Abstract
Fuel resource diversification is a global effort to deviate from non-renewable fossil fuels. Biomass has been identified as an alternative solid biofuel source due to its desirable properties and carbon neutrality. As reported in the literature, biomass can positively contribute towards combating climate change while providing alleviation for energy security issue. As part of efforts to diversify biomass resources, this work intends to explore the potential of Napier grass, one type of energy crop, for the production of renewable syngas via gasification. This energy crop is originally from Africa, which is highly productive with low cost (40 tonnes per year per hectare). Limited studies were conducted to analyze the potential of such an energy crop as a fuel source, which is the subject of this work. In order to analyze the full potential of such energy crop, the physical and chemical characteristics of this biomass was first analyzed. To determine the productivity of syngas from this biomass, fluidized bed gasifier was used in this work. The effects of gasification process parameters (i.e., equivalence ratio and temperature) on product yield and producer gas compositions were examined. Besides, the effects of equivalence ratio towards higher heating value of syngas and carbon conversion efficiency were analyzed. Based on the ultimate analysis results, the molecular formula of Napier gas was CH1.56O0.81N0.0043. Meanwhile, the higher heating value of such biomass was determined as 16.73 MJ/kg, which was comparable to other biomasses. It is noted that in this work, the volatile matter was determined as 85.52% and this promoted gasification process remarkably. The dynamics of the reactions involved were observed as a significant variation in product yield and biogas components were recorded at varying equivalence ratio and gasifier operating temperature.
1. Introduction
The increasing scarcity of conventional fossil fuels has led to diversification of energy resources. In addition, the combustion process of fossil fuels for electricity generation emits greenhouse gasses and criteria pollutants, which are harmful to both living organisms and the planet. The global carbon emission has been increasing at an alarming rate. Average annual global carbon dioxide emission from burning of fossil fuels was 3.1 GtC per year in the 1960s. Recently, the rate has recorded an increment higher than threefold where 9.4 GtC per year was emitted during 2008–2017 [1]. Combustion of finite non-renewable fossil fuels for energy production in various sectors such as transportation and industrial activities has been reported to be the main perpetrator to this worrying situation. This dire situation prompts for cooperative and collective effort at a global scale as manifested by the Kyoto Protocol and Paris Agreement. Many countries around the world are phasing out and rendering non-renewable fossil fuels as an obsolete option for energy production.
Reducing our reliance on finite fossil fuels and exploration of potential renewable resources for energy generation have become a focus at the global scale, and Malaysia is not left behind in this worldwide trend. The initiative has gained support at the governmental level as evidenced by the introduction of the Five-Fuel Diversification Policy. Under this policy, renewable energy is included as the fifth fuel in the supply mix where utilization of abundant biomass is one of the strategies being encouraged [2,3]. According to the Malaysia Energy Commission, 100,721 ktoe of energy was supplied in 2015 where 95.5% of the energy was generated from non-renewable resources, mainly natural gas (61.7%) and crude oil (32.2%). Biomass, on the other hand, contributed a small fraction of 0.2% to the total energy supply for that year [4]. Biomass is derived from living organisms through the photosynthesis process where solar energy is converted into carbohydrates. A wide range of biomass is available that entails significant variation in their properties, characteristics and chemical compositions. In general, major constituents of biomass consist of oxygen, carbon and hydrogen. The use of biomass for energy production is considered carbon neutral due to carbon fixation process during photosynthesis [5].
Conversion processes (physical, biological, thermochemical, etc.) of the ample biomass produce renewable syngas, which provides alleviation for both energy security and global warming issues. Additionally, various types of value-added products can be produced from the conversion processes. For this purpose, a range of thermochemical conversion processes is available such as pyrolysis, gasification, liquefaction and direct combustion. The main difference between thermochemical technologies is the availability of oxygen during the process. In some applications, more than one thermochemical conversion process is combined to enhance the quality of producer gas as conducted by Alipour Moghadam, et al. [6] where both pyrolysis and air-steam gasification processes are integrated together. Four possible biomass thermochemical conversion routes for renewable energy production have been discussed and compared by Mohammed, Salmiaton, Wan Azlina, Mohammad Amran, Fakhru’l–Razi and Taufiq–Yap [5].
Thermochemical conversion of biomass produces syngas with half energy density of natural gas. The reactions involved during biomass conversion process are summarized in Table 1 [7].

Table 1.
Chain of reactions involved in the biomass thermochemical process.
In Malaysia, many work related to the thermochemical conversion of biomass has been concentrated on palm oil derived biomass due to its abundancy and wide availability [8,9]. In order to broaden the range of biomass utilized for renewable energy generation, which directly supports the fuel diversification policy of Malaysia, new potential renewable energy resources are being explored. Napier grass (NG) has gained considerable attention in recent years due to its desirable characteristics as potential renewable fuel. This energy crop of African origin is highly productive with low establishment cost [10]. The annual yield is 40 tonnes per hectare with multiple harvest frequency. There is limited information on the potential of producing green energy from Napier grass reported in the literature where the works have been concentrated on using the pyrolysis conversion process [11,12,13].
Fluidized bed gasification has been reported to be a versatile technology for biomass conversion. Intensive mixing in the bed enhances heat and mass transfer that leads to a high reaction rate [14]. Abdoulmoumine, et al. [15] reported that operation parameters have a major influence on the kinetics of reactions involved, which directly affect yield and the quality of producer gas. To our knowledge, the potential of generating renewable fuel from gasification of Napier grass has never been conducted. It is the aim of this study to evaluate the feasibility of syngas production from Napier grass via the bench-scale gasifier system at varying operating conditions.
2. Materials and Methods
2.1. Sample Preparation
Mature Napier grass was sourced from Crops for the Future Research Centre (CFFRC), Semenyih, Selangor, Malaysia. The biomass was dried in an oven at 105 °C according to BS EN12048 standard prior to size reduction by using the Retsch rotor beater mill. The sample size was reduced to 0.2 and 2 mm and kept in air-tight plastic bags for further analysis.
2.2. Proximate Analysis of Mature Napier Grass
Proximate analysis was conducted on the shredded form of Napier grass by using a thermogravimetric analyzer (TGA; TGA/SDTA851, Mettler Toledo, Columbus, OH, USA) to determine fixed carbon, volatile matter, moisture and ash contents in Napier grass. The details of the experimental procedure can be found elsewhere [16].
2.3. Ultimate Analysis of Mature Napier Grass
An ultimate analysis was conducted to determine elemental composition of mature Napier grass by using the CHNS/O analyzer (model LECO CHN628 and 628S, St. Joseph, MI, USA) according to the ASTM D-5291 standard method.
2.4. Measurement of the Higher Heating Value of Napier Grass
The gross calorific value of mature Napier grass was measured by using the Parr 6100 oxygen bomb calorimeter (Moline, IL, USA) according to BS EN 14918.
2.5. Gasification of Napier Grass for Syngas Production
The gasification of the shredded Napier grass was conducted in a fluidized bed gasifier. The reactor was cylindrical with 370 mm high and 54 mm wide, made of stainless steel. The schematic of the experimental rig is shown in Figure 1.
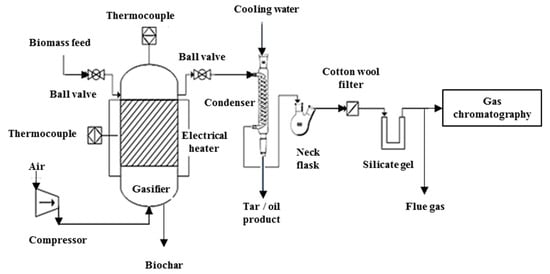
Figure 1.
Process flow diagram of lab scale gasifier setup.
The procedure began with charging the reactor with 20 g of sand as the bed material to obtain good temperature distribution, to stabilize the fluidization and to prevent coking inside the reactor. Air stream and biomass feedstock were introduced from the bottom and top of the reactor respectively as the bed temperature achieved the steady state condition. The experiment was carried out at five different temperatures between 650 °C and 850 °C at 50 °C temperature increment and three different equivalence ratio (ER; 0.2, 0.3 and 0.4).
2.5.1. Gas Analysis
Syngas produced from gasification process were analyzed using gas chromatography (GC) (model Agilent Technologies 6890N, Mundelein, IL, USA) equipped with a thermal conductivity detector (TCD).
2.5.2. Condensed Liquid Characterization
A visually brown liquid was produced from gasification of Napier grass. Prior to characterization, the brown liquid was decanted and diluted with dichloromethane solvent (99.8%) at a volume ratio of 1:1. The mixed solvent and brown liquid product were then centrifuged at 4000 rpm for 5 min by using the Hettich EBA 21 Centrifuge (Tuttlingen, Germany) to separate the organic phase from aqueous phase and char traces. The upper layer of the solution (bio-oil and solvent) was extracted and analyzed using gas chromatography mass spectrometry (GC–MS model QP2010 Plus SHIMADZU, Japan) equipped with a Zebron ZB-5MS capillary column (30 m long, 0.25 mm inner diameter and 0.25 µm thick). The injection and detector temperatures were set at 250 °C and 200 °C respectively. The flow rate of the carrier gas, He, was 1.0 mL/min.
2.5.3. Analysis of Inorganic Compounds in Ash
The inorganic compounds in the Napier grass ash were analyzed by using an energy dispersive X-ray fluorescence spectrometer (model SHIMADZU EDX-720, Japan).
3. Results and Discussion
3.1. Proximate and Ultimate Analysis of Napier Grass Feedstock
The results of the ultimate and proximate analysis of NG and other biomasses reported in the literature are presented in Table 2. TGA was conducted at three different heating rates (5, 10 and 20 °C/min) and almost similar results were produced. Therefore, the result acquired at 10 °C/min was considered for further discussion. As shown in Figure 2, NG in air atmosphere recorded a higher moisture content (8.78%) compared to the N2 atmosphere (7.73%). The same pattern was observed for volatile matter where a high content was recorded in air (81.49%) compared to nitrogen (62.70%). This is due to the presence of oxygen in the air that promotes biomass devolatilization. In contrast, lower fixed carbon content was recorded in air atmosphere (3.54%) compared to the N2 atmosphere (9.69%). Ash content was three times higher under N2 as compared to air atmospheres. According to Ptasinski [17] substantial variation in characteristics of biomass might have a direct effect on syngas composition. In addition, due to the environmental conditions and harvesting techniques, the biomass composition may vary remarkably [18].
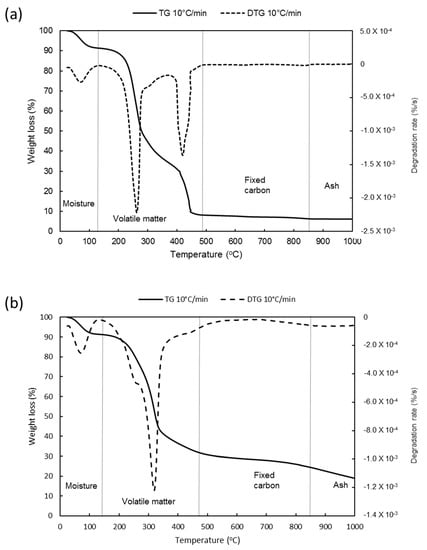
Figure 2.
Proximate analysis of Napier grass in (a) air and (b) nitrogen at a constant flowrate of 25 mL/min.
NG contains a high fraction of volatile matter (>80%) and moisture (30%) but low ash content (<7%). The high content of volatile matter in the Napier grass is desirable since this feature enhances the gasification process and reduces the amount of char produced [7]. High moisture content (>40 wt%) of biomass tends to degrade the gasification performance as more energy is required in the drying process [19]. Besides, moderate fixed carbon compared to other biomasses indicates shorter burning time is required as the solid-gas combustion reactions are slower than gas–gas reactions [7].
Ultimate analysis of NG found that NG contained 48.52% oxygen, 45.10% carbon, 5.94% hydrogen, 0.45% nitrogen and 0% sulphur. These values were comparable to NG, Miscanthus and coconut shell reported in literature. However, in comparison to all biomasses except for the NG in literature, NG had higher oxygen but lower carbon content, indicating a lower calorific value. Higher oxygen composition (carbon–oxygen bonds) would have a significant negative effect on the heating value of biomass [20]. NG is considered as an environmentally friendly feedstock as it contains a low amount of nitrogen and no sulphur, which may improve fuel quality and reduce toxic gas emission. The heating value of NG (16.73 MJ/kg) was comparable to coconut shell but slightly lower than other biomasses in literature because of its high level of moisture and oxygen. These desirable characteristics indicate that NG is a good solid biofuel candidate for energy generation via gasification.

Table 2.
Comparison of Napier grass (NG) feedstock characteristics with various types of biomass in literature.
Table 2.
Comparison of Napier grass (NG) feedstock characteristics with various types of biomass in literature.
| Property | Napier Grass (Current Study) | Napier Grass [13] | Cardoon [21] | Miscanthus [21] | Rubber–Wood–Sawdust [22] | Palm Kernel Shell [23] | Coconut Shell [23] |
|---|---|---|---|---|---|---|---|
| Moisture (wt%) | 30.07 | 9.43 | 12.00 | 6.48 | - | 7.96 | 4.89 |
| Proximate analysis (wt%, dry basis) | |||||||
| Volatile matter | 85.52 | 72.58 | 76.02 | 78.36 | 51.39 | 72.47 | 30.62 |
| Fixed carbon | 8.17 | 8.35 | 9.19 | 14.90 | 14.29 | 18.56 | 26.41 |
| Ash content | 6.31 | 9.68 | 14.80 | 6.74 | 22.67 | 8.97 | 42.98 |
| Ultimate analysis (wt%, dry basis) | |||||||
| C | 45.10 | 42.40 | 56.01 | 46.97 | 53.40 | 51.63 | 45.24 |
| H | 5.94 | 5.96 | 6.46 | 5.57 | 6.70 | 5.52 | 5.04 |
| N | 0.45 | 1.71 | 0.99 | 1.37 | 3.10 | 1.89 | 1.46 |
| S | 0.00 | 0.09 | 0.22 | 0.28 | 0.00 | 0.05 | 0.06 |
| O (by difference) | 48.52 | 45.32 | 36.10 | 45.82 | 36.80 | 40.91 | 48.2 |
| Calorific value (MJ/kg) | 16.73 | - | 17.33 | 18.73 | 18.30 | 22.97 | 16.07 |
3.2. Inorganic Compounds in Napier Grass Ash
Table 3 compares inorganic compounds in ash of NG and other biomasses reported in literature. More than half of inorganic compounds detected in NG ash were potassium (54.39%), with some iron (15.53%), silicon (9.81%), chlorine (8.84%), calcium (8.20%) and sulphur (2.30%). The ash composition of NG in literature is rich in silicon (43.00%), potassium (30.5%), magnesium (9.90%) and phosphorus (7.20%). The differences of NG ash properties in the current study as compared to the literature were due to the fact that the trees were dependent on growth conditions and other environmental factors. The ash composition has a negative effect on the gasification performance reactor when subjected to high temperature combustion [24].
According to Obernberger and Thek [25], a high potassium content in ash causes agglomeration at high temperature, which may bring severe damage to the gasifier system. Arvelakis, et al. [26] reported that potassium content in ash could react with bed material such as silica sand and break Si–O–Si bonds to form silicates that deposit on the reactor wall and on the bed particle surface, causing agglomeration. According to Arvelakis, Gehrmann, Beckmann and Koukios [26], high proportion of SiO2 found in the ash of Miscanthus (62.21%) and agricultural residues (89.57%) would cause severe agglomeration when the potassium reacts with SiO2.

Table 3.
Inorganic contents in ash of Napier grass and other types of biomass.
Table 3.
Inorganic contents in ash of Napier grass and other types of biomass.
| Ash Basis (wt%) | Napier Grass (Current Study) | Olive Tree Residue [27] | EFB [28] | Napier Grass [12] | Cardoon [21] | Miscanthus [21] | Agricultural Residue [29] |
|---|---|---|---|---|---|---|---|
| K2O | 54.39 | 9.26 | 44.00 | 30.5 | 24.91 | 14.00 | 1.65 |
| Fe2O3 | 15.53 | 1.38 | 3.00 | 1.4 | 1.77 | 2.63 | 2.95 |
| SiO2 | 9.81 | 11.84 | 27.00 | 43.0 | 8.34 | 62.21 | 89.57 |
| Cl | 8.84 | - | 5.30 | - | - | - | 1.30 |
| CaO | 8.20 | 54.82 | 8.00 | 1.9 | 38.33 | 8.32 | 0.77 |
| SO3 | 2.03 | - | 2.70 | - | - | - | - |
| MnO | 0.44 | 0.10 | 0.11 | - | - | - | - |
| Rb2O | 0.37 | - | 0.12 | - | - | - | - |
| Br | 0.14 | - | 0.018 | - | - | - | - |
| CuO | 0.10 | - | 0.039 | - | - | - | - |
| ZnO | 0.10 | - | 0.092 | 0.08 | - | - | - |
| As2O3 | 0.66 | - | - | - | - | - | - |
| Al2O3 | - | 2.60 | 0.97 | <0.1 | 3.50 | 5.47 | 1.32 |
| MgO | - | 4.36 | 4.80 | 9.9 | 5.74 | 3.16 | 0.76 |
| Na2O | - | 0.16 | 0.55 | <0.01 | 13.08 | 0.53 | 1.15 |
| TiO2 | - | 0.35 | 0.08 | 0.03 | 0.10 | 0.32 | 7.56 |
| P2O5 | - | 3.40 | 3.60 | 7.2 | 4.23 | 3.37 | 1.04 |
| NiO | - | - | 0.01 | - | - | - | - |
| SrO | - | - | 0.03 | 0.03 | - | - | - |
| BaO | - | - | - | 0.08 | - | - | - |
Mohammed, Salmiaton, WanAzlina and Mohamad Amran [16] stated that CaO acts as a CO2 adsorbent where its presence might accelerate the secondary reaction and therefore improve hydrogen content in the syngas. In addition, Mahishi and Goswami [30] supported that the presence of CaO has a significant effect on hydrogen production during the gasification process at an elevated temperature. In relation to iron content, NG in the current study contains a high amount of iron, which was 15.53%. According to Lahijani and Zainal [28], magnesium, iron and calcium are good agents for reducing agglomeration. Low silica content (9.81%) was found in Napier grass in this current study and this amount was comparable to olive residues (11.84%) and cardoon (8.34%). Mohammed, Salmiaton, Wan Azlina and Mohamad Amran [16] mentioned that the combination of low silica content with reasonable amount of MgO, Fe2O and Al2O3 is effective in reducing agglomeration. Furthermore, NG in the current study contained a significant amount of Cl (8.84%), which could react with potassium to form potassium chloride and subsequently promote potassium devolatilization [26].
3.3. Components of Bio-Liquid
Gasification of biomass generates bio-liquid as one of the by-products. The liquid is visually dark brown, usually comprised of water, oxygenated hydrocarbons and other hydrocarbons. Bio-liquid generated from pyrolysis and gasification can be used for fuel in direct combustion or as chemical products after further treatment processes. Table 4 presents the fraction of individual compounds detected in the bio-liquid produced from gasification of Napier grass. The liquid contains a mixture of hydrocarbon, oxygenated and nitrogenated compounds. Phosphonic acid, (p-hydroxyphenyl) was found to be the major constituent (31.94%), followed by pentane, 2,2-dimethyl (16.10%). The oxygenated compounds in bio-liquid were corrosive in nature with low pH value. Bio-liquid with high amount of oxygenated compounds should undergo further treatment such as hydrothermal processing to produce biofuels or value-added chemicals [13].

Table 4.
Chemical compounds detected in bio-liquid obtained from gasification of Napier grass at 850 °C and equivalence ratio (ER) of 0.2.
3.4. Effects of ER on the Product Yield and Composition of Producer Gas
Equivalence ratio (ER) is defined as the ratio of the amount of air supplied during the thermochemical process to the amount of air required for stoichiometric combustion of the fuel (Equation (1)). The concept of ER is applicable in gasification when air or oxygen is injected for partial combustion of the biomass feedstock. In comparison to the combustion process, which requires excess air and ER to be greater than 1 for complete fuel combustion, the range of ER for gasification is usually limited to a value below 0.4.
ER = (Actual weight air/weight of dry biomass)/(Stoichiometric air/biomass ratio).
The formula for stoichiometric combustion with oxygen is:
where:
CH1.56O0.81 + 0.985O2 → 0.78H2O + CO2,
ACstq = (MO2 + 3.76MN2)/100 × (C/Mc + H/2MH2 + S/MS − O/MO2) = 5.12.
Taking the information below into consideration.
Density of air, ρ = m/V.
1.18 = 0.032/V.
V = 0.027 m3 = 27 liters.
Superficial velocity = V/A.
Diameter of reactor, D = 0.054 m.
Surface area of reactor, A = π × (D/2)2 = 2.29 × 10−3 m2.
Superficial velocity = 3.0 L/min × 1 m3/1000 L × 1/2.29 × 10−3 m2 × 1 min/60 s = 0.0218 m/s.
In this study, the ER was manipulated by varying the air flowrate while the biomass feeding rate remained unchanged. The effects of ER on product yield are shown in Figure 3. Bio-gas and bio-liquid yields recorded an upward trend with increasing ER while the bio-char demonstrated the opposite. The ER is an indicator of the quantity of oxygen supplied to the reactor and gasification temperature under autothermal operation [29]. Higher ER leads to higher gasification temperature, accelerates oxidation reactions and leads to enhanced product quality. Conversely, lower ER limits the amount of oxygen available for gasification reactions and therefore is not a favorable condition [31].
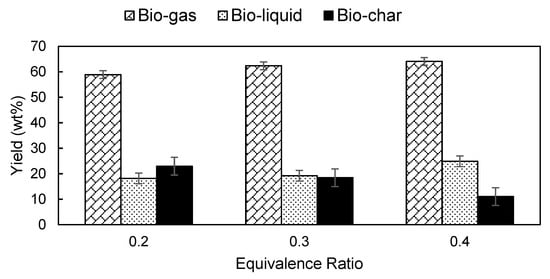
Figure 3.
Effect of ER on the NG gasification product yield.
Figure 4 shows the gas composition at different ER. As ER increased from 0.20 to 0.40, H2 and CH4 contents in the producer gas decreased from 11.54 mol% to 8.29 mol% and 9.85 mol% to 7.09 mol% respectively. The effect of ER on gas composition is attributed to the oxidation reactions. Higher ER implies that more air (oxygen) is injected into the reactor, which in turn promotes combustion of CH4 with O2 while the CH4 formation by methanation reaction is inhibited at high temperature [32]. Therefore, the volume fraction of CH4 decreases as ER increases. Furthermore, Kuo, et al. [33] also reported that the fraction of H2, CO and CH4 decreases as ER increases. As the amount of oxygen supplied decreases with decreasing ER, the carbon converts to CO instead of CO2 through oxidation and partial combustion reactions [30]. As the amount of CO increased, more CH4 and H2 are formed through methanation and water-gas shift reactions. Hence, higher concentration of CH4 and H2 are detected in producer gas at lower ER.
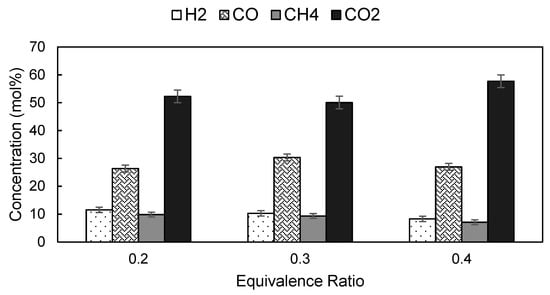
Figure 4.
Effect of ER on the composition of producer gas produced from the gasification of Napier grass at a temperature of 850 °C.
At ER of 0.20, the concentration of CO and CO2 were 26.36 mol% and 52.26 mol%, respectively. As ER increased to 0.30, the concentration of CO increased to 30.32 mol% and decreased to 26.94 mol% as ER was further increased to 0.40. The observation is in good agreement with finding reported by Ghassemi and Shahsavan–Markadeh [34]. Meanwhile, the concentration of CO2 decreased slightly to 50.04 mol% as ER increased to 0.3, and increased to 57.68 mol% as ER was further increased to 0.40. The phenomena could be deciphered by reversible water-gas shift and dry reforming reactions. Excess air would promote oxidation of bio-char and other combustible species and consequently leads to elevated CO2 production.
3.5. Effects of Temperature on Product Yield and Quality of Producer Gas
In air gasification of biomass, gasification temperature is one of the crucial operating parameters, which is usually manipulated to investigate thermodynamic behavior of the reactions. Production of syngas consists of multi-step chemical reactions where temperature has significant impacts on the kinetics of the reactions involved. The influence of temperature on syngas production from the gasification of Napier grass was investigated at five different reactor temperatures (650 °C, 700 °C, 750 °C, 800 °C and 850 °C) while ER was fixed at 0.25.
The experimental results are shown in Figure 5 and Figure 6. As observed in Figure 5, the bio-gas yield increased from 56.92 wt% to 67.56 wt% while the bio-char yield decreased from 27.40 wt% to 17.88 wt% with the rise of temperature, showing a divergent trend. The yield of bio-liquid showed an upward trend with increasing temperature and recorded a peak of 20.12 wt% at 800 °C. The yield subsequently declined as the temperature was further increased. A high operating temperature provides a conducive condition and supplies sufficient thermal energy for Boudouard, water-gas and methanation reactions, consuming more solid carbon to produce combustible gases. Furthermore, as the temperature increased from 650 °C to 800 °C, more H2 was produced and reacted with O2 to form water, and thus increased the yield of bio-liquid. As the temperature further increased to 850 °C, secondary reactions such as tar-cracking consume water and therefore reduce the yield of bio-liquid.
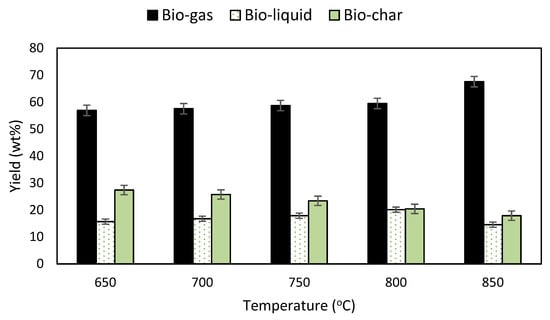
Figure 5.
Effects of temperature on Napier grass gasification yield.
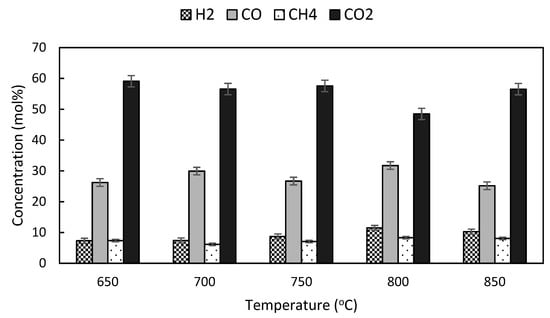
Figure 6.
Effects of temperature on gas composition at ER of 0.25.
Figure 6 illustrates the effects of temperature on the composition of H2, CO, CH4 and CO2 in the gases produced from gasification of NG at ER of 0.25. H2 gas concentration increased remarkably from 7.31 mol% to 11.47 mol% as the operating temperature increased from 650 °C to 800 °C and dropped slightly to 10.27 mol% at 850 °C. The high operating temperature provides favorable conditions for endothermic reversible steam methane reforming, water-gas and dry reforming reactions. The production of hydrogen is enhanced as the heat absorbing reactions shift the equilibrium to the right [28].
No distinctive trend can be observed for CO and CO2 production. CO concentration increased from 26.23 mol% to 29.93 mol% as the temperature was increased from 650 °C to 700 °C. Subsequently the CO content decreased to 26.69 mol% at 750 °C and dropped further to 25.16 mol% at 850 °C. Within the gasifier operating temperature range of 650–850 °C, the recorded CO2 concentration was within the range of 48.48–59.07 mol%. The pattern in CO2 production at varying operating temperatures was opposite to CO. The high bed temperature stimulated complete combustion while deterring incomplete combustion processes, and accelerated solid carbon burning to produce CO2 instead of CO.
3.6. Effects of ER on the Higher Heating Value (HHV) of Syngas and Carbon Conversion Efficiency (CCE)
The calculation of the gross calorific value or higher heating value (HHV) of Napier grass can be performed by using Equation (11) [35]. Carbon conversion efficiency (CCE) is one of the key indicators of gasification performance, which provides the information pertaining to the degree of reaction completion. CCE can be determined from carbon element content in biomass feedstock and syngas composition, and can be calculated by using Equation (12) [36].
where CO%, H2% and CH4% are molar fraction of syngas components.
where Ma and M0 are final and initial total mass of biomass respectively.
HHV (MJ/Nm3) = (CO% × 3018 + H2% × 3052 + CH4% × 9500)(0.01 × 4.1868),
CCE (%) = (1 − Ma/M0) × 100,
The use of air as a gasifying agent is cheap and widely practiced. However, the presence of abundant nitrogen in air dilutes the concentration of syngas and consequently reduces the syngas heating value. The values of producer gas HHV and biomass CCE at varying ER are summarized in Table 5. The calculation of HHV is performed for both with and without taking nitrogen content in the air into consideration. With the presence of nitrogen, the highest HHV was found at ER of 0.20, and the value decreased as ER was further increased. According to Lv, et al. [37], ER is more than just a measurement of oxygen supply. ER represents the border-line between combustion and gasification reactions in the gasification system. Jayathilake and Rudra [38] reported that lower ER values resulted in higher CH4 and H2 concentration. The higher amount of air supplied at higher ER promotes combustion of H2 and CH4 components in the syngas. In addition, CH4 formation by methanation reaction is retarded at higher temperature that comes with higher ER. Consequently, the reduction of H2 and CH4 concentration in syngas at higher ER will directly reduce the HHV of the producer gas. The findings are in good agreement with research done by Sheth and Babu [39]. Syngas HHV under N2 free condition increased slightly from 8.72 MJ/m3 to 8.78 MJ/m3 as ER increased from 0.20 to 0.30, and decreased to 7.28 MJ/m3 with a further increase in the ER to 0.4. On the other hand, as shown in Table 5, higher CCE is achieved at higher ER. The increased amount of air supplied during gasification improves the combustion process and contact with solid carbon, thus enhances the carbon conversion rate.

Table 5.
Effects of ER on higher heating value (HHV) and carbon conversion efficiency (CCE) at a gasification temperature of 850 °C.
The effects of varying gasification temperature on producer gas HHV and biomass CCE are presented in Table 6. The HHV appeared to be increasing with increasing temperature. A peak was recorded at a gasification temperature of 800 °C where HHV subsequently decreased with a further temperature rise. Gasification temperature had a significant effect on syngas composition, which directly influenced the HHV of producer gas. As reported by Wu, et al. [40], elevated CO2 concentration from high temperature biomass combustion process dilutes the concentration of H2 and CH4, results in a lower calorific value. As depicted in Table 6, CCE demonstrates a positive correlation with gasification temperature. The highest CCE is recorded to be 82.12% at 850 °C. The findings are in good agreement with work reported by Lv, Xiong, Chang, Wu, Chen and Zhu [37]. As shown in Table 5 and Table 6, the dilution of the syngas by nitrogen can degrade the HHV of the syngas by at least twofold. The syngas produced from gasification thermochemical conversion of biomass can be used to produce heat and electricity in the combined heat and power (CHP) system, internal combustion engines or other applications.

Table 6.
Effects of temperature on HHV and CCE.
4. Conclusions
Napier grass energy crop demonstrates a good potential as a renewable solid biofuel. Its calorific value of 16.73 MJ/kg is comparable to other biomasses reported in literature. The high content of volatile matter in Napier grass is highly desirable as this feature promotes the gasification process. Thermochemical gasification of Napier grass produces syngas and value-added by-products such as bio-char and bio-liquid. The presence of a high amount of potassium in Napier grass ash might impose a further problem for the long-term operation. The dynamics of the reactions involved were observed as a significant variation in product yield and biogas components were recorded at varying ER and gasifier operating temperatures. There was a positive correlation between ER and gasification temperature with CCE. Enhancement of combustion process at elevated temperature and air supply produced CO2 that degraded the syngas quality and resulted in low HHV. The highest HHV was recorded at a gasification temperature of 800 °C with ER of 0.2 and thus these conditions were determined as the optimum operational conditions for bench-scale gasification of Napier grass. The findings from this study were encompassed within the limited explored range and further research (scale-up) will be carried out as future development.
Author Contributions
Conceptualization, T.H.B. and W.A.W.A.K.G.; methodology, W.A.W.A.K.G. and D.K.S.N.; formal analysis, T.H.B.; investigation, T.H.B.; resources, D.K.S.N.; writing—original draft preparation, M.S.M.S. and T.H.B.; writing—review and editing, M.S.M.S. and W.A.W.A.K.G.; supervision, W.A.W.A.K.G. and S.A.H.; funding acquisition, W.A.W.A.K.G.
Funding
This research was funded by Ministry of Education Malaysia, grant number LRGS/2013/UKM/KPT.
Acknowledgments
Feedstocks provided by Crops for the Future Research Centre (CFFRC) are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Hauck, J.; Pongratz, J.; Pickers, P.A.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; et al. Global Carbon Budget 2018. Earth Syst. Sci. Data 2018, 10, 2141–2194. [Google Scholar] [CrossRef]
- Bujang, A.S.; Bern, C.J.; Brumm, T.J. Summary of energy demand and renewable energy policies in Malaysia. Renew. Sustain. Energy Rev. 2016, 53, 1459–1467. [Google Scholar] [CrossRef]
- Rebitanim, N.Z.; Wan Ab Karim Ghani, W.A.; Rebitanim, N.A.; Amran Mohd Salleh, M. Potential applications of wastes from energy generation particularly biochar in Malaysia. Renew. Sustain. Energy Rev. 2013, 21, 694–702. [Google Scholar] [CrossRef]
- Energy Commission. 2017 Malaysia Energy Statistics Handbook; Suruhanjaya Tenaga (Energy Commission): Putrajaya, Malaysia, 2017. [Google Scholar]
- Mohammed, M.A.A.; Salmiaton, A.; Wan Azlina, W.A.K.G.; Mohammad Amran, M.S.; Fakhru’l-Razi, A.; Taufiq-Yap, Y.H. Hydrogen rich gas from oil palm biomass as a potential source of renewable energy in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 1258–1270. [Google Scholar] [CrossRef]
- Alipour Moghadam, R.; Yusup, S.; Azlina, W.; Nehzati, S.; Tavasoli, A. Investigation on syngas production via biomass conversion through the integration of pyrolysis and air–steam gasification processes. Energy Convers Manag. 2014, 87, 670–675. [Google Scholar] [CrossRef]
- Mallick, D.; Mahanta, P.; Moholkar, V.S. Co-gasification of coal and biomass blends: Chemistry and engineering. Fuel 2017, 204, 106–128. [Google Scholar] [CrossRef]
- Ismail, W.M.S.W.; Mohd Thaim, T.; Abdul Rasid, R. Biomass gasification of oil palm fronds (OPF) and Koompassia malaccensis (Kempas) in an entrained flow gasifier: A performance study. Biomass Bioenergy 2019, 124, 83–87. [Google Scholar] [CrossRef]
- Chan, Y.H.; Quitain, A.T.; Yusup, S.; Uemura, Y.; Sasaki, M.; Kida, T. Liquefaction of palm kernel shell in sub- and supercritical water for bio-oil production. J. Energy Inst. 2018, 91, 721–732. [Google Scholar] [CrossRef]
- Hlavsová, A.; Corsaro, A.; Raclavská, H.; Juchelková, D.; Škrobánková, H.; Frydrych, J. Syngas Production from Pyrolysis of Nine Composts Obtained from Nonhybrid and Hybrid Perennial Grasses. Sci. World J. 2014, 2014, 11. [Google Scholar] [CrossRef]
- Suntivarakorn, R.; Treedet, W.; Singbua, P.; Teeramaetawat, N. Fast pyrolysis from Napier grass for pyrolysis oil production by using circulating Fluidized Bed Reactor: Improvement of pyrolysis system and production cost. Energy Rep. 2018, 4, 565–575. [Google Scholar] [CrossRef]
- Strezov, V.; Evans, T.J.; Hayman, C. Thermal conversion of elephant grass (Pennisetum Purpureum Schum) to bio-gas, bio-oil and charcoal. Bioresour. Technol. 2008, 99, 8394–8399. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K.; Tsai, W.-T.; Tsai, Y.-L.; Lin, S.-H. Pyrolysis of napier grass in an induction-heating reactor. J. Anal. Appl. Pyrol. 2010, 88, 110–116. [Google Scholar] [CrossRef]
- Farzad, S.; Mandegari, M.A.; Görgens, J.F. A critical review on biomass gasification, co-gasification, and their environmental assessments. Biofuel Res. J. 2016, 3, 483–495. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Kulkarni, A.; Adhikari, S. Effects of temperature and equivalence ratio on mass balance and energy analysis in loblolly pine oxygen gasification. Energy Sci. Eng. 2016, 4, 256–268. [Google Scholar] [CrossRef]
- Mohammed, M.A.A.; Salmiaton, A.; Wan Azlina, W.A.K.G.; Mohamad Amran, M.S. Gasification of oil palm empty fruit bunches: A characterization and kinetic study. Bioresour. Technol. 2012, 110, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Ptasinski, K.J. Thermodynamic efficiency of biomass gasification and biofuels conversion. Biofuel. Bioprod. Biorefin. 2008, 2, 239–253. [Google Scholar] [CrossRef]
- Monti, A.; Di Virgilio, N.; Venturi, G. Mineral composition and ash content of six major energy crops. Biomass Bioenergy 2008, 32, 216–223. [Google Scholar] [CrossRef]
- Hosseini, M.; Dincer, I.; Rosen, M.A. Steam and air fed biomass gasification: Comparisons based on energy and exergy. Int. J. Hydrog. Energy 2012, 37, 16446–16452. [Google Scholar] [CrossRef]
- Sher, F.; Pans, M.A.; Sun, C.; Snape, C.; Liu, H. Oxy-fuel combustion study of biomass fuels in a 20 kWth fluidized bed combustor. Fuel 2018, 215, 778–786. [Google Scholar] [CrossRef]
- Karampinis, E.; Vamvuka, D.; Sfakiotakis, S.; Grammelis, P.; Itskos, G.; Kakaras, E. Comparative study of combustion properties of five energy crops and Greek lignite. Energy Fuels 2012, 26, 869–878. [Google Scholar] [CrossRef]
- Ghani, W.A.W.A.K.; Mohd, A.; da Silva, G.; Bachmann, R.T.; Taufiq-Yap, Y.H.; Rashid, U.; Ala’a, H. Biochar production from waste rubber-wood-sawdust and its potential use in C sequestration: Chemical and physical characterization. Ind. Crop. Prod. 2013, 44, 18–24. [Google Scholar] [CrossRef]
- Wan Ab Karim Ghani, W.; Moghadam, R.A.; Salleh, M.; Alias, A. Air gasification of agricultural waste in a fluidized bed gasifier: Hydrogen production performance. Energies 2009, 2, 258–268. [Google Scholar] [CrossRef]
- González-Vázquez, M.P.; García, R.; Gil, M.V.; Pevida, C.; Rubiera, F. Unconventional biomass fuels for steam gasification: Kinetic analysis and effect of ash composition on reactivity. Energy 2018, 155, 426–437. [Google Scholar] [CrossRef]
- Obernberger, I.; Thek, G. Physical characterisation and chemical composition of densified biomass fuels with regard to their combustion behaviour. Biomass Bioenergy 2004, 27, 653–669. [Google Scholar] [CrossRef]
- Arvelakis, S.; Gehrmann, H.; Beckmann, M.; Koukios, E.G. Preliminary results on the ash behavior of peach stones during fluidized bed gasification: Evaluation of fractionation and leaching as pre-treatments. Biomass Bioenergy 2005, 28, 331–338. [Google Scholar] [CrossRef]
- Cuenca, J.; Rodríguez, J.; Martín-Morales, M.; Sánchez-Roldán, Z.; Zamorano, M. Effects of olive residue biomass fly ash as filler in self-compacting concrete. Constr. Build. Mater. 2013, 40, 702–709. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A. Gasification of palm empty fruit bunch in a bubbling fluidized bed: A performance and agglomeration study. Bioresour. Technol. 2011, 102, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Ghani, W.; Alias, A.; Savory, R.; Cliffe, K. Co-combustion of agricultural residues with coal in a fluidised bed combustor. Waste Manag. 2009, 29, 767–773. [Google Scholar] [CrossRef]
- Mahishi, M.R.; Goswami, D.Y. An experimental study of hydrogen production by gasification of biomass in the presence of a CO2 sorbent. Int. J. Hydrog. Energy 2007, 32, 2803–2808. [Google Scholar] [CrossRef]
- Miccio, F.; Moersch, O.; Spliethoff, H.; Hein, K.R.G. Generation and conversion of carbonaceous fine particles during bubbling fluidised bed gasification of a biomass fuel. Fuel 1999, 78, 1473–1481. [Google Scholar] [CrossRef]
- Guo, F.; Dong, Y.; Zhang, T.; Dong, L.; Guo, C.; Rao, Z. Experimental Study on Herb Residue Gasification in an Air-Blown Circulating Fluidized Bed Gasifier. Ind. Eng. Chem. Res. 2014, 53, 13264–13273. [Google Scholar] [CrossRef]
- Kuo, J.-H.; Lin, C.-L.; Wey, M.-Y. Effect of agglomeration/defluidization on hydrogen generation during fluidized bed air gasification of modified biomass. Int. J. Hydrog. Energy 2012, 37, 1409–1417. [Google Scholar] [CrossRef]
- Ghassemi, H.; Shahsavan-Markadeh, R. Effects of various operational parameters on biomass gasification process; a modified equilibrium model. Energy Convers. Manag. 2014, 79, 18–24. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, M.; Jin, B.; Huang, Y.; Zhou, H. High-temperature air/steam-blown gasification of coal in a pressurized spout-fluid bed. Energy Fuels 2006, 20, 715–720. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda-Almansa, G.; Bula, A. Gasification of biomass wastes in an entrained flow gasifier: Effect of the particle size and the residence time. Fuel Process. Technol. 2010, 91, 681–692. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air–steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jayathilake, R.; Rudra, S. Numerical and Experimental Investigation of Equivalence Ratio (ER) and Feedstock Particle Size on Birchwood Gasification. Energies 2017, 10, 1232. [Google Scholar] [CrossRef]
- Sheth, P.N.; Babu, B.V. Experimental studies on producer gas generation from wood waste in a downdraft biomass gasifier. Bioresour. Technol. 2009, 100, 3127–3133. [Google Scholar] [CrossRef]
- Wu, C.Z.; Yin, X.L.; Ma, L.L.; Zhou, Z.Q.; Chen, H.P. Operational characteristics of a 1.2-MW biomass gasification and power generation plant. Biotechnol. Adv. 2009, 27, 588–592. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).