Thermochemical Conversion of Napier Grass for Production of Renewable Syngas
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
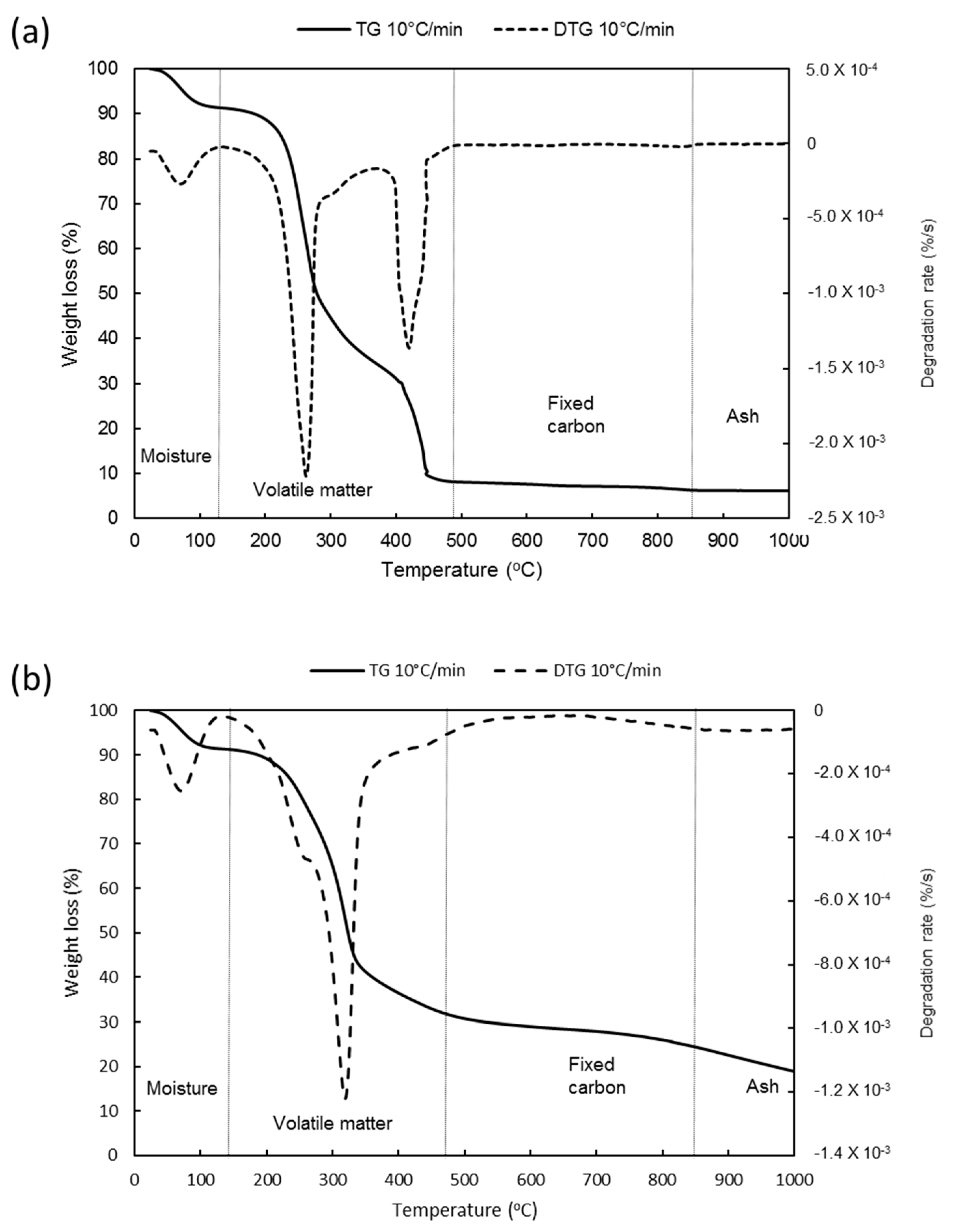
2.2. Proximate Analysis of Mature Napier Grass
2.3. Ultimate Analysis of Mature Napier Grass
2.4. Measurement of the Higher Heating Value of Napier Grass
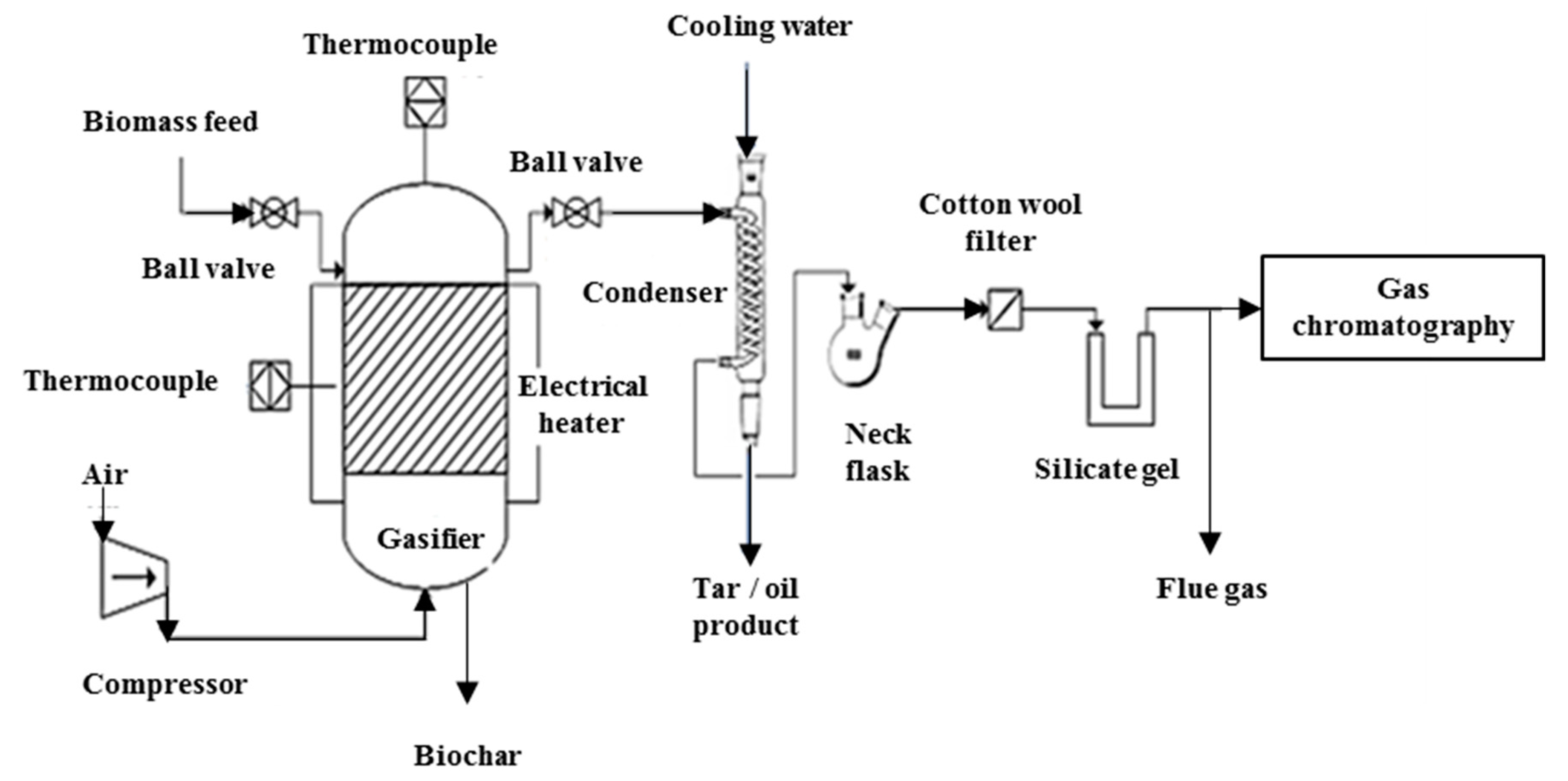
2.5. Gasification of Napier Grass for Syngas Production
2.5.1. Gas Analysis
2.5.2. Condensed Liquid Characterization
2.5.3. Analysis of Inorganic Compounds in Ash
3. Results and Discussion
3.1. Proximate and Ultimate Analysis of Napier Grass Feedstock
| Property | Napier Grass (Current Study) | Napier Grass [13] | Cardoon [21] | Miscanthus [21] | Rubber–Wood–Sawdust [22] | Palm Kernel Shell [23] | Coconut Shell [23] |
|---|---|---|---|---|---|---|---|
| Moisture (wt%) | 30.07 | 9.43 | 12.00 | 6.48 | - | 7.96 | 4.89 |
| Proximate analysis (wt%, dry basis) | |||||||
| Volatile matter | 85.52 | 72.58 | 76.02 | 78.36 | 51.39 | 72.47 | 30.62 |
| Fixed carbon | 8.17 | 8.35 | 9.19 | 14.90 | 14.29 | 18.56 | 26.41 |
| Ash content | 6.31 | 9.68 | 14.80 | 6.74 | 22.67 | 8.97 | 42.98 |
| Ultimate analysis (wt%, dry basis) | |||||||
| C | 45.10 | 42.40 | 56.01 | 46.97 | 53.40 | 51.63 | 45.24 |
| H | 5.94 | 5.96 | 6.46 | 5.57 | 6.70 | 5.52 | 5.04 |
| N | 0.45 | 1.71 | 0.99 | 1.37 | 3.10 | 1.89 | 1.46 |
| S | 0.00 | 0.09 | 0.22 | 0.28 | 0.00 | 0.05 | 0.06 |
| O (by difference) | 48.52 | 45.32 | 36.10 | 45.82 | 36.80 | 40.91 | 48.2 |
| Calorific value (MJ/kg) | 16.73 | - | 17.33 | 18.73 | 18.30 | 22.97 | 16.07 |
3.2. Inorganic Compounds in Napier Grass Ash
| Ash Basis (wt%) | Napier Grass (Current Study) | Olive Tree Residue [27] | EFB [28] | Napier Grass [12] | Cardoon [21] | Miscanthus [21] | Agricultural Residue [29] |
|---|---|---|---|---|---|---|---|
| K2O | 54.39 | 9.26 | 44.00 | 30.5 | 24.91 | 14.00 | 1.65 |
| Fe2O3 | 15.53 | 1.38 | 3.00 | 1.4 | 1.77 | 2.63 | 2.95 |
| SiO2 | 9.81 | 11.84 | 27.00 | 43.0 | 8.34 | 62.21 | 89.57 |
| Cl | 8.84 | - | 5.30 | - | - | - | 1.30 |
| CaO | 8.20 | 54.82 | 8.00 | 1.9 | 38.33 | 8.32 | 0.77 |
| SO3 | 2.03 | - | 2.70 | - | - | - | - |
| MnO | 0.44 | 0.10 | 0.11 | - | - | - | - |
| Rb2O | 0.37 | - | 0.12 | - | - | - | - |
| Br | 0.14 | - | 0.018 | - | - | - | - |
| CuO | 0.10 | - | 0.039 | - | - | - | - |
| ZnO | 0.10 | - | 0.092 | 0.08 | - | - | - |
| As2O3 | 0.66 | - | - | - | - | - | - |
| Al2O3 | - | 2.60 | 0.97 | <0.1 | 3.50 | 5.47 | 1.32 |
| MgO | - | 4.36 | 4.80 | 9.9 | 5.74 | 3.16 | 0.76 |
| Na2O | - | 0.16 | 0.55 | <0.01 | 13.08 | 0.53 | 1.15 |
| TiO2 | - | 0.35 | 0.08 | 0.03 | 0.10 | 0.32 | 7.56 |
| P2O5 | - | 3.40 | 3.60 | 7.2 | 4.23 | 3.37 | 1.04 |
| NiO | - | - | 0.01 | - | - | - | - |
| SrO | - | - | 0.03 | 0.03 | - | - | - |
| BaO | - | - | - | 0.08 | - | - | - |
3.3. Components of Bio-Liquid
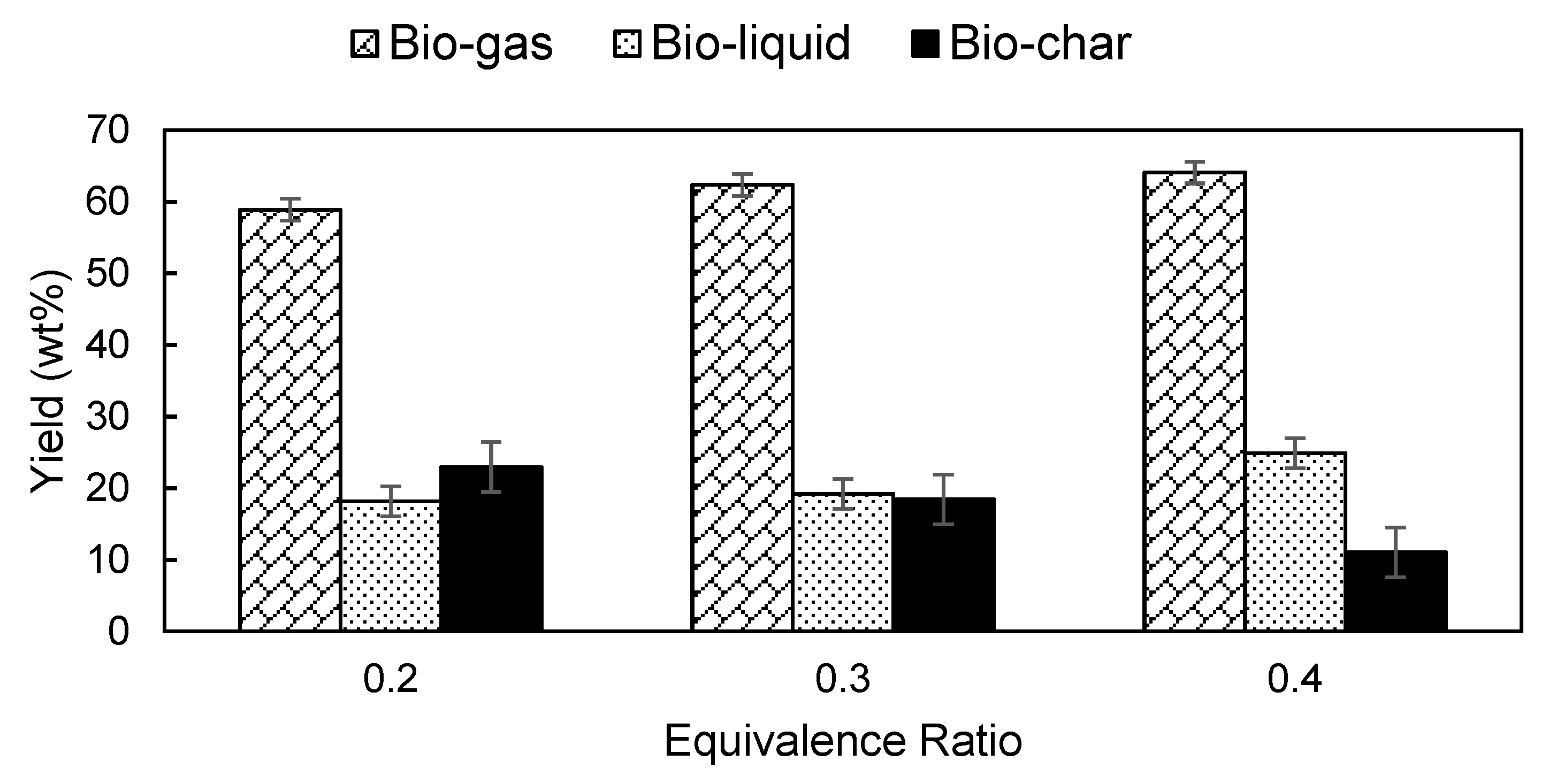
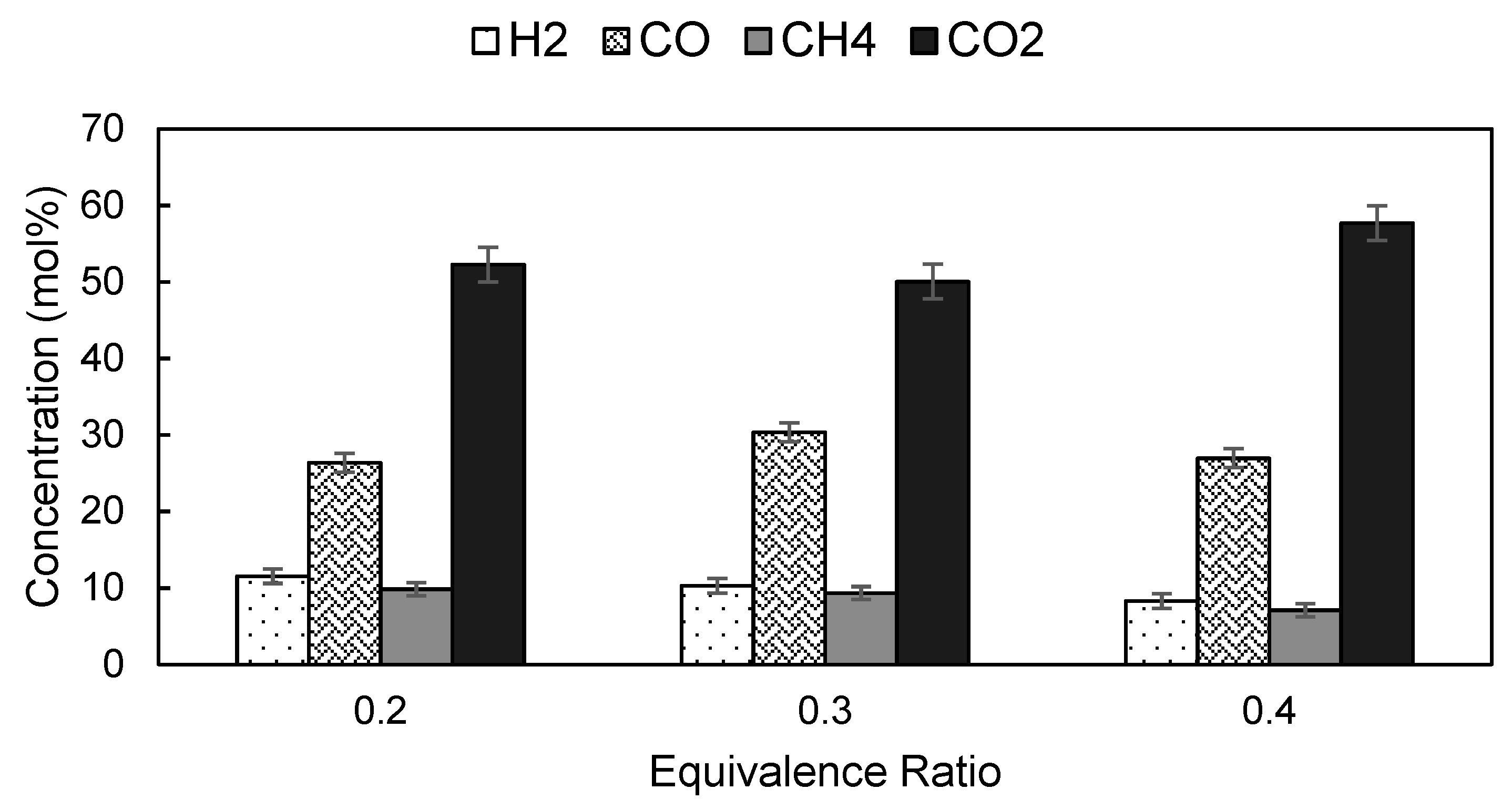
3.4. Effects of ER on the Product Yield and Composition of Producer Gas
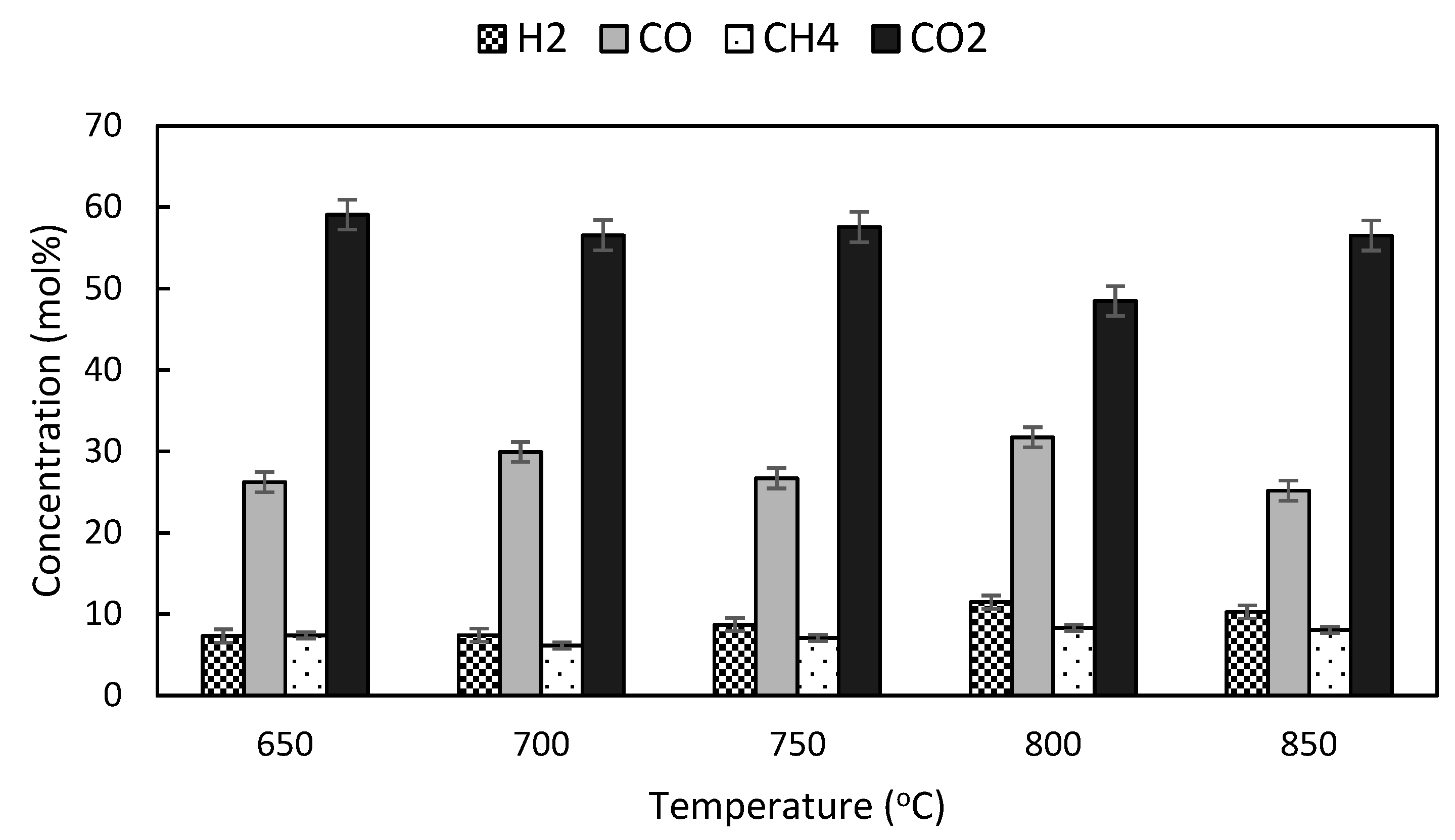
3.5. Effects of Temperature on Product Yield and Quality of Producer Gas
3.6. Effects of ER on the Higher Heating Value (HHV) of Syngas and Carbon Conversion Efficiency (CCE)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.A.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; et al. Global Carbon Budget 2018. Earth Syst. Sci. Data 2018, 10, 2141–2194. [Google Scholar] [CrossRef]
- Bujang, A.S.; Bern, C.J.; Brumm, T.J. Summary of energy demand and renewable energy policies in Malaysia. Renew. Sustain. Energy Rev. 2016, 53, 1459–1467. [Google Scholar] [CrossRef]
- Rebitanim, N.Z.; Wan Ab Karim Ghani, W.A.; Rebitanim, N.A.; Amran Mohd Salleh, M. Potential applications of wastes from energy generation particularly biochar in Malaysia. Renew. Sustain. Energy Rev. 2013, 21, 694–702. [Google Scholar] [CrossRef]
- Energy Commission. 2017 Malaysia Energy Statistics Handbook; Suruhanjaya Tenaga (Energy Commission): Putrajaya, Malaysia, 2017. [Google Scholar]
- Mohammed, M.A.A.; Salmiaton, A.; Wan Azlina, W.A.K.G.; Mohammad Amran, M.S.; Fakhru’l-Razi, A.; Taufiq-Yap, Y.H. Hydrogen rich gas from oil palm biomass as a potential source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 1258–1270. [Google Scholar] [CrossRef]
- Alipour Moghadam, R.; Yusup, S.; Azlina, W.; Nehzati, S.; Tavasoli, A. Investigation on syngas production via biomass conversion through the integration of pyrolysis and air–steam gasification processes. Energy Convers Manag. 2014, 87, 670–675. [Google Scholar] [CrossRef]
- Mallick, D.; Mahanta, P.; Moholkar, V.S. Co-gasification of coal and biomass blends: Chemistry and engineering. Fuel 2017, 204, 106–128. [Google Scholar] [CrossRef]
- Ismail, W.M.S.W.; Mohd Thaim, T.; Abdul Rasid, R. Biomass gasification of oil palm fronds (OPF) and Koompassia malaccensis (Kempas) in an entrained flow gasifier: A performance study. Biomass Bioenergy 2019, 124, 83–87. [Google Scholar] [CrossRef]
- Chan, Y.H.; Quitain, A.T.; Yusup, S.; Uemura, Y.; Sasaki, M.; Kida, T. Liquefaction of palm kernel shell in sub- and supercritical water for bio-oil production. J. Energy Inst. 2018, 91, 721–732. [Google Scholar] [CrossRef]
- Hlavsová, A.; Corsaro, A.; Raclavská, H.; Juchelková, D.; Škrobánková, H.; Frydrych, J. Syngas Production from Pyrolysis of Nine Composts Obtained from Nonhybrid and Hybrid Perennial Grasses. Sci. World J. 2014, 2014, 11. [Google Scholar] [CrossRef]
- Suntivarakorn, R.; Treedet, W.; Singbua, P.; Teeramaetawat, N. Fast pyrolysis from Napier grass for pyrolysis oil production by using circulating Fluidized Bed Reactor: Improvement of pyrolysis system and production cost. Energy Rep. 2018, 4, 565–575. [Google Scholar] [CrossRef]
- Strezov, V.; Evans, T.J.; Hayman, C. Thermal conversion of elephant grass (Pennisetum Purpureum Schum) to bio-gas, bio-oil and charcoal. Bioresour. Technol. 2008, 99, 8394–8399. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K.; Tsai, W.-T.; Tsai, Y.-L.; Lin, S.-H. Pyrolysis of napier grass in an induction-heating reactor. J. Anal. Appl. Pyrol. 2010, 88, 110–116. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. A critical review on biomass gasification, co-gasification, and their environmental assessments. Biofuel Res. J. 2016, 3, 483–495. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Kulkarni, A.; Adhikari, S. Effects of temperature and equivalence ratio on mass balance and energy analysis in loblolly pine oxygen gasification. Energy Sci. Eng. 2016, 4, 256–268. [Google Scholar] [CrossRef]
- Mohammed, M.A.A.; Salmiaton, A.; Wan Azlina, W.A.K.G.; Mohamad Amran, M.S. Gasification of oil palm empty fruit bunches: A characterization and kinetic study. Bioresour. Technol. 2012, 110, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Ptasinski, K.J. Thermodynamic efficiency of biomass gasification and biofuels conversion. Biofuel. Bioprod. Biorefin. 2008, 2, 239–253. [Google Scholar] [CrossRef]
- Monti, A.; Di Virgilio, N.; Venturi, G. Mineral composition and ash content of six major energy crops. Biomass Bioenergy 2008, 32, 216–223. [Google Scholar] [CrossRef]
- Hosseini, M.; Dincer, I.; Rosen, M.A. Steam and air fed biomass gasification: Comparisons based on energy and exergy. Int. J. Hydrog. Energy 2012, 37, 16446–16452. [Google Scholar] [CrossRef]
- Sher, F.; Pans, M.A.; Sun, C.; Snape, C.; Liu, H. Oxy-fuel combustion study of biomass fuels in a 20 kWth fluidized bed combustor. Fuel 2018, 215, 778–786. [Google Scholar] [CrossRef]
- Karampinis, E.; Vamvuka, D.; Sfakiotakis, S.; Grammelis, P.; Itskos, G.; Kakaras, E. Comparative study of combustion properties of five energy crops and Greek lignite. Energy Fuels 2012, 26, 869–878. [Google Scholar] [CrossRef]
- Ghani, W.A.W.A.K.; Mohd, A.; da Silva, G.; Bachmann, R.T.; Taufiq-Yap, Y.H.; Rashid, U.; Ala’a, H. Biochar production from waste rubber-wood-sawdust and its potential use in C sequestration: Chemical and physical characterization. Ind. Crop. Prod. 2013, 44, 18–24. [Google Scholar] [CrossRef]
- Wan Ab Karim Ghani, W.; Moghadam, R.A.; Salleh, M.; Alias, A. Air gasification of agricultural waste in a fluidized bed gasifier: Hydrogen production performance. Energies 2009, 2, 258–268. [Google Scholar] [CrossRef]
- González-Vázquez, M.P.; García, R.; Gil, M.V.; Pevida, C.; Rubiera, F. Unconventional biomass fuels for steam gasification: Kinetic analysis and effect of ash composition on reactivity. Energy 2018, 155, 426–437. [Google Scholar] [CrossRef]
- Obernberger, I.; Thek, G. Physical characterisation and chemical composition of densified biomass fuels with regard to their combustion behaviour. Biomass Bioenergy 2004, 27, 653–669. [Google Scholar] [CrossRef]
- Arvelakis, S.; Gehrmann, H.; Beckmann, M.; Koukios, E.G. Preliminary results on the ash behavior of peach stones during fluidized bed gasification: Evaluation of fractionation and leaching as pre-treatments. Biomass Bioenergy 2005, 28, 331–338. [Google Scholar] [CrossRef]
- Cuenca, J.; Rodríguez, J.; Martín-Morales, M.; Sánchez-Roldán, Z.; Zamorano, M. Effects of olive residue biomass fly ash as filler in self-compacting concrete. Constr. Build. Mater. 2013, 40, 702–709. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A. Gasification of palm empty fruit bunch in a bubbling fluidized bed: A performance and agglomeration study. Bioresour. Technol. 2011, 102, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Ghani, W.; Alias, A.; Savory, R.; Cliffe, K. Co-combustion of agricultural residues with coal in a fluidised bed combustor. Waste Manag. 2009, 29, 767–773. [Google Scholar] [CrossRef]
- Mahishi, M.R.; Goswami, D.Y. An experimental study of hydrogen production by gasification of biomass in the presence of a CO2 sorbent. Int. J. Hydrog. Energy 2007, 32, 2803–2808. [Google Scholar] [CrossRef]
- Miccio, F.; Moersch, O.; Spliethoff, H.; Hein, K.R.G. Generation and conversion of carbonaceous fine particles during bubbling fluidised bed gasification of a biomass fuel. Fuel 1999, 78, 1473–1481. [Google Scholar] [CrossRef]
- Guo, F.; Dong, Y.; Zhang, T.; Dong, L.; Guo, C.; Rao, Z. Experimental Study on Herb Residue Gasification in an Air-Blown Circulating Fluidized Bed Gasifier. Ind. Eng. Chem. Res. 2014, 53, 13264–13273. [Google Scholar] [CrossRef]
- Kuo, J.-H.; Lin, C.-L.; Wey, M.-Y. Effect of agglomeration/defluidization on hydrogen generation during fluidized bed air gasification of modified biomass. Int. J. Hydrog. Energy 2012, 37, 1409–1417. [Google Scholar] [CrossRef]
- Ghassemi, H.; Shahsavan-Markadeh, R. Effects of various operational parameters on biomass gasification process; a modified equilibrium model. Energy Convers. Manag. 2014, 79, 18–24. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, M.; Jin, B.; Huang, Y.; Zhou, H. High-temperature air/steam-blown gasification of coal in a pressurized spout-fluid bed. Energy Fuels 2006, 20, 715–720. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda-Almansa, G.; Bula, A. Gasification of biomass wastes in an entrained flow gasifier: Effect of the particle size and the residence time. Fuel Process. Technol. 2010, 91, 681–692. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air–steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jayathilake, R.; Rudra, S. Numerical and Experimental Investigation of Equivalence Ratio (ER) and Feedstock Particle Size on Birchwood Gasification. Energies 2017, 10, 1232. [Google Scholar] [CrossRef]
- Sheth, P.N.; Babu, B.V. Experimental studies on producer gas generation from wood waste in a downdraft biomass gasifier. Bioresour. Technol. 2009, 100, 3127–3133. [Google Scholar] [CrossRef]
- Wu, C.Z.; Yin, X.L.; Ma, L.L.; Zhou, Z.Q.; Chen, H.P. Operational characteristics of a 1.2-MW biomass gasification and power generation plant. Biotechnol. Adv. 2009, 27, 588–592. [Google Scholar] [CrossRef]

| Eq. | Reaction | Chemical Equation | Enthalpy (Negative Value Indicates Exothermicity) |
|---|---|---|---|
| 1 | Combustion (complete) | C + O2 → CO2 | −283 MJ/kmol |
| 2 | Combustion (incomplete) | C + 1/2O2 → CO | −111 MJ/kmol |
| 3 | Boudouard | C + CO2 ↔ 2CO | +172 MJ/kmol |
| 4 | Water-gas | C + H2O ↔ CO + H2 | +131 MJ/kmol |
| 5 | Methanation | C + 2H2 ↔ CH4 | −75 MJ/kmol |
| 6 | Water-gas shift | CO + H2O ↔ CO2 + H2 | −41 MJ/kmol |
| 7 | Steam-methane reforming | CH4 + H2O ↔ CO + 3H2 | +206 MJ/kmol |
| 8 | Dry reforming | CH4 + CO2 ↔ 2H2 + 2CO | +260 MJ/kmol |
| 9 | Methanation | C + 2H2O ↔ CH4 + CO2 | +103 MJ/kmol |
| Compound Name | Area (%) | Formula |
|---|---|---|
| Pentane, 2,2-dimethyl- (Al) | 16.10 | C7H16 |
| Dimethoxydimethylsilane (E) | 4.01 | C4H12O2Si |
| Pentane, 3,3-dimethyl- (Al) | 2.36 | C7H16 |
| Cyclohexane (Al) | 7.12 | C6H12 |
| Pentane, 2,3-dimethyl- (Al) | 1.33 | C7H16 |
| Hexane, 1-chloro- (Al) | 3.63 | C6H13Cl |
| Hexane, 3-methyl- (Al) | 6.09 | C7H16 |
| Pyridine (N) | 2.79 | C5H5N |
| Pyrrole (N) | 1.57 | C4H5N |
| 2,2-Dimethoxybutane (K) | 1.41 | C6H14O2 |
| Phosphonic acid, (p-hydroxyphenyl)- (A) | 31.94 | C6H7O4P |
| Phenol, 2-methyl- (P) | 4.01 | C7H8O |
| Phenol, 3-methyl- (P) | 9.49 | C7H8O |
| Total | 91.85 |
| ER | 0.20 | 0.30 | 0.40 |
|---|---|---|---|
| HHV (MJ/kg) | 3.37 | 2.68 | 1.99 |
| HHV (N2 free) (MJ/kg) | 8.72 | 8.78 | 7.28 |
| CCE (%) | 77.04 | 81.00 | 89.08 |
| Temperature (°C) | 650 | 700 | 750 | 800 | 850 |
|---|---|---|---|---|---|
| HHV (MJ/kg) | 2.42 | 2.29 | 2.70 | 3.37 | 3.07 |
| HHV (N2 free) (MJ/kg) | 7.19 | 7.43 | 7.81 | 8.95 | 8.51 |
| CCE (%) | 72.60 | 74.56 | 76.32 | 77.16 | 82.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md Said, M.S.; Wan Abdul Karim Ghani, W.A.; Hong Boon, T.; Hussain, S.A.; Ng, D.K.S. Thermochemical Conversion of Napier Grass for Production of Renewable Syngas. Processes 2019, 7, 705. https://doi.org/10.3390/pr7100705
Md Said MS, Wan Abdul Karim Ghani WA, Hong Boon T, Hussain SA, Ng DKS. Thermochemical Conversion of Napier Grass for Production of Renewable Syngas. Processes. 2019; 7(10):705. https://doi.org/10.3390/pr7100705
Chicago/Turabian StyleMd Said, Mohamad Syazarudin, Wan Azlina Wan Abdul Karim Ghani, Tan Hong Boon, Siti Aslina Hussain, and Denny Kok Sum Ng. 2019. "Thermochemical Conversion of Napier Grass for Production of Renewable Syngas" Processes 7, no. 10: 705. https://doi.org/10.3390/pr7100705
APA StyleMd Said, M. S., Wan Abdul Karim Ghani, W. A., Hong Boon, T., Hussain, S. A., & Ng, D. K. S. (2019). Thermochemical Conversion of Napier Grass for Production of Renewable Syngas. Processes, 7(10), 705. https://doi.org/10.3390/pr7100705






