Synthesis of Magnesium Aluminate Spinel Powder from the Purified Sodium Hydroxide Leaching Solution of Black Dross
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedure
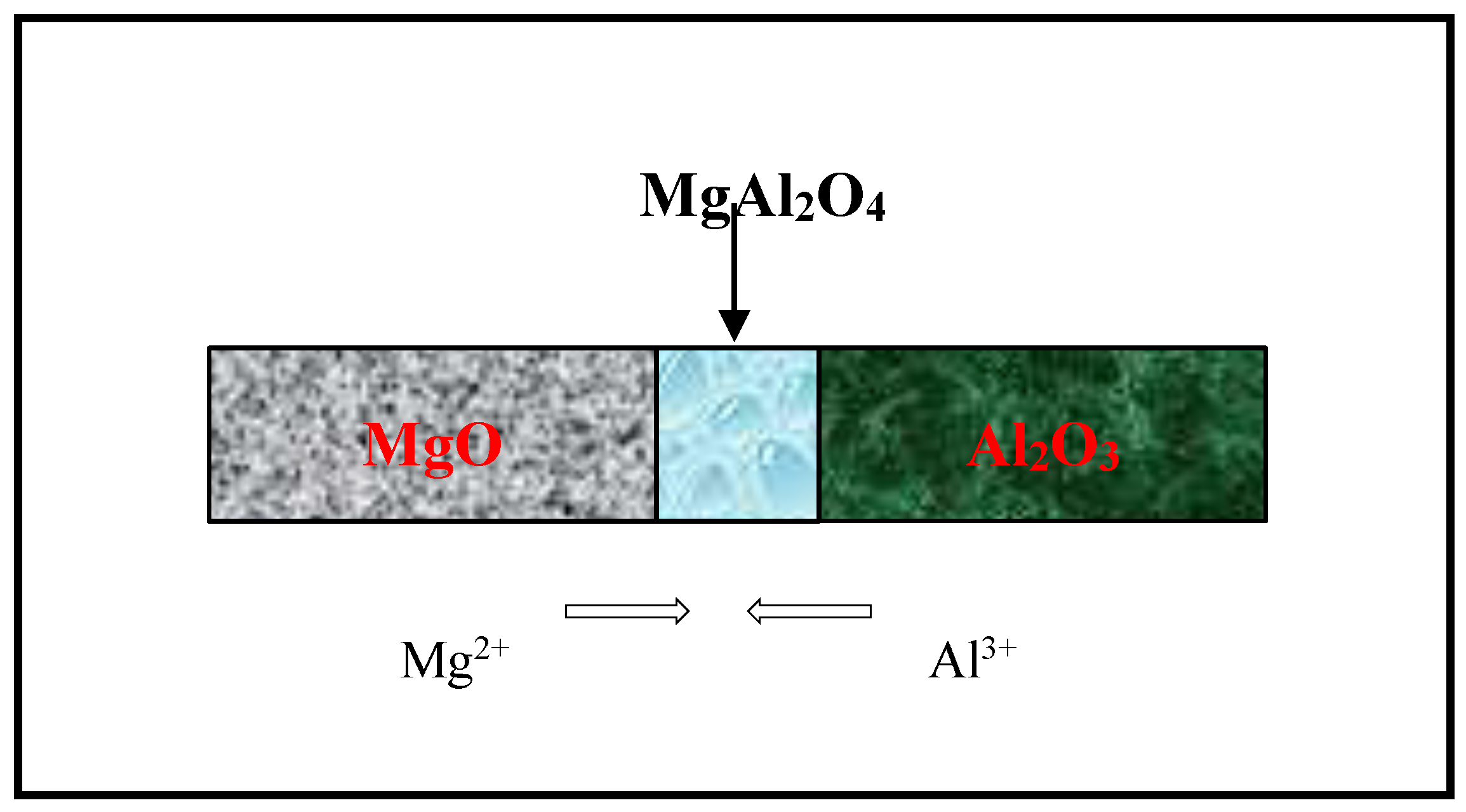
2.2.1. Formation of Magnesium Aluminate Spinel by Ball Milling
2.2.2. Formation of Magnesium Aluminate Spinel by Co-Precipitation in Acid
2.2.3. Apparatus
3. Results
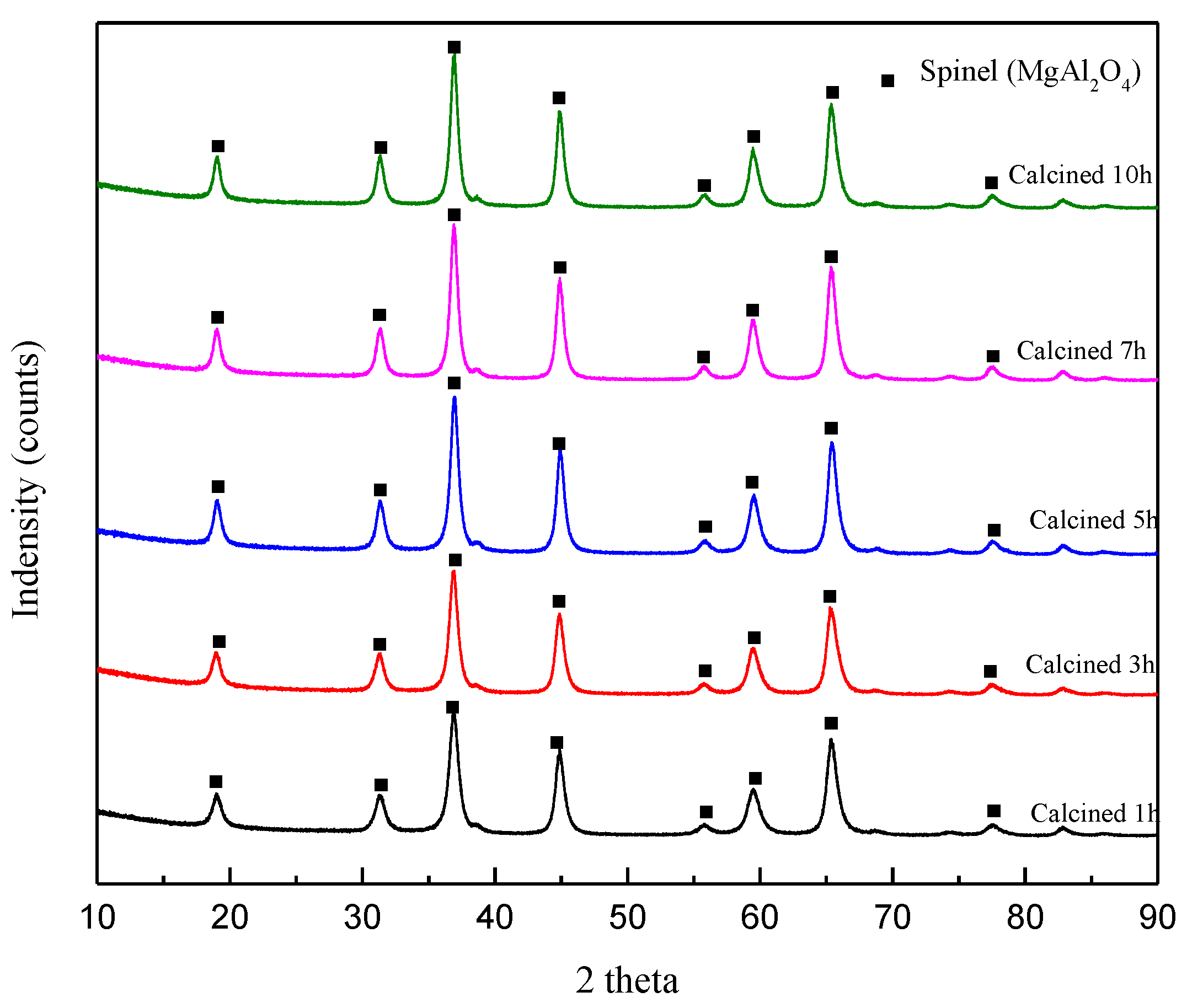
3.1. Formation of Magnesium Aluminate Spinel by Ball Milling
3.1.1. Effect of Temperature
3.1.2. Effect of Reaction Time during Calcination
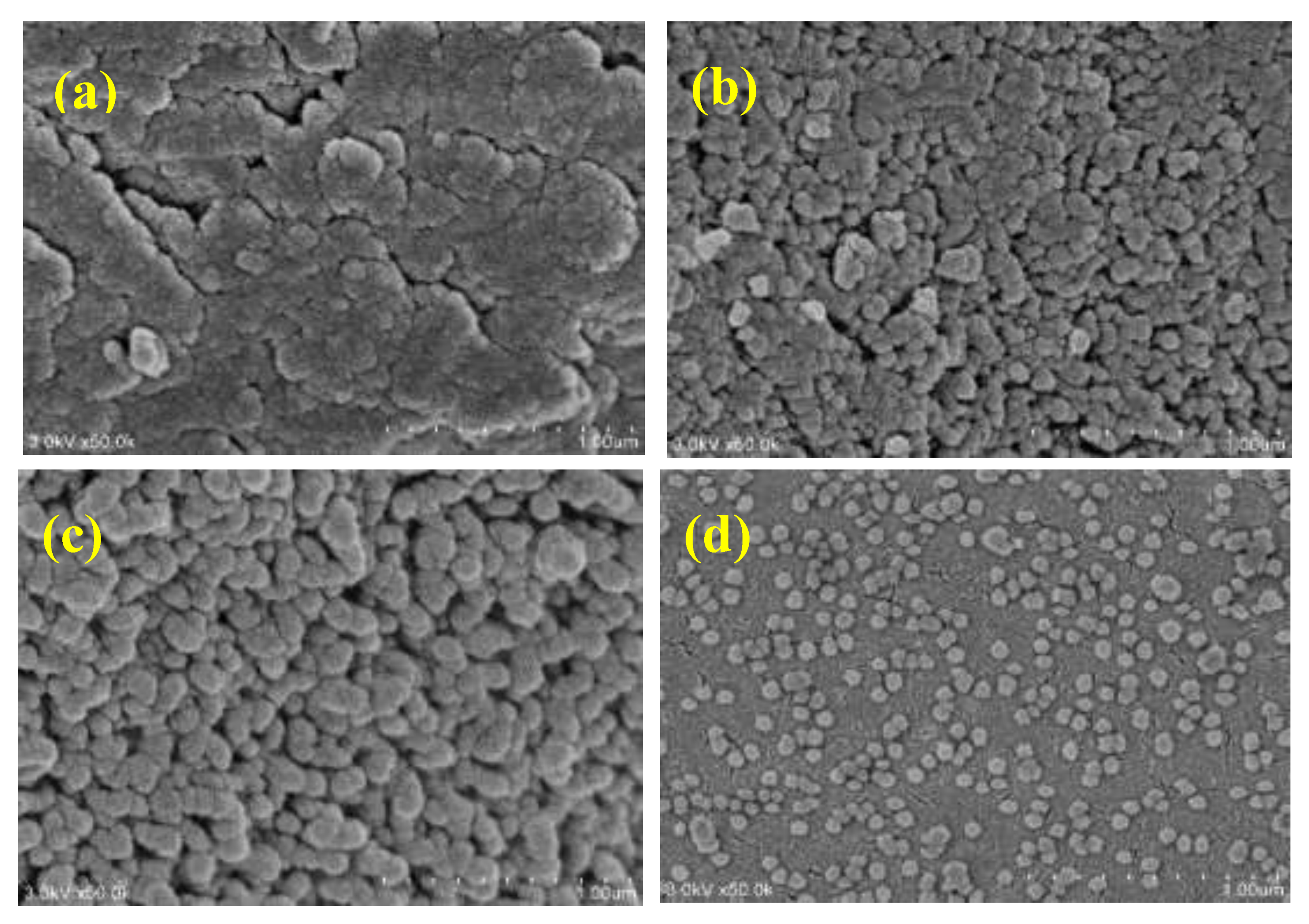
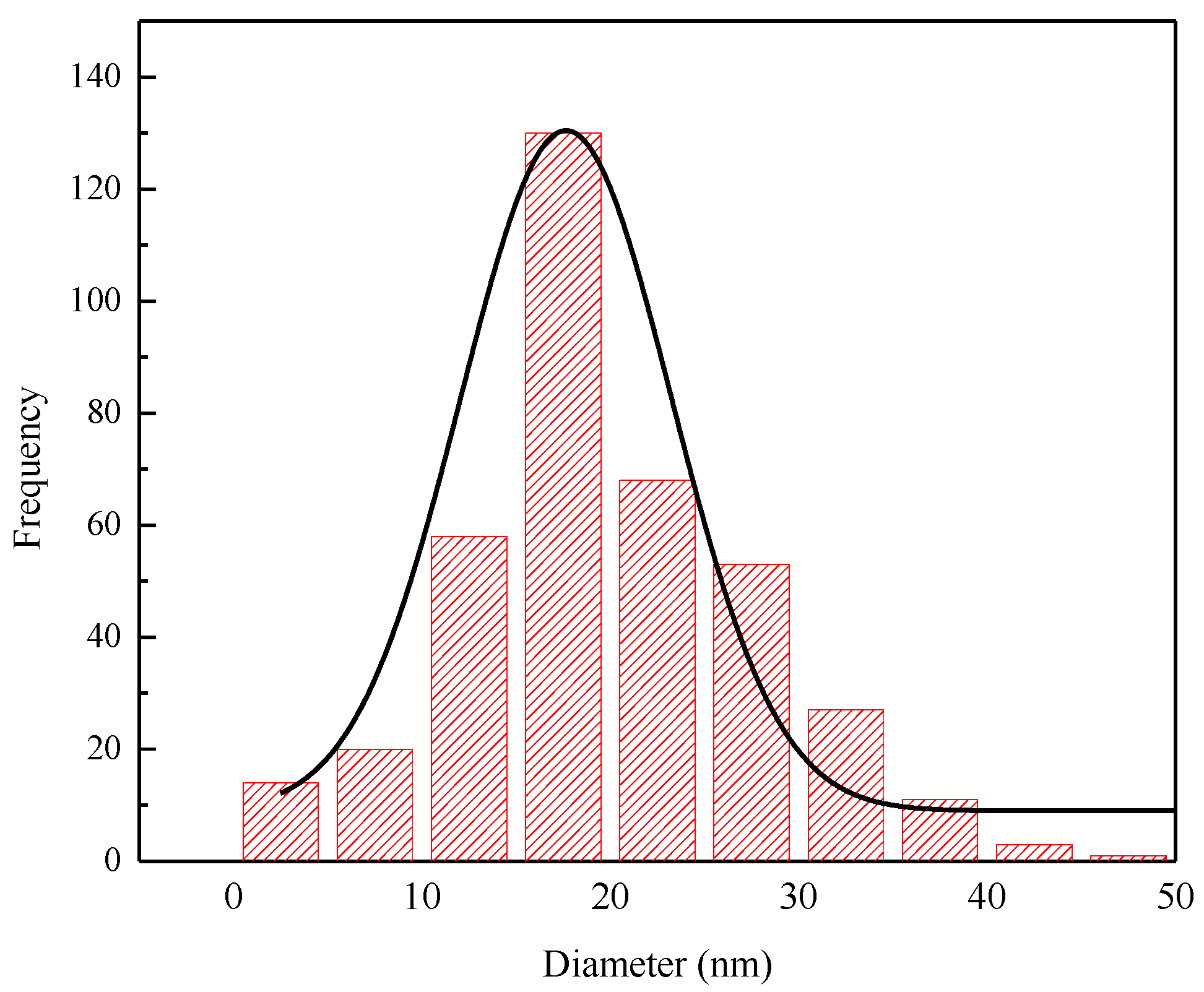
3.2. Formation of Magnesium Aluminate Spinel by Co-Precipitation in Acid
3.2.1. Effect of Temperature
3.2.2. Effect of Reaction Time
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manfredi, O.; Wuth, W.; Bohlinger, I. Characterizing the Physical and Chemical Properties of Aluminum Dross. JOM 1997, 49, 48–51. [Google Scholar] [CrossRef]
- Seyed Ghasemi, S.M.; Azizi, A. Alkaline leaching of lead and zinc by sodium hydroxide: Kinetics modeling. J. Mater. Res. Technol. 2018, 118–125. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Lee, M.S.; Nguyen, T.H. Ball Milling Treatment of Black Dross for Selective Dissolution of Alumina in Sodium Hydroxide Leaching. Processes 2019, 6, 29. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Lee, M.S. Purification of the Sodium Hydroxide Leaching Solution of Black Dross by Removal of Silicate (IV) with Polyacrylamide (PAM). Miner. Process. Extr. Metall. Rev. 2019, 1–8. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Lee, M.S. Recovery of Alumina from Sodium Hydroxide Leaching Solution by Precipitation with Hydrogen Peroxide. J. Korean Inst. Resour. Recycl. 2019, 28, 23–29. [Google Scholar]
- Zhi, W.; Liu, Y.; Juan, Z. Adjustment on gibbsite and boehmite co-precipitation from supersaturated sodium aluminate solutions. Trans. Nonferrous Met. Soc. China 2010, 20, 521–527. [Google Scholar] [CrossRef]
- Vollweiler, L.; Jost, H.; Hausner, H. Precipitation and properties of spinel rich of magnesium and aluminum. Key Eng. Mater. 1997, 132–136, 1814–1817. [Google Scholar] [CrossRef]
- Mohapatra, D.; Sarkar, D. Effect of in situ spinel seeding on synthesis of MgO-rich MgAl2O4 composite. J. Mater. Sci. 2007, 7286–7293. [Google Scholar] [CrossRef]
- Baudin, C.; Martinez, R.; Pena, P. High-Temperature Mechanical Behavior of Stoichiometric Magnesium Spinel. J. Am. Ceram. Soc. 1995, 78, 1857–1862. [Google Scholar] [CrossRef]
- Tavangarian, F.; Emadi, R. Synthesis and characterization of pure nanocrystalline magnesium aluminate spinel powder. J. Alloys Compd. 2010, 489, 600–604. [Google Scholar] [CrossRef]
- Zawrah, M.F.; Hamaad, H.; Meky, S. Synthesis and characterization of nano MgAl2O4 spinel by the co-precipitated method. Ceram. Int. 2007, 33, 969–978. [Google Scholar] [CrossRef]
- Tsung, C.; Fukuzumi, S. Mesoporous Nickel Ferrites with Spinel Structure Prepared by an Aerosol Spray Pyrolysis Method for Photocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2014, 2, 2588–2594. [Google Scholar]
- Shiono, T.; Shiono, K.; Miyamoto, K.; Pezzotti, G. Synthesis and characterization of MgAl2O4 spinel precursor from heterogeneous Alkoxide Solution Containing Fine MgO Powder. J. Am. Ceram. Soc. 2000, 37, 235–237. [Google Scholar] [CrossRef]
- Montouillout, V.; Massiot, D.; Douy, A.; Coutures, J.P. Characterization of MgAl2O4 Precursor Powders Prepared by Aqueous Route. J. Am. Ceram. Soc. 1999, 82, 3299–3304. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.H.; Han, Z.Y.; Xiao, X.Y.; Peng, C. Feasibility of aluminum recovery and MgAl2O4 spinel synthesis from secondary aluminum dross. Int. J. Miner. Metall. Mater. 2019, 26, 309–318. [Google Scholar] [CrossRef]
- Yongvanich, N.; Emtip, B.; Hengprayoon, B.; Jankat, E. Synthesis of spinel color pigments from aluminum dross waste. Key Eng. Mater. 2018, 766 KEM, 282–287. [Google Scholar] [CrossRef]
- Ghanbari-Ahari, K.; Lee, E.; Habesch, S. Spinel formation in cement-free castable bond systems. In Proceedings of the 44th International Colloquium on Refractories, Refractories in Steelmaking, Aachen, Germany, 26–27 Semtemper 2001; pp. 160–163. [Google Scholar]
- Fuhrer, M.; Hey, A.; Lee, W.E. Microstructural Spinel/Calcium Refractories Evolution in Self-forming Aluminate-Bonded Castable. J. Eur. Ceram. Soc. 1998, 18, 813–820. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials. J. Gen. Intern. Med. 1954, 14, 485–487. [Google Scholar]
- George, M.; Nair, S.S.; Malini, K.A.; Joy, P.A.; Anantharaman, M.R. Finite size effects on the electrical properties of sol–gel synthesized CoFe2O4 powders: Deviation from Maxwell–Wagner theory and evidence of surface polarization effects. J. Phys. D Appl. Phys. 2007, 40, 1593–1602. [Google Scholar] [CrossRef]
- Mohammad, A.M.; Ridha, S.M.A.; Mubarak, T.H. Dielectric Properties of Cr-Substituted Cobalt Ferrite Nanoparticles Synthesis by Citrate-Gel Auto Combustion Method. Int. J. Appl. Eng. Res. 2018, 13, 6026–6035. [Google Scholar]
- Carter, R.E. Mechanism of Solid-State Reaction between Magnesium-Oxide and Aluminum Oxide and Between Magnesium Oxide and Ferric Oxide. J. Am. Ceram. Soc. 1960, 14, 116–120. [Google Scholar] [CrossRef]
- Salem, S. Technical aspect for oxidation of magnesium and aluminum nitrates to manufacture nano- and micro-sized MgAl2O4 spinel by combustion method. J. Adv. Ceram. 2017, 6, 187–195. [Google Scholar] [CrossRef]
- Prabhakaran, K.; Joseph, J.; Gokhale, N.M.; Sharma, S.C.; Lal, R. Synthesis of Nanocrystalline Lanthanum Strontium Manganite Powder. J. Am. Ceram. Soc. 2006, 2337, 2335–2337. [Google Scholar] [CrossRef]
- Biswas, M.; Prabhakaran, K.; Gokhale, N.M.; Sharma, S.C. Synthesis of nanocrystalline yttria doped ceria powder by urea–formaldehyde polymer gel auto-combustion process. Mater. Res. Bull. 2007, 42, 609–617. [Google Scholar] [CrossRef]
- Fu, P.; Lu, W.; Lei, W.; Wu, K.; Xu, Y.; Wu, J. Thermal Stability and Microstructure Characterization of MgAl2O4 Nanoparticles Synthesized by Reverse Microemulsion Method. Mater. Res. 2013, 16, 844–849. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with Image. J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar] [CrossRef]




| Calcination Temperature (°C) | Average Crystallite Size (nm) |
|---|---|
| 1000 | 15 |
| 1200 | 24 |
| 1500 | 38 |
| Calcination Temperature (°C) | Average Crystallite Size (nm) |
|---|---|
| 600 | 3 |
| 800 | 4 |
| 1000 | 16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.N.; Lee, M.S. Synthesis of Magnesium Aluminate Spinel Powder from the Purified Sodium Hydroxide Leaching Solution of Black Dross. Processes 2019, 7, 741. https://doi.org/10.3390/pr7100741
Nguyen TTN, Lee MS. Synthesis of Magnesium Aluminate Spinel Powder from the Purified Sodium Hydroxide Leaching Solution of Black Dross. Processes. 2019; 7(10):741. https://doi.org/10.3390/pr7100741
Chicago/Turabian StyleNguyen, Thi Thuy Nhi, and Man Seung Lee. 2019. "Synthesis of Magnesium Aluminate Spinel Powder from the Purified Sodium Hydroxide Leaching Solution of Black Dross" Processes 7, no. 10: 741. https://doi.org/10.3390/pr7100741
APA StyleNguyen, T. T. N., & Lee, M. S. (2019). Synthesis of Magnesium Aluminate Spinel Powder from the Purified Sodium Hydroxide Leaching Solution of Black Dross. Processes, 7(10), 741. https://doi.org/10.3390/pr7100741






