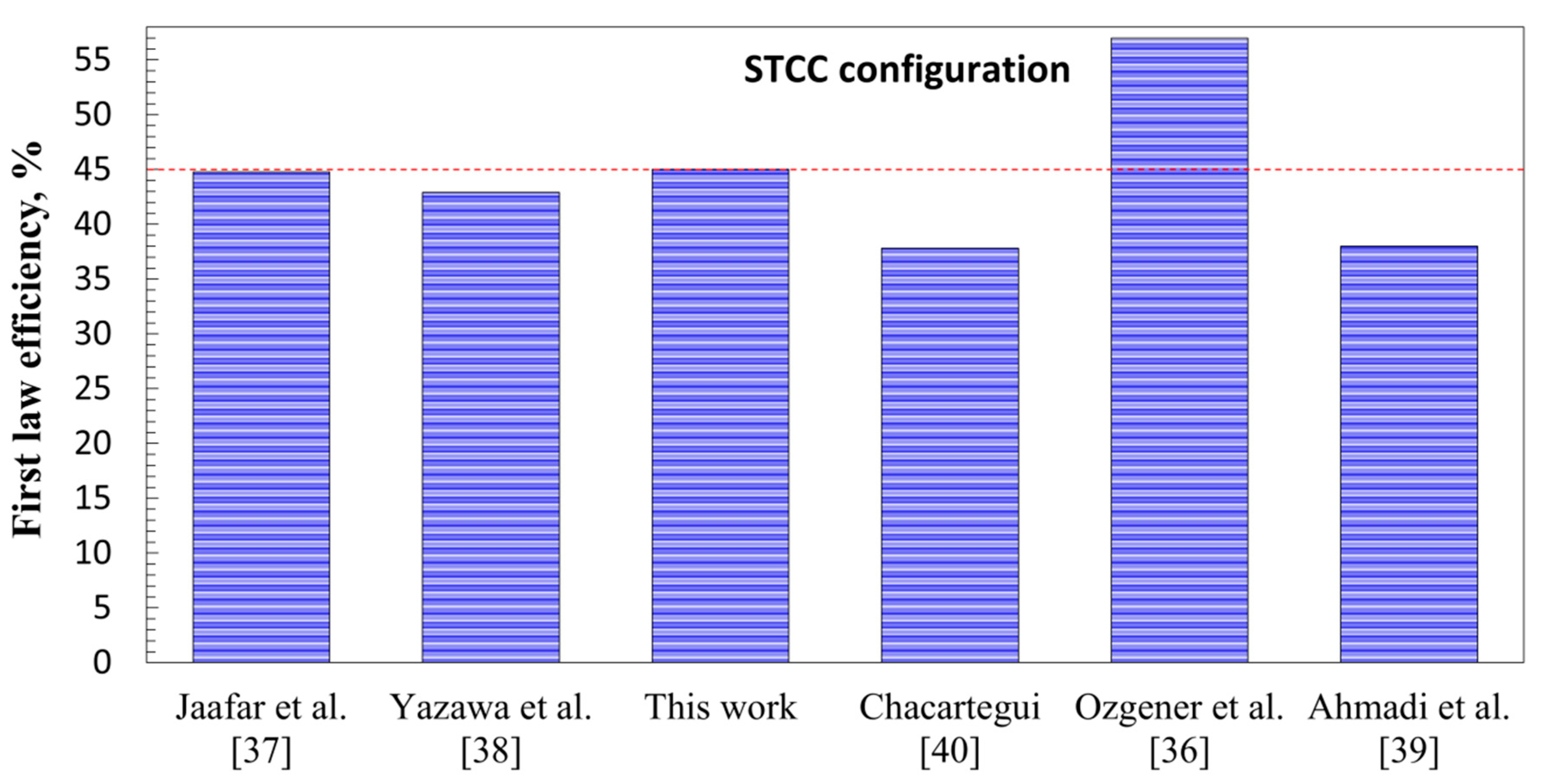
3.4. Comparison of the Proposed Power Cycles and Those Available in the Literature
4. Conclusions
- (1)
- Exergy analysis of the LCLG system working with molten lead oxide showed that had a strong influence on the quality and level of exergy carried by syngas. For , more exergy was carried by the syngas produced, while for values , hot air carried the higher exergy as the quality of syngas reduced. This meant that the LCLG system working at could provide more work by power block, while syngas could provide more work in Fischer–Tropsch or other applications.
- (2)
- When copper oxide was used in LCLG, for , more exergy was carried by syngas, and at , the system was capable of producing more work by power block.
- (3)
- The STCC integrated with the LCLG system working with lead oxide showed that the first law and exergy efficiency for achieved values of 0.43 and 0.68, respectively. It provided a cold and clean stream of high-quality syngas, which can be used in a Fischer–Tropsch unit.
- (4)
- The IGCC integrated with the LCLG system working with both copper oxide and lead oxide showed lower efficiency in comparison with the STCC and GTCC power blocks. This is because in outlet syngas, nitrogen is omitted and the quality of syngas is high enough for other chemical applications rather than combustion or producing work.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Ex | Exergy, kJ/kmol |
| W | Work, kJ |
| Molar flow rate, kmol/hr | |
| Greek letters | |
| Liquid oxygen carrier to fuel ratio or steam to fuel ratio | |
| Difference | |
| First law efficiency, % | |
| Exergy efficiency, % | |
| Abbreviations | |
| AR | Air Reactor |
| Eq. | Equation |
| FR | Fuel Reactor |
| GT | Gas Turbine |
| GTCC | Gas Turbine Combined Cycle |
| ST | Steam Turbine |
| STCC | Steam Turbine Combined Cycle |
| LHV | Lower Heating Value, MJ/kg |
| LOC | Liquid Oxygen Carrier |
References
- Ge, H.; Zhang, H.; Guo, W.; Song, T.; Shen, L. System simulation and experimental verification: Biomass-based integrated gasification combined cycle (BIGCC) coupling with chemical looping gasification (CLG) for power generation. Fuel 2019, 241, 118–128. [Google Scholar] [CrossRef]
- Hu, J.; Li, C.; Guo, Q.; Dang, J.; Zhang, Q.; Lee, D.-J.; Yang, Y. Syngas production by chemical-looping gasification of wheat straw with Fe-based oxygen carrier. Bioresour. Technol. 2018, 263, 273–279. [Google Scholar] [CrossRef]
- Li, F.; Kim, H.R.; Sridhar, D.; Wang, F.; Zeng, L.; Chen, J.; Fan, L.-S. Syngas chemical looping gasification process: Oxygen carrier particle selection and performance. Energy Fuels 2009, 23, 4182–4189. [Google Scholar] [CrossRef]
- Dadsetani, R.; Sheikhzadeh, G.A.; Safaei, M.R.; Alnaqi, A.A.; Amiriyoon, A. Exergoeconomic optimization of liquefying cycle for noble gas argon. Heat Mass Transf. 2019, 55, 1995–2007. [Google Scholar] [CrossRef]
- Fan, L.; Li, F.; Ramkumar, S. Utilization of chemical looping strategy in coal gasification processes. Particuology 2008, 6, 131–142. [Google Scholar] [CrossRef]
- Scott, S.; Dennis, J.; Hayhurst, A.; Brown, T. In situ gasification of a solid fuel and CO2 separation using chemical looping. AIChE J. 2006, 52, 3325–3328. [Google Scholar] [CrossRef]
- Safaiy, M.; Maghmoumi, Y.; Karimipour, A. Economic evaluation of utilization of electro-feed water pump and turbo-feed water pump and compare them in a 12.5-megawatts steam unit thermal cycle and provide the optimum solution. AIP Conf. Proc. 2012, 1440, 550–555. [Google Scholar]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; Luis, F. Progress in chemical-looping combustion and reforming technologies. Progress Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Jafarian, M.; Arjomandi, M.; Nathan, G.J. Potential use of liquid metal oxides for chemical looping gasification: A thermodynamic assessment. Appl. Energy 2017, 195, 702–712. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Jafarian, M.; Arjomandi, M.; Nathan, G.J. The relative performance of alternative oxygen carriers for liquid chemical looping combustion and gasification. Int. J. Hydrogen Energy 2017, 42, 16396–16407. [Google Scholar] [CrossRef]
- Cormos, A.-M.; Cormos, C.-C. Investigation of hydrogen and power co-generation based on direct coal chemical looping systems. Int. J. Hydrogen Energy 2014, 39, 2067–2077. [Google Scholar] [CrossRef]
- Cormos, C.-C. Evaluation of syngas-based chemical looping applications for hydrogen and power co-generation with CCS. Int. J. Hydrogen Energy 2012, 37, 13371–13386. [Google Scholar] [CrossRef]
- Maurstad, O. An Overview of Coal Based Integrated Gasification Combined Cycle (IGCC. In Proceedings of Technology; MIT LFEE 2005-002 WP; Laboratory for Energy and the Environment, Massachusetts Institute of Technology: Cambridge, MA, USA, 2005. [Google Scholar]
- Ståhl, K.; Neergaard, M. IGCC power plant for biomass utilisation, Värnamo, Sweden. Biomass Bioenergy 1998, 15, 205–211. [Google Scholar] [CrossRef]
- Gasification, I. An Overview of Coal Based Integrated Gasification Combined Cycle (IGCC) Technology; Cambridge, MA, USA, 2005. Available online: citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.208.5891&rep=rep1&type=pdf (accessed on 22 September 2019).
- Manente, G.; Lazzaretto, A. Innovative biomass to power conversion systems based on cascaded supercritical CO2 Brayton cycles. Biomass Bioenergy 2014, 69, 155–168. [Google Scholar] [CrossRef]
- Traverso, A.; Massardo, A.F.; Scarpellini, R. Externally fired micro-gas turbine: Modelling and experimental performance. Appl. Therm. Eng. 2006, 26, 1935–1941. [Google Scholar] [CrossRef]
- Bracht, M.; Alderliesten, P.; Kloster, R.; Pruschek, R.; Haupt, G.; Xue, E.; Ross, J.; Koukou, M.; Papayannakos, N. Water gas shift membrane reactor for CO2 control in IGCC systems: Techno-economic feasibility study. Energy Convers. Manag. 1997, 38, S159–S164. [Google Scholar] [CrossRef]
- Chiesa, P.; Kreutz, T.G.; Lozza, G.G. CO2 sequestration from IGCC power plants by means of metallic membranes. J. Eng. Gas Turbines Power 2007, 129, 123–134. [Google Scholar] [CrossRef]
- Holt, N.; Booras, G.; Todd, D. A summary of recent IGCC studies of CO2 capture for sequestration. In Proceedings of the Gasification Technologies Conference, San Francisco, CA, USA, 12–15 October 2003; pp. 12–15. [Google Scholar]
- Mukherjee, S.; Kumar, P.; Yang, A.; Fennell, P. Energy and exergy analysis of chemical looping combustion technology and comparison with pre-combustion and oxy-fuel combustion technologies for CO2 capture. J. Environ. Chem. Eng. 2015, 3, 2104–2114. [Google Scholar] [CrossRef]
- Jafarian, M.; Arjomandi, M.; Nathan, G.J. The energetic performance of a novel hybrid solar thermal & chemical looping combustion plant. Appl. Energy 2014, 132, 74–85. [Google Scholar] [CrossRef]
- Abbott, M.M.; Smith, J.M.; Van Ness, H.C. Introduction to Chemical Engineering Thermodynamics; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Shishin, D.; Decterov, S.A. Critical assessment and thermodynamic modeling of the Cu–O and Cu–O–S systems. Calphad 2012, 38, 59–70. [Google Scholar] [CrossRef]
- Risold, D.; Nagata, J.-I.; Suzuki, R. Thermodynamic description of the Pb-O system. J. Phase Equilibria 1998, 19, 213–233. [Google Scholar] [CrossRef]
- Hong, H.; Han, T.; Jin, H. A low temperature solar thermochemical power plant with CO2 recovery using methanol-fueled chemical looping combustion. J. Sol. Energy Eng. 2010, 132, 031002. [Google Scholar] [CrossRef]
- Gou, C.; Cai, R.; Hong, H. A novel hybrid oxy-fuel power cycle utilizing solar thermal energy. Energy 2007, 32, 1707–1714. [Google Scholar] [CrossRef]
- Naqvi, R.; Wolf, J.; Bolland, O. Part-load analysis of a chemical looping combustion (CLC) combined cycle with CO2 capture. Energy 2007, 32, 360–370. [Google Scholar] [CrossRef]
- Álvaro, Á.J.; Paniagua, I.L.; Fernández, C.G.; Martín, J.R.; Carlier, R.N. Simulation of an integrated gasification combined cycle with chemical-looping combustion and carbon dioxide sequestration. Energy Convers. Manag. 2015, 104, 170–179. [Google Scholar]
- Anantharaman, R.; Bolland, O.; Booth, N.; van Dorst, E.; Ekstrom, C.; Sanchez Fernandes, E.; Franco, F.; Macchi, E.; Manzolini, G.; Nikolic, D. European best practice guidelines for assessment of CO2 capture technologies. SINTEF Trondheim Norway. 2007, 1. Available online: www.academia.edu/download/45301000/D_1.4.3_Euro_BP_Guid_for_Ass_CO2_Cap_Tech_280211.pdf (accessed on 22 September 2019).
- Coal, B.; Gas, N. Fossil Energy Power Plant Desk Reference; USA, 2007. Available online: citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.222.3202&rep=rep1&type=pdf (accessed on 22 September 2019).
- Cormos, C.-C. Integrated assessment of IGCC power generation technology with carbon capture and storage (CCS). Energy 2012, 42, 434–445. [Google Scholar] [CrossRef]
- Huang, Y.; Rezvani, S.; McIlveen-Wright, D.; Minchener, A.; Hewitt, N. Techno-economic study of CO2 capture and storage in coal fired oxygen fed entrained flow IGCC power plants. Fuel Process. Technol. 2008, 89, 916–925. [Google Scholar] [CrossRef]
- Giuffrida, A.; Romano, M.C.; Lozza, G. Efficiency enhancement in IGCC power plants with air-blown gasification and hot gas clean-up. Energy 2013, 53, 221–229. [Google Scholar] [CrossRef]
- Aichmayer, L.; Spelling, J.; Laumert, B. Thermoeconomic Analysis of a Solar Dish Micro Gas-turbine Combined-cycle Power Plant. Energy Procedia 2015, 69, 1089–1099. [Google Scholar] [CrossRef]
- Taimoor, A.A.; Muhammad, A.; Saleem, W. Humidified exhaust recirculation for efficient combined cycle gas turbines. Energy 2016, 106, 356–366. [Google Scholar] [CrossRef]
- Aminov, Z.; Nakagoshi, N.; Xuan, T.D.; Higashi, O.; Alikulov, K. Evaluation of the energy efficiency of combined cycle gas turbine. Case study of Tashkent thermal power plant, Uzbekistan. Appl. Therm. Eng. 2016, 103, 501–509. [Google Scholar] [CrossRef]
- Del Rio, M.S.; Lucquiaud, M.; Gibbins, J. Maintaining the Power Output of an Existing Coal Plant with the Addition of CO2 Capture: Retrofits Options With Gas Turbine Combined Cycle Plants. Energy Procedia 2014, 63, 2530–2541. [Google Scholar]
- Ersayin, E.; Ozgener, L. Performance analysis of combined cycle power plants: A case study. Renew. Sustain. Energy Rev. 2015, 43, 832–842. [Google Scholar] [CrossRef]
- Ganjehkaviri, A.; Jaafar, M.M.; Hosseini, S. Optimization and the effect of steam turbine outlet quality on the output power of a combined cycle power plant. Energy Convers. Manag. 2015, 89, 231–243. [Google Scholar] [CrossRef]
- Yazawa, K.; Koh, Y.R.; Shakouri, A. Optimization of thermoelectric topping combined steam turbine cycles for energy economy. Appl. Energy 2013, 109, 1–9. [Google Scholar] [CrossRef]
- Ahmadi, G.R.; Toghraie, D. Energy and exergy analysis of Montazeri Steam Power Plant in Iran. Renew. Sustain. Energy Rev. 2016, 56, 454–463. [Google Scholar] [CrossRef]
- Chacartegui, R.; Sánchez, D.; De Escalona, J.M.; Jimenez-Espadafor, F.; Munoz, A.; Sánchez, T. SPHERA project: Assessing the use of syngas fuels in gas turbines and combined cycles from a global perspective. Fuel Process. Technol. 2012, 103, 134–145. [Google Scholar] [CrossRef]







| Operating Condition | Range | Source |
|---|---|---|
| Gasifier | ||
| Operating pressure (for lead oxide) Operating pressure (for copper oxide) | 1–10 bar 1–10 bar | - |
| Operating temperature (for lead oxide) Operating temperature (for copper oxide) | 900–1000 °C 1100–1300 °C | - |
| LOC to fuel ratio (for lead oxide) | 0.01–0.5 | - |
| LOC to fuel ratio (for copper oxide) | 0.01–2 | - |
| Air reactor | ||
| Pressure (for lead oxide) Pressure (for copper oxide) | 1 bar 1 bar | - |
| Temperature (for lead oxide) Temperature (for copper oxide) | 900 °C 1300 °C | - |
| Power cycles | ||
| Gas turbine’s isentropic efficiency | 0.91 | [22,26] |
| Efficiency of the heat exchangers | 0.75 | - |
| Steam turbine’s isentropic efficiency | 0.9 | [27] |
| Pump’s efficiency | 0.75 | [22,28] |
| Gas turbine’s mechanical efficiency | 0.99 | [22] |
| Steam turbine’s mechanical efficiency | 0.99 | [22] |
| No. | IGCC (Copper and Lead Oxide) | GTCC (Only Copper Oxide) | STCC (Copper and Lead Oxide) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| T | P | T | P | T | P | ||||
| 1 | 1300 °C (Cu) 1000 °C (Pb) | 10 bar 10 bar | 1000 tonnes/hr (Cu) 1000 tonnes/hr (Pb) | 1300 °C | 10 bar | 1000 tonnes/hr | 1300 °C (Cu) 1000 °C (Pb) | 1 bar | 1000 tonnes/hr |
| 2 | 1300 °C (Cu) 1000 °C (Pb) | 10 bar 10 bar | 998 tonnes/hr (Cu) and (Pb) | 1300 °C | 1 bar | 1000 tonnes/hr | 1300 °C (Cu) 1000 °C (Pb) | 1 bar | 1000 tonnes/hr |
| 3 | 1250 °C | 10 bar | 81,290 kmol/hr | 1084 | 1 bar | 34,541.2 kmol/hr | 35 °C | 1 bar | 52,158.7 kmol/hr |
| 4 | 1300 °C (Cu) 1000 °C (Pb) | 10 bar | 495 tonnes/hr (Cu) 422 tonnes/hr (Pb) | 1300 °C | 1 bar | 1000 tonnes/hr | 166 °C | 1 bar | 24,590.2 kmol/hr |
| 5 | 42 °C | 1 bar | 495 tonnes/hr (Cu) 422 tonnes/hr (Pb) | 159 °C | 5 bar | 34,541.2 kmol/hr | 66 °C | 1 bar | 16,375 kmol/hr |
| 6 | 610 °C | 60 bar | 30,017 kmol/hr | 600 °C | 60 bar | 20,108 kmol/hr | 600 °C | 60 bar | 16,375 kmol/hr |
| 7 | 158 °C | 1 bar | 30,017 kmol/hr | 164 °C | 1 bar | 29,586 kmol/hr | 163 °C | 1 bar | 16375 kmol/hr |
| 8 | 160 °C | 1 bar | 81,290 kmol/hr | 58 °C | 1 bar | 29,586 kmol/hr | 54 °C | 1 bar | 16,375 kmol/hr |
| 9 | 38 °C | 1 bar | 30,017 kmol/hr | 58 °C | 60 bar | 20,108 kmol/hr | 66 °C | 60 bar | 10,500 kmol/hr |
| 10 | 43 °C | 60 bar | 30,017 kmol/hr | - | - | - | 1300 °C (Cu) 1000 °C (Pb) | 1 bar | 500 tonnes/hr |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarafraz, M.m.; Safaei, M.R.; Leon, A.S.; Khaled, U.; Goodarzi, M.; Meer, R. Energetic Analysis of Different Configurations of Power Plants Connected to Liquid Chemical Looping Gasification. Processes 2019, 7, 763. https://doi.org/10.3390/pr7100763
Sarafraz Mm, Safaei MR, Leon AS, Khaled U, Goodarzi M, Meer R. Energetic Analysis of Different Configurations of Power Plants Connected to Liquid Chemical Looping Gasification. Processes. 2019; 7(10):763. https://doi.org/10.3390/pr7100763
Chicago/Turabian StyleSarafraz, Mohammad mohsen, Mohammad Reza Safaei, Arturo S. Leon, Usama Khaled, Marjan Goodarzi, and Rashed Meer. 2019. "Energetic Analysis of Different Configurations of Power Plants Connected to Liquid Chemical Looping Gasification" Processes 7, no. 10: 763. https://doi.org/10.3390/pr7100763
APA StyleSarafraz, M. m., Safaei, M. R., Leon, A. S., Khaled, U., Goodarzi, M., & Meer, R. (2019). Energetic Analysis of Different Configurations of Power Plants Connected to Liquid Chemical Looping Gasification. Processes, 7(10), 763. https://doi.org/10.3390/pr7100763









