Study on the Compatibility of Gas Adsorbents Used in a New Insulating Gas Mixture C4F7N/CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
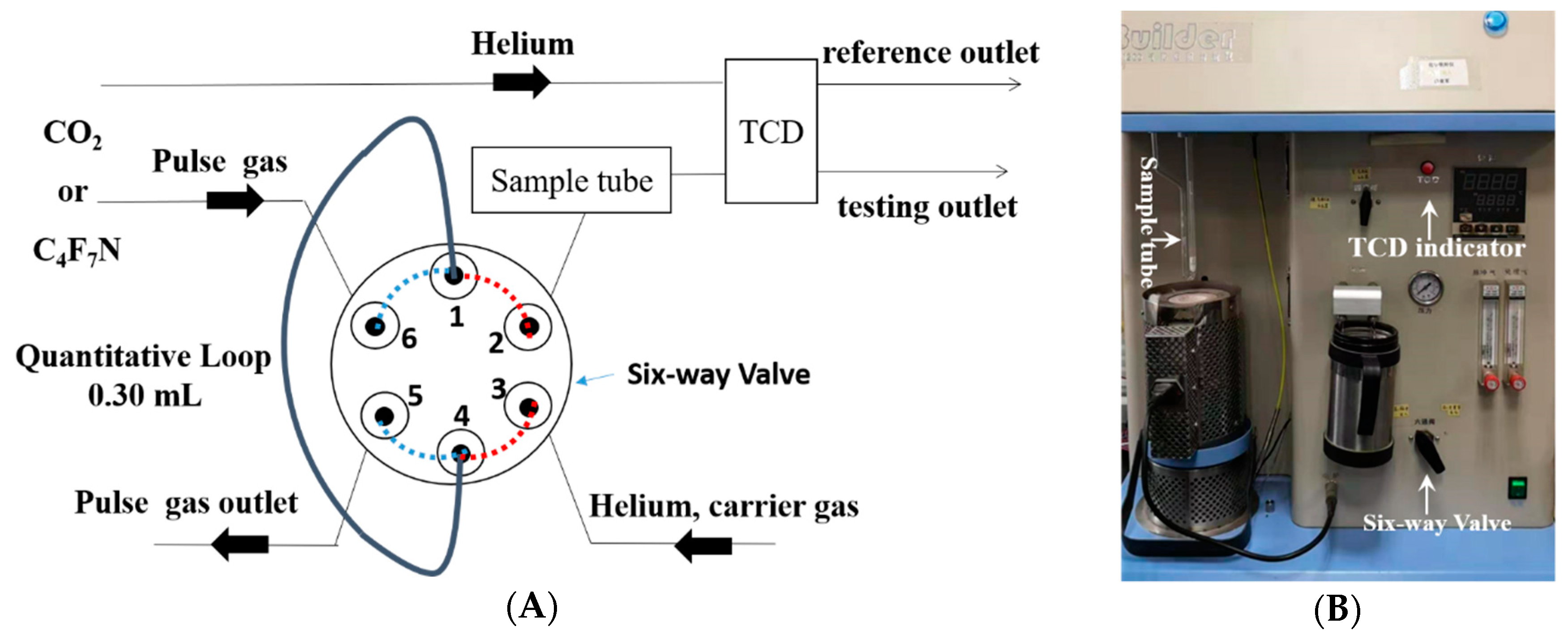
2.2. Adsorption Characterization
2.3. Data Analysis
3. Results and Discussion
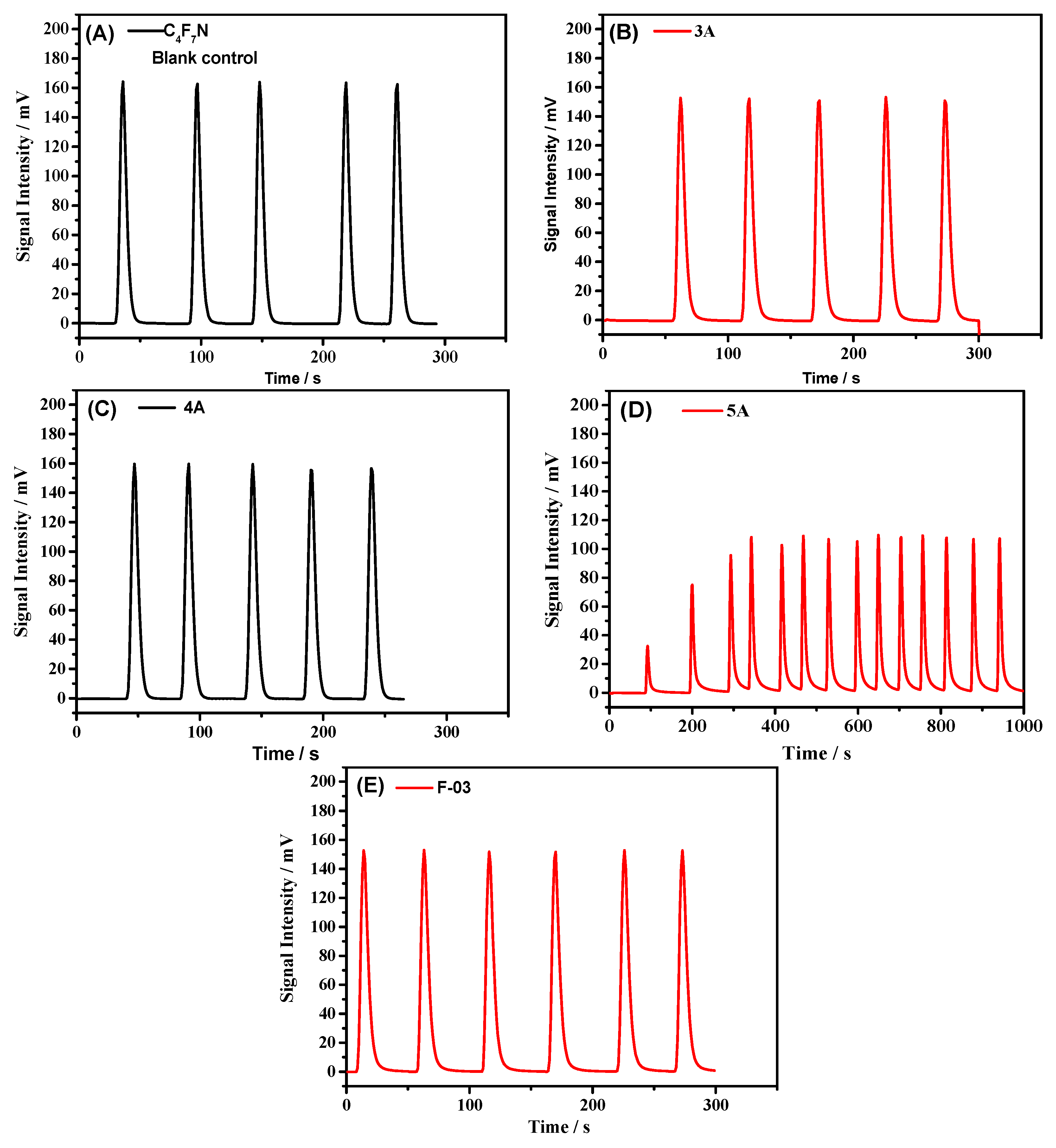
3.1. Compatibility of Samples with C4F7N
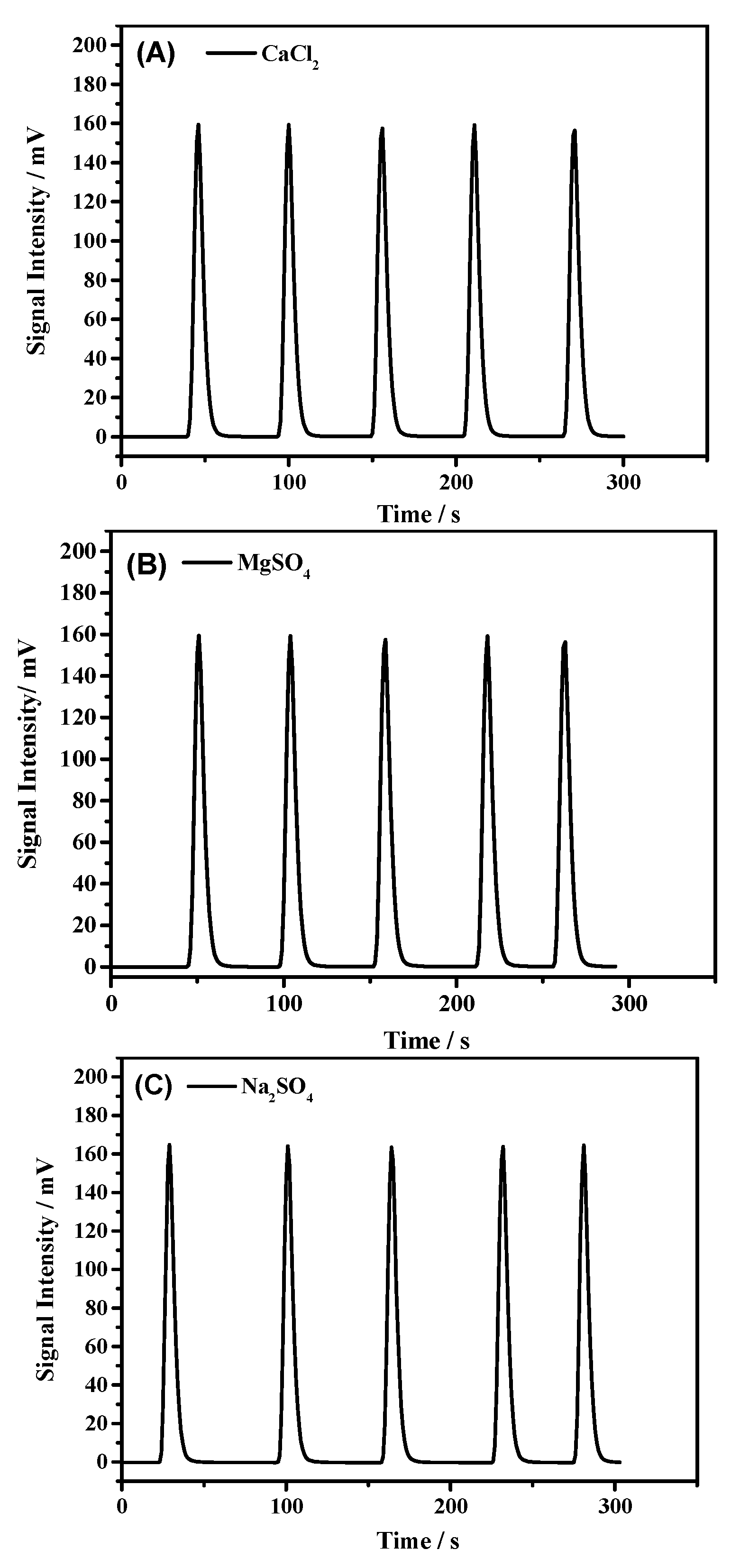
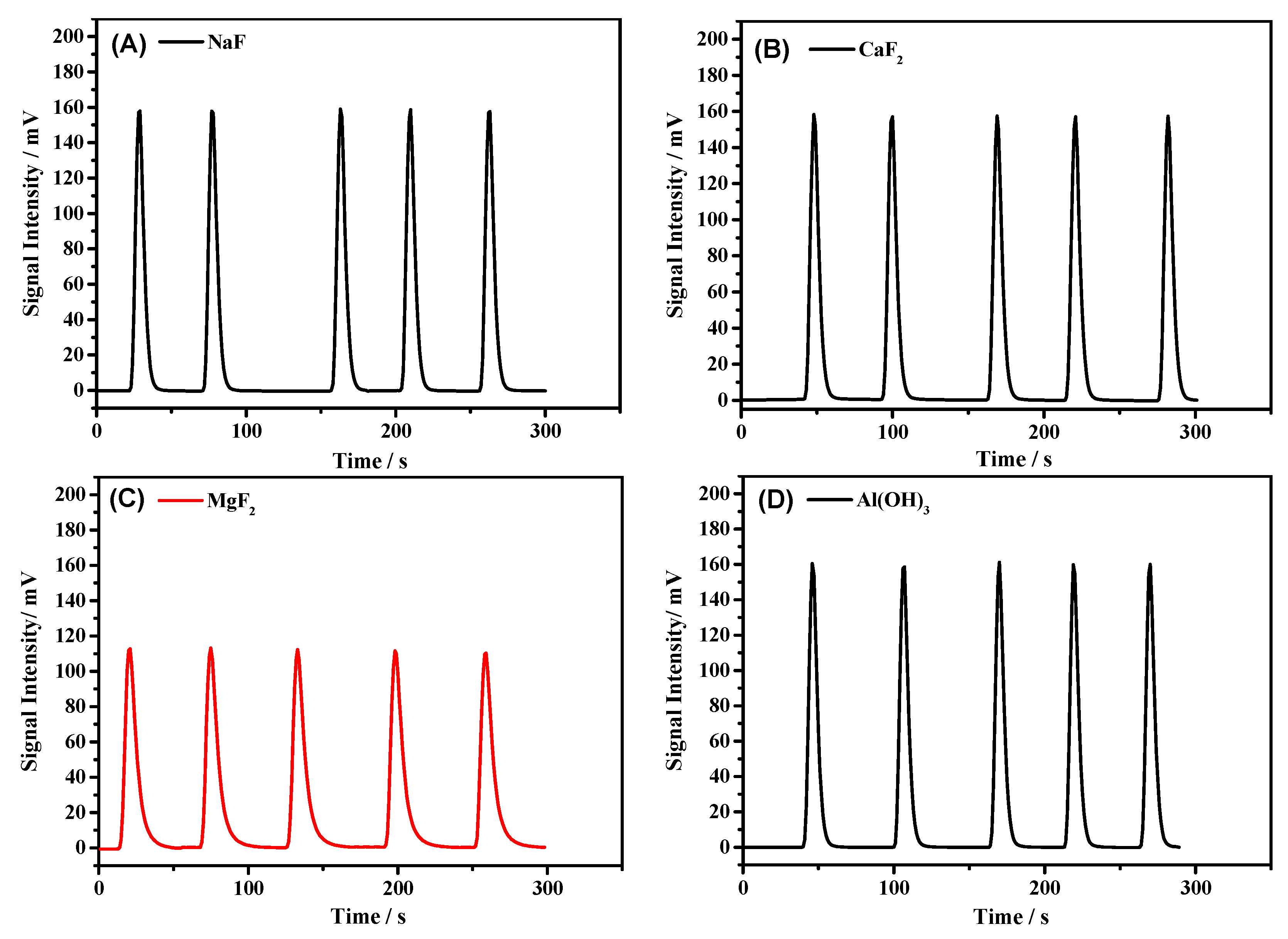
3.2. Compatibility of Samples with CO2
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Huang, D.; Liu, J.; Zhang, Y.; Zeng, L. Alternative Environmentally Friendly Insulating Gases for SF6. Processes 2019, 7, 216. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Xiao, S.; Chen, Q.; Tang, J.; Chen, D.; Wang, D. Decomposition Properties of C4F7N/N2 Gas Mixture: An Environmentally Friendly Gas to Replace SF6. Ind. Eng. Chem. Res. 2018, 57, 5173−5182. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, J.; Xiao, S.; Xie, B.; Chen, D.; Gao, Y.; Tang, J. Assessment on the toxicity and application risk of C4F7N: A new SF6 alternative gas. J. Hazard. Mater. 2019, 368, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Zhang, J.; Fu, M.; Zhuo, R.; Luo, Y.; Chen, D.; Xiao, S. Experimental study on the partial discharge and AC breakdown properties of C4F7N/CO2 mixture. High Voltage 2019, 4, 12–17. [Google Scholar] [CrossRef]
- Zhong, L.; Ji, S.; Wang, F.; Sun, Q.; Chen, S.; Liu, J.; Hai, B.; Tang, L. Theoretical study of the chemical decomposition mechanism and model of Sulfur hexafluorid (SF6) under corona discharge. J. Fluorine Chem. 2019, 220, 61–68. [Google Scholar] [CrossRef]
- Park, J.-H.; Shin, I.H.; Seo, S.H.; Choi, C.Y.; Son, Y.-S. The optimization of SF6 decomposition process using an electron beam. Radiat. Phys. Chem. 2018, 151, 192–197. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, D.; Cui, H.; Dong, X.; Xiao, S.; Tang, J. Understanding of SF6 decompositions adsorbed on cobalt-doped SWCNT: A DFT study. Appl. Surf. Sci. 2017, 420, 371–382. [Google Scholar] [CrossRef]
- Kieffel, Y. Characteristics of g3- an alternative to SF6. In Proceedings of the 2016 IEEE International Conference on Dielectrics (ICD), Montpellier, France, 3–7 July 2016; p. 880. [Google Scholar]
- Zhang, X.; Li, Y.; Xiao, S.; Tian, S.; Deng, Z.; Tang, J. Theoretical study of the decomposition mechanism of environmentally friendly insulating medium C4F7N in the presence of H2O in a discharge. J. Phys. D Appl. Phys. 2017, 50, 325201. [Google Scholar] [CrossRef]
- Pakseresht, S.; Kazemeini, M.; Akbarnejad, M.M. Equilibrium isotherms for CO, CO2, CH4 and C2H4 on the 5A molecular sieve by a simple volumetric apparatus. Sep. Purif. Technol. 2002, 28, 53–60. [Google Scholar] [CrossRef]
- AbdulKareem, F.A.; Mohd Shariff, A.; Ullah, S.; See, T.L.; Keong, L.K.; Mellon, N. Adsorption performance of 5A molecular sieve zeolite in water vapor–binary gas environment: Experimental and modeling evaluation. J. Ind. Eng. Chem. 2018, 64, 173–187. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, X.X.; Chen, D.; Fu, M.L.; Tang, J.; Li, Y. Adsorption Characteristics of γ-Al2O3 for the Environment-friendly Insulating Medium C4F7N/N2 and Its Decomposition Products. High Voltage Eng. 2018, 44, 3135–3140. [Google Scholar]
- Lin, R.; Ladshaw, A.; Nan, Y.; Liu, J.; Yiacoumi, S.; Tsouris, C.; DePaoli, D.W.; Tavlarides, L.L. Isotherms for Water Adsorption on Molecular Sieve 3A: Influence of Cation Composition. Ind. Eng. Chem. Res. 2015, 54, 10442–10448. [Google Scholar] [CrossRef]
- Liu, X.; Wang, R. Effective removal of hydrogen sulfide using 4A molecular sieve zeolite synthesized from attapulgite. J. Hazard. Mater. 2017, 326, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Gabruś, E.; Witkiewicz, K.; Nastaj, J. Modeling of regeneration stage of 3A and 4A zeolite molecular sieves in TSA process used for dewatering of aliphatic alcohols. Chem. Eng. J. 2018, 337, 416–427. [Google Scholar] [CrossRef]
- Hussein, M.S.; Ahmed, M.J. Fixed bed and batch adsorption of benzene and toluene from aromatic hydrocarbons on 5A molecular sieve zeolite. Mater. Chem. Phys. 2016, 181, 512–517. [Google Scholar] [CrossRef]
- Afzal, S.; Rahimi, A.; Ehsani, M.R.; Tavakoli, H. Experimental study of hydrogen fluoride adsorption on sodium fluoride. J. Ind. Eng. Chem. 2010, 16, 147–151. [Google Scholar] [CrossRef]
- Kaawar, Z.; Paulus, B. Adsorption of hydrogen fluoride on alkaline earth fluoride surfaces: A first-principles study. J. Fluorine Chem. 2019, 224, 67–72. [Google Scholar] [CrossRef]
- Ju, J.; Liu, R.; He, Z.; Liu, H.; Zhang, X.; Qu, J. Utilization of aluminum hydroxide waste generated in fluoride adsorption and coagulation processes for adsorptive removal of cadmium ion. Front. Environ. Sci. Eng. 2016, 10, 467–476. [Google Scholar] [CrossRef]
- Hiremath, C.R.; Kadoli, R.; Katti, V.V. Experimental and theoretical study on dehumidification potential of clay-additives based CaCl2 composite desiccants. Appl. Therm. Eng. 2018, 129, 70–83. [Google Scholar] [CrossRef]
- Fergus, J.W.; Hsu, T. Integrating humidity sensor based on a polybutadiene—MgSO4 composite. Meas. Sci. Technol. 2005, 16, 1255–1260. [Google Scholar] [CrossRef]
- Zhang, X.X.; Zhang, Q.C.; Zhang, J.; Li, Y.; Xiao, S.; Zhuo, R.; Tang, J. Experimental study on power frequency breakdown characteristics of C4F7N/CO2 gas mixture under quasi-homogeneous electric field. IEEE Access. 2019, 7, 19100–19108. [Google Scholar] [CrossRef]
- Li, Z.; Ding, W.; Liu, Y.; Li, Y.; Zheng, Z.; Liu, W.; Gao, K. Surface flashover characteristics of epoxy insulator in C4F7N/CO2 mixtures in a uniform field under AC voltage. IEEE Trans. Dielectr. Electr Insul. 2019, 26, 1065–1072. [Google Scholar] [CrossRef]
- Saheb, V.; Javanmardi, M. Theoretical studies on the mechanism and kinetics of the reaction of CF3 radical with oxygen molecule. J. Fluorine Chem. 2018, 211, 154–158. [Google Scholar] [CrossRef]
- Tavakoli, H.; Ghasemi, M.R. Equilibrium, kinetics and breakthrough studies for adsorption of hydrogen fluoride on sodium fluoride. Chem. Eng. Process: Process Intensif. 2010, 49, 435–440. [Google Scholar] [CrossRef]
- Li, C.X.; He, S.J.; Jiang, D.Y.; Li, Q. Hydrogen Fluoride Adsorption Ability of some Inorganic Compounds. Adv. Mater. Res. 2012, 412, 1–4. [Google Scholar] [CrossRef]
- McIntosh, G.J.; Agbenyegah, G.E.; Hyland, M.M.; Metson, J.B. Adsorptive capacity and evolution of the pore structure of alumina on reaction with gaseous hydrogen fluoride. Langmuir 2015, 31, 5387–5397. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wu, Z.-X. Experimental Study on Adsorption of Carbon Dioxide by 5A Molecular Sieve for Helium Purification of High-Temperature Gas-Cooled Reactor. Ind. Eng. Chem. Res. 2009, 48, 4466–4473. [Google Scholar] [CrossRef]

| Molecular Sieve | Chemical Composition | Pore Size/nm |
|---|---|---|
| 3A | Na6.6K5.4-[(AlO2)12(SiO2)12] | 0.3 |
| 4A | Na12·[(AlO2)12·(SiO2)12] | 0.4 |
| 5A | Ca6·[(AlO2)12·(SiO2)12] | 0.5 |
| F-03 | Na12·[(AlO2)12·(SiO2)15] | 1.0 |
| Items | Average Peak Area Ab/mV·s | IC/mV·s/mL | Sample Mass/g | Vad/mL/g |
|---|---|---|---|---|
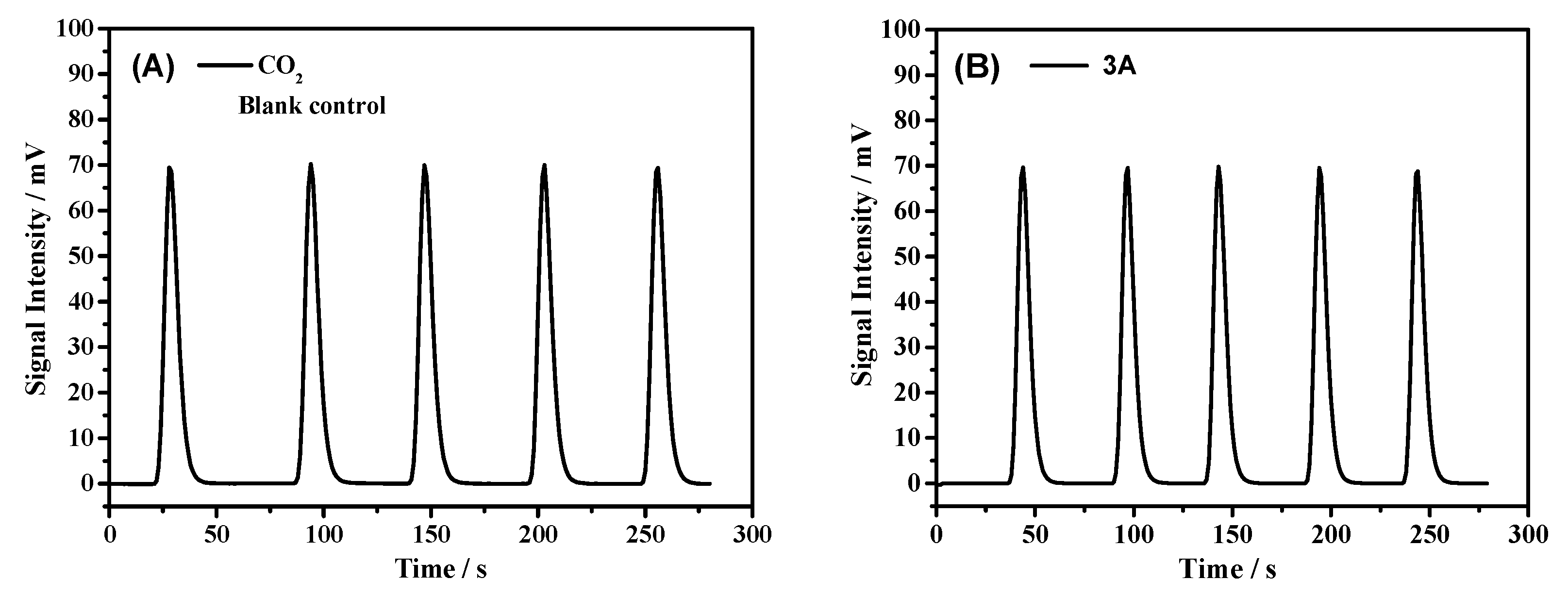
| Blank control | 1059 ± 13 | 3530 | - | - |
| 3A | 1047 ± 3 | 3490 | 0.1437 | 0.39 |
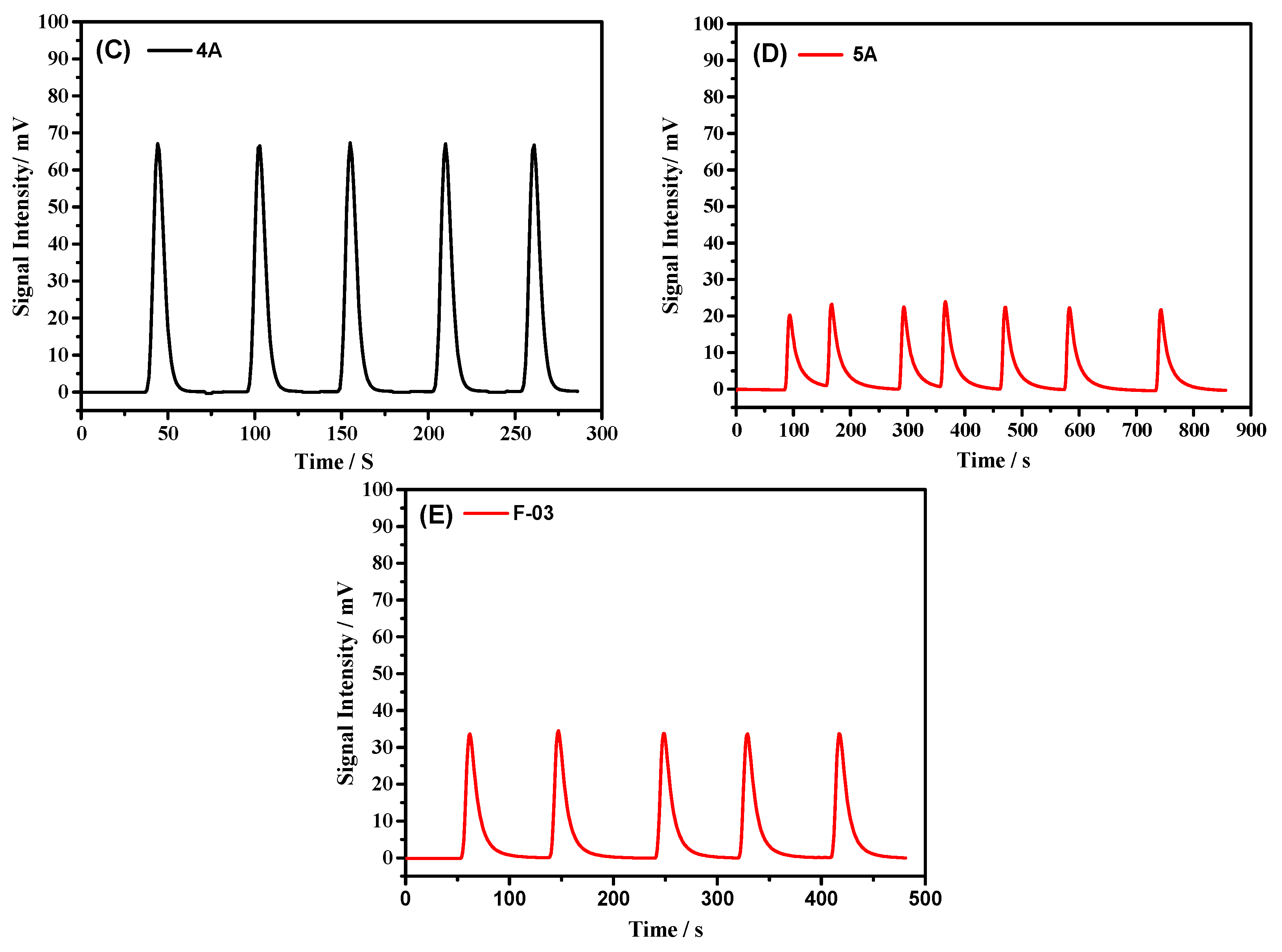
| 4A | 1045 ± 8 | 3483 | 0.0463 | 1.44 |
| 5A | 741 | 2470 | 0.0658 | 22.82 |
| F-03 | 1055 ± 10 | 3516 | 0.1028 | 0.19 |
| CaCl2 | 1060 ± 9 | 3533 | 0.1236 | - |
| MgSO4 | 1055 ± 3 | 3516 | 0.1327 | 0.15 |
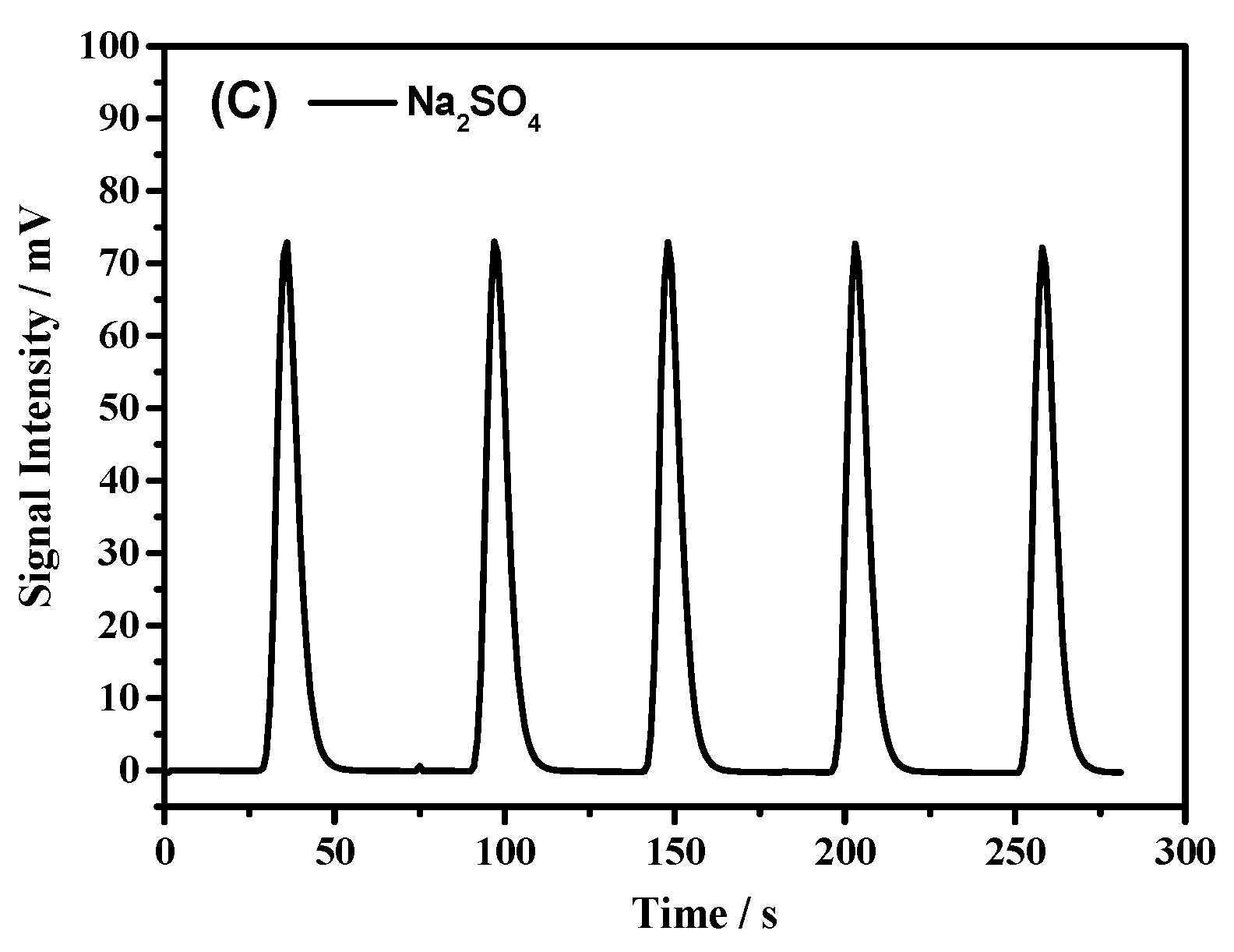
| Na2SO4 | 1056 ± 6 | 3520 | 0.1636 | 0.09 |
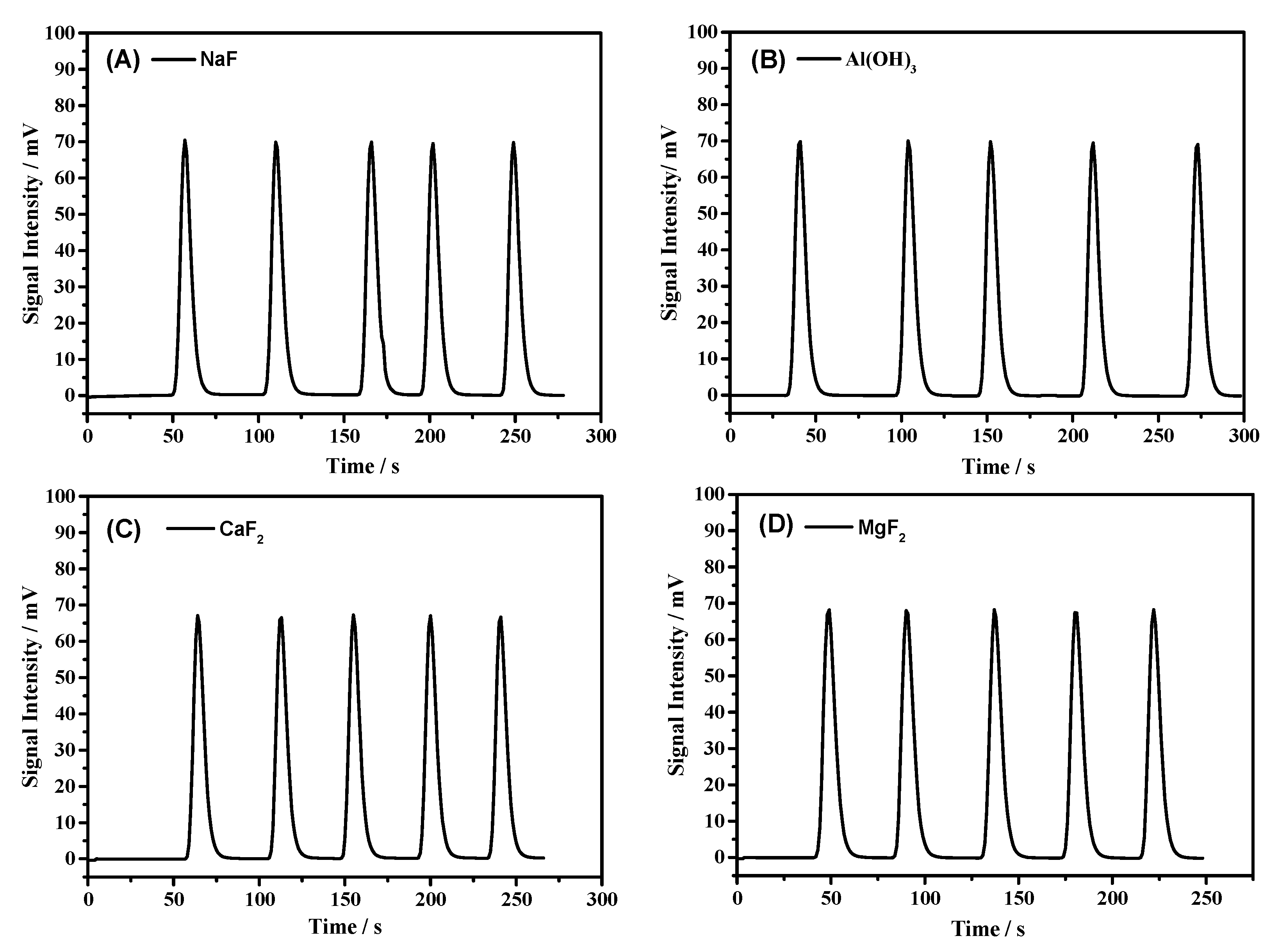
| Al(OH)3 | 1056 ± 6 | 3520 | 0.1018 | 0.14 |
| NaF | 1057 ± 9 | 3523 | 0.1138 | 0.09 |
| CaF2 | 1058 ± 5 | 3526 | 0.1042 | 0.05 |
| MgF2 | 1049 ± 19 | 3496 | 0.1445 | 0.33 |
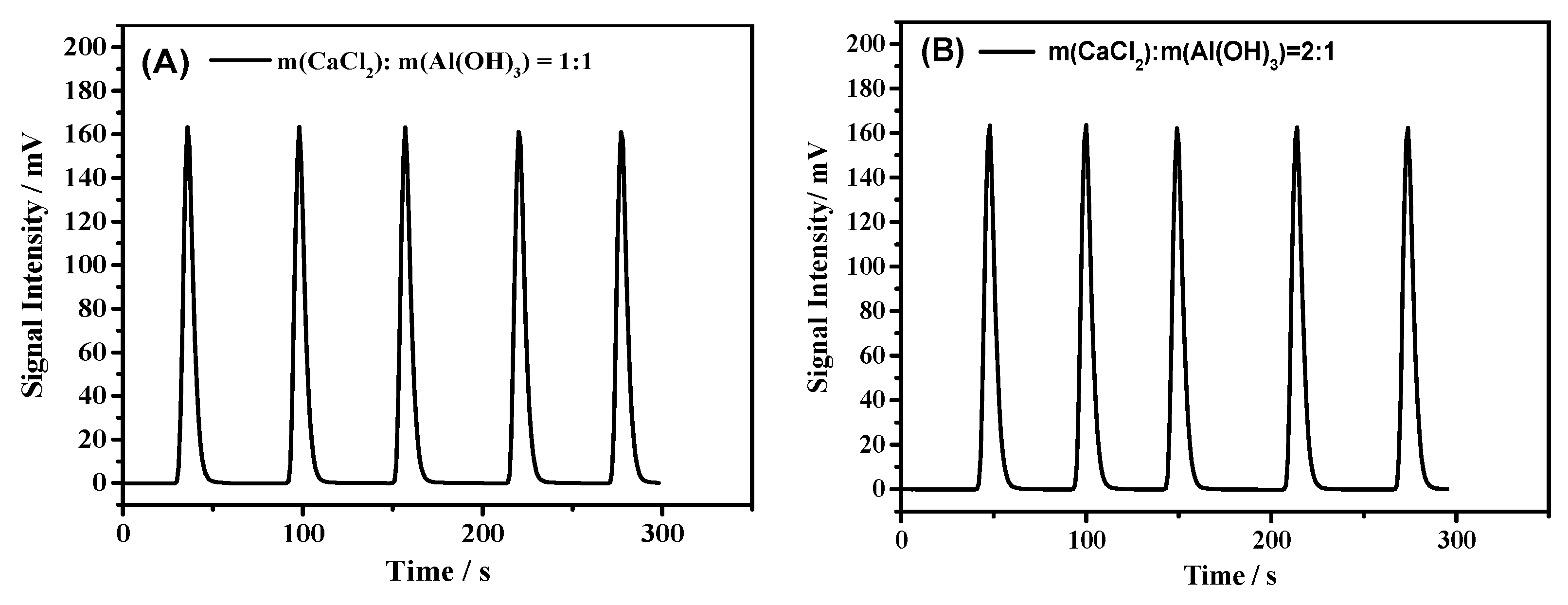
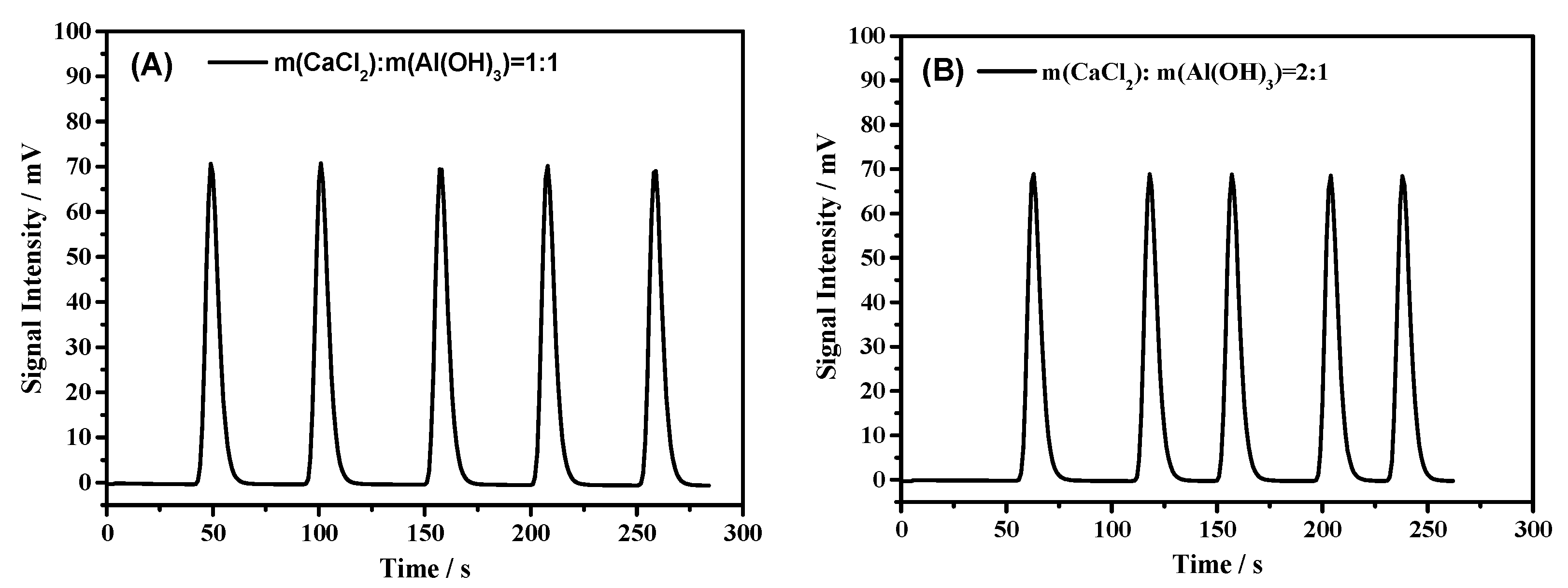
| m(CaCl2):m(Al(OH)3) = 1:1 | 1058 ± 10 | 3526 | 0.1426 | 0.04 |
| m(CaCl2):m(Al(OH)3) = 2:1 | 1057 ± 8 | 3523 | 0.1329 | 0.07 |
| Items | Average Peak Area Ab/mV·s | IC/mV·s/mL | Sample Mass/g | Vad/mL/g |
|---|---|---|---|---|
| Blank control | 523 ± 4 | 1743 | - | - |
| 3A | 516 ± 4 | 1720 | 0.0824 | 0.80 |
| 4A | 499 ± 4 | 1663 | 0.073 | 3.13 |
| 5A * | 430 | 1433 | 0.0407 | 43.66 |
| F-03 * | 427 | 1423 | 0.07 | 26.20 |
| CaCl2 | 524 ± 6 | 1747 | 0.0506 | 0 |
| MgSO4 | 525 ± 3 | 1750 | 0.0682 | 0 |
| Na2SO4 | 528 ± 5 | 1760 | 0.1317 | 0 |
| Al(OH)3 | 522 ± 2 | 1740 | 0.1285 | 0.07 |
| NaF | 520 ± 4 | 1733 | 0.1328 | 0.21 |
| CaF2 | 518 ± 7 | 1727 | 0.1233 | 0.38 |
| MgF2 | 518 ± 6 | 1727 | 0.1428 | 0.33 |
| m(CaCl2):m(Al(OH)3) = 1:1 | 519 ± 3 | 1730 | 0.1235 | 0.30 |
| m(CaCl2):m(Al(OH)3) = 2:1 | 512 ± 3 | 1707 | 0.1326 | 0.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Wang, Y.; Liu, J.; Zhang, Y.; Zeng, L. Study on the Compatibility of Gas Adsorbents Used in a New Insulating Gas Mixture C4F7N/CO2. Processes 2019, 7, 698. https://doi.org/10.3390/pr7100698
Huang Q, Wang Y, Liu J, Zhang Y, Zeng L. Study on the Compatibility of Gas Adsorbents Used in a New Insulating Gas Mixture C4F7N/CO2. Processes. 2019; 7(10):698. https://doi.org/10.3390/pr7100698
Chicago/Turabian StyleHuang, Qingdan, Yong Wang, Jing Liu, Yaru Zhang, and Lian Zeng. 2019. "Study on the Compatibility of Gas Adsorbents Used in a New Insulating Gas Mixture C4F7N/CO2" Processes 7, no. 10: 698. https://doi.org/10.3390/pr7100698
APA StyleHuang, Q., Wang, Y., Liu, J., Zhang, Y., & Zeng, L. (2019). Study on the Compatibility of Gas Adsorbents Used in a New Insulating Gas Mixture C4F7N/CO2. Processes, 7(10), 698. https://doi.org/10.3390/pr7100698




